1. Introduction
N-3 long-chain polyunsaturated fatty acids (LC-PUFAs) such as eicosapentaenoic acid (EPA, 20:5n-3), docosapentaenoic acid (DPA, 22:5n-3), and docosahexaenoic acid (DHA, 22:6n-3) are crucial for proper animal growth and development [
1]. Many vertebrates possess the enzymatic repertoire to biosynthesize n-3 LC-PUFAs from their dietary C18 precursor alfa-linolenic acid (ALA, 18:3n-3) through elongation and desaturation processes [
2]. However, humans are relatively inefficient at synthesizing n-3 LC-PUFAs, which, therefore, must necessarily be obtained through the diet [
3]. Marine fish are the primary source of n-3 LC-PUFAs for humans. Nevertheless, the sustainability challenges of aquaculture and the global depletion of most fish stocks due to overfishing make it essential to explore alternative sources of n-3 fatty acids (FAs) from terrestrial animals [
4].
In recent decades, poultry meat consumption has risen globally due to its affordability as a source of protein and bioactive substances, including vitamins and antioxidants [
5]. Poultry meat is considered the most cost-effective and sustainable terrestrial animal protein source because of its high efficiency in converting feed into meat [
6]. Moreover, poultry has been regarded as a viable source of LC-PUFAs due to its inherent enzymatic capability for synthesizing these physiologically important FAs [
7,
8]. However, as current livestock diets are especially rich in linoleic acid (LA, 18:2n-6), poultry products such as meat and eggs are common sources of n-6 PUFAs, including LA and arachidonic acid (ARA, 20:4n-6) [
9]. The biosynthesis of ARA and EPA from LA and ALA, respectively, involves the same desaturase and elongase enzymes, resulting in a competition between the two groups of PUFAs. Therefore, reducing the dietary n-6/n-3 ratio (LA/ALA) could increase the content of beneficial n-3 LC-PUFAs in poultry products [
10].
The search for sources of n-3 LC-PUFAs has become a significant focus for the food industry. In this context, several strategies have been conducted to improve the nutritional quality of chicken meat, including feeding animals with vegetable oils richer in ALA such as LO [
11,
12,
13]. However, the initial step in the biosynthesis of EPA and DHA from ALA to generate stearidonic acid (SDA, 18:4n-3) requires the Δ6 desaturase enzyme that is considered rate limiting [
7]. Therefore, utilizing vegetable sources containing SDA may also represent a more effective strategy to enhance the levels of EPA, DPA, and DHA in the final product [
14]. Although SDA is present in relatively low amounts in most plant oils, it is present at an important ratio in the Primulaceae, Cannabaceae, and Boraginaceae families, this last including the
Echium genus [
15]. In fact,
Echium oil (EO) contains significant amounts of ALA, SDA, and the nutraceutical γ-linolenic acid (GLA, 18:3n-6), and moderate levels of LA [
16,
17].
The Canary Islands are recognized as a biodiversity hotspot, boasting over 680 endemic plant species and being the largest center for the
Echium genus, with 28 endemic taxa [
18]. Approximately 28% of the 13,000 plant and animal species in the Canaries are unique to the region, making it one of the world’s foremost areas of endemism [
19]. As a result of the unique climate and location isolation, livestock animals such as chicken that were introduced during the XV and XVI centuries have generated, over time, a distinct breed with specific features [
20]. Canarian native chickens are well adapted to their local environmental conditions and traditional breeding practices, linked to sustainable management models. In contrast to most genetically selected poultry breeds, the Canarian genotype is distinguished by its dual-purpose nature, well suited for fattening and egg laying [
21]. Unlike commercial crossbreeds, local chicken breeds represent an important genetic heritage that must be conserved to maintain biodiversity and could be exploited in genetic selection programs to improve resilience to environmental stresses [
22].
Previous studies [
23,
24,
25] have examined the effects of SDA-rich diets on the lipid composition of chicken tissues. These investigations were conducted using fast-growing (FG) genotypes in intensive farming systems, where high growth rates prioritize muscle development and influence polyunsaturated fatty acids (PUFAs) accumulation [
26]. In contrast, free-range systems favor animal welfare and sustainability, providing chickens with a better life quality [
27]. While this approach may be seen as economically disadvantageous, it can represent added value to consumers by offering a more acceptable and sustainable method for poultry production [
28]. Recent data published by Villora et al. [
14] demonstrated that slow-growing Canarian chickens fed diets supplemented with EO exhibited increased expression of hepatic elongases, which correlated with higher n-3 LC-PUFAs levels in thigh meat. This research aims to complement these findings by investigating the growth, performance, FA composition, and sensory qualities of Canarian cockerel breast meat. Specifically, the study addresses two key issues: (1) whether a diet containing higher proportions of SDA and ALA is more effective than a richer one in ALA, and (2) whether the benefits are more pronounced in dual-purpose native breeds like Canarian chicken than in intensively reared broilers, based on available data.
4. Discussion
The dietary supplementation with SO, LO, or EO resulted in similar chicken growth performance, as the final live bird weights were all around 3 kg (
Table 2). Although overall data on the Canarian chicken are limited, Torres et al. [
41] evaluated the morphological characteristics of the local rooster and hen populations in the Canary Islands, establishing a mean weight of 3.5 kg in adult males. In this study, the carcass weight was approximately 2.1 kg, yielding a dressing percentage of 70–73%. This is notably higher than the 64.34% reported by Torres et al. [
21] for male Canarian cockerels slaughtered at 15 weeks, likely because our measurements were taken at 24 weeks. LO and EO did not vary performance outcomes such as carcass weights and yields of main joints when compared to the SO-d. Our findings are consistent with those by Kitessa and Young [
23] when using EO-supplemented diets in Australian broilers and also align well with other studies that reported no impact of dietary LO on carcass yields [
42,
43,
44]. Similarly, no differences in animal weight, carcass yield, or yield of different meat cuts were evident when incorporating oils high in SDAs, such as SDA-enriched soybean oil [
24] or ahiflower [
25].
Breast meat pH at 24 h post-slaughter (pH
24) remained fairly constant regardless of the dietary group (5.77–5.82;
Table 3). These values are within the expected limits of 5.75 and 5.96 at the end of post-mortem process outlined by Castellini et al. [
45]. Additionally, and in agreement with our results, previous research reported no difference in pH
24 after the inclusion of LO in the chicken diet [
46,
47,
48]. Likewise, Torres at al. [
21] stated a mean pH
24 value of 5.85 from Canarian cockerel meat fed with a commercial diet. Muscle pH is linked to various important attributes of meat quality, including color, cooking losses, and tenderness [
49]. As reviewed by Wideman et al. [
50], meat color is highly correlated with the amount of myoglobin in the muscle but is also influenced by pH and muscle type. There are contradictory results when studying the correlation between the dietary inclusion of n-3 rich oils and meat physical properties. While some studies have found no significant relationship between both factors [
46,
51,
52], others have shown some association. For instance, Qi et al. [
53] observed that reducing the n-6/n-3 ratio decreased breast muscle luminosity (L*). In our present research, there was no significant correlation between the dietary n-6/n-3 ratio and meat color parameters. However, the dietary inclusion of EO increased luminosity (L*) with respect to LO-d, resulting in lighter breast meat. This suggests that while the n-6/n-3 ratio itself may not directly influence meat color, the specific type of oil incorporated into the diet can differentially affect color characteristics.
The cooking loss values registered in our study are slightly lower than those reported by Torres et al. [
21] for the Canarian genotype, but they are in line with those obtained in the same research for Les Blues, another dual-purpose breed, at 15 weeks of age. Consistently, and regardless of the dietary group, meat shear forces were barely lower than those by Torres et al. [
21], despite their research using younger birds. This evidence contradicts the general trend described for different bird species where shear force typically increases with age since muscle protein content increases more sharply than other components, and the composition of the connective tissue becomes denser as the livestock mature [
26,
54,
55]. However, and in agreement with our results, some studies did not find differences in drip losses [
56] or shear force [
48,
52,
57] after the inclusion of dietary sources rich in PUFAs.
Under our experimental conditions, the dietary FA profile did not vary the chemical composition of chicken breast meat, including moisture, protein, fat, and ash (
Table 3). Our data confirm earlier findings that the dietary inclusion of LO did not significantly alter the protein content of birds’ meat [
43], nor did the lipid content change with the incorporation of n-3 PUFA-rich sources such as SDA-enriched soy oil [
58], linseed oil [
44], or chia seeds [
56]. Likewise, Kitessa and Young [
23] indicated that intramuscular fat remained constant when broilers were fed 3 g/kg of EO. The slightly higher meat protein proportions (26.42–27.33%), and the less than half total fat content (0.57–0.61%) recorded in our experiment compared to those of Canarian cockerels raised under free-range conditions [
21], might be attributed to a different age of slaughter [
59]. In this context, the lipid class composition of the muscle was stable, independently of the dietary treatment (
Table 4) with PC and PE as the dominant phospholipids [
8,
60], as both are integral essential components of biological membranes. To our knowledge, scientific data on the impact of omega-3-rich vegetable oils on the lipid class composition of poultry meat remain very limited.
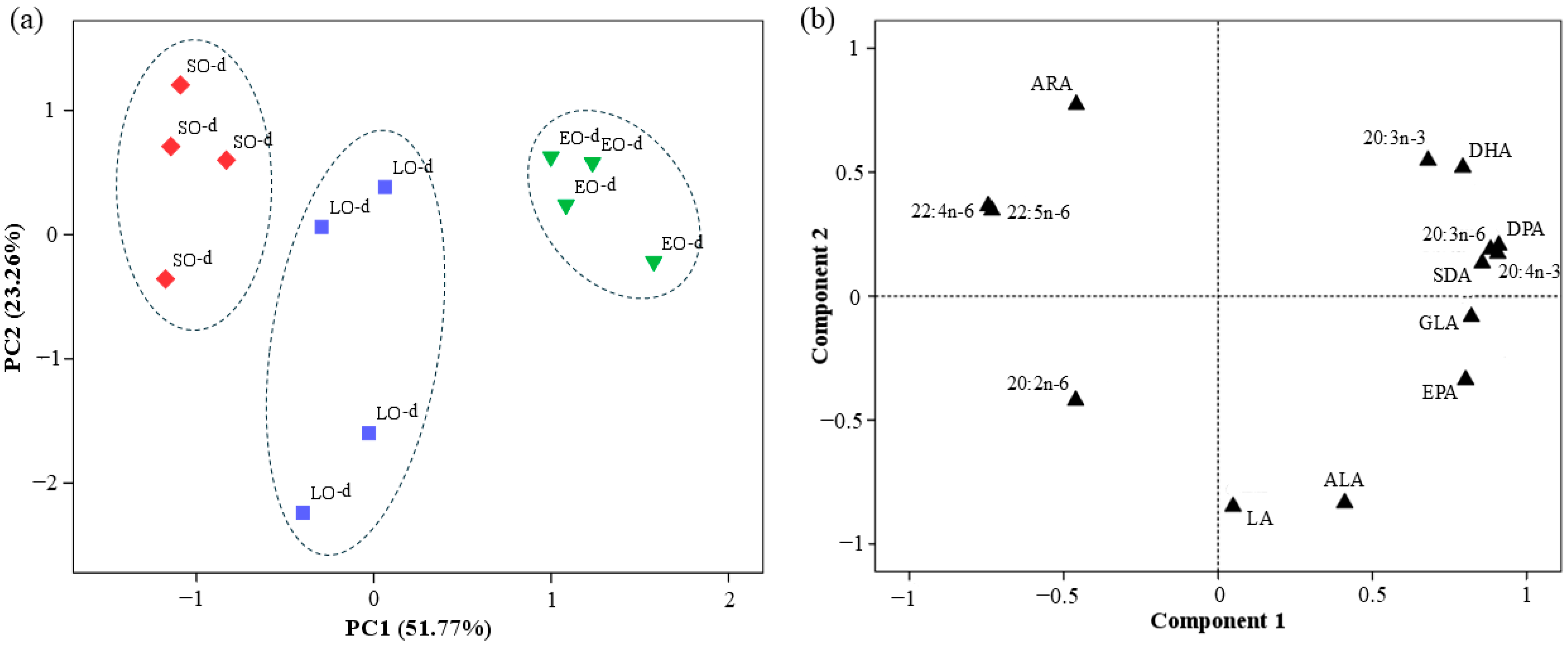
The LA levels of breast meat were similar in the three groups of Canarian cockerels (44.46–52.49 mg/100 fresh meat;
Table 5), despite the different dietary contents (~38% in the SO-d and ~29% in the LO and EO diets,
Table 1). Nevertheless, dietary LO and EO reduced ARA and its elongation and desaturation products with respect to SO. In agreement with our results, Rymer et al. [
58] and Elkin et al. [
24] demonstrated that lowering dietary LA led to a reduction of ARA in chicken breast meat. A reduction in the ingestion of ARA is associated with reduced inflammatory responses and improved cardiovascular health [
61]. In contrast, EO increased GLA and 20:3n-6 compared to the other experimental diets (
Table 5). Sergeant et al. [
62] highlighted the potential of GLA, especially when combined with n-3 LC-PUFAs, to mitigate inflammation and to improve symptoms of inflammatory diseases. Additionally, 20:3n-6 has been reported to produce bioactive compounds with anti-inflammatory, vasodilatory, and anti-neoplastic effects while also lowering blood pressure and inhibiting smooth muscle cell proliferation [
63]. Hence, the FA profile of breast meat from birds fed EO might be considered the most beneficial one for human health.
Previous studies found no evidence that birds converted SDA to DHA more efficiently than ALA [
23,
24,
25,
58]. A regulatory step after the initial hepatic Δ6 desaturation, possibly the second Δ6 step in the Sprecher pathway (desaturation of 24:5n-3 to 24:6n-3), might be limiting the production of DHA. In this study, chickens fed the EO-d exhibited superior efficiency versus LO in promoting the accumulation of n-3 LC-PUFAs, including DPA and DHA (7.03 vs. 9.41 mg/100 g, respectively,
Table 5), indicating a more effective role in enriching poultry meat. The discrepancies between previous studies and the present research may be due to genetic selection. While earlier studies focused on FG broilers, our study examined the Canarian breed, a slow-growing (SG) and dual-purpose local genotype. In this regard, Boschetti et al. [
64] confirmed that the SG genotype exhibited enhanced expression of FADS1 and FADS2 genes and higher Δ6 and Δ5 desaturase activity. Additionally, recent studies by Cartoni-Mancinelli et al. [
65] have demonstrated higher expression of FADS2 and increased Δ6 desaturase activity in SG (Leghorn) compared to FG (Ross 308), enabling more efficient synthesis of n-3 LC-PUFAs. These findings highlight the role of genetic factors in LC-PUFA synthesis and suggest that selecting slow-growing genotypes with higher elongase and desaturase activity could be a promising strategy for improving the nutritional quality of poultry products.
Most health organizations recommend adults to daily consume between 250 and 1000 mg of EPA + DHA for overall health and well-being, although specific guidelines for different ages and physiological groups still require further research [
66]. In Europe, current legislation requires that foods must contain a minimum of 40 mg per 100 g and 100 kcal of EPA + DHA to be considered a source of omega-3 [
67]. Breast meat from chicken fed the EO-supplemented diet contained approximately 12.5 mg of EPA and DHA per 100 g of fresh tissue, which falls below the threshold required to be regarded as an omega-3 source (
Table 5). Nevertheless, chicken breast meat might still represent an important dietary contribution of n-3 LC-PUFAs, particularly for populations with limited access to marine products. Moreover, it is important to note that other n-3 LC-PUFAs such as DPA were the predominant n-3 FAs in the breast meat of EO-supplemented birds (14.4 mg/100 g). DPA possesses some beneficial and potentially unique properties such as reducing the expression of inflammatory genes, preventing angiogenesis, and inhibiting platelet aggregation more effectively than EPA and DHA [
68]. In concordance, the inclusion of EO resulted in breast meat with a lower TI compared to SO-d and LO-d (
Table 6), which has a protective action against coronary heart diseases [
69].
The inclusion of vegetable oils richer in n-3 PUFAs did not lead to variations in the sensory perception evaluated by the trained panel compared to samples from animals fed with higher n-6 FA proportions (
Table 7). Odor and flavor, alongside texture, are key sensory quality attributes for consumers when determining the acceptability of chicken meat [
70]. Although LO and EO diets resulted in slightly lower scores for breast odor and flavor compared to the SO-d, these differences were not statistically significant. Similarly, Rymer et al. [
60] reported that slight differences perceived in the breast meats of birds fed SDA-enriched SO were associated with the texture and appearance of the meat, but not its flavor, aroma, or aftertaste. Consistent with our findings, other authors observed no significant variations in the sensory quality attributes of chicken meat due to the dietary inclusion of different LO levels [
11,
40,
71,
72]. It is noteworthy that all groups received high scores across the analyzed variables. Of particular interest is the appearance, which consistently scored close to 7 points for all diets. These values are moderately higher than those reported by Torres et al. [
21], not only for the Canarian chicken genotype but also for other breeds evaluated in that study, such as Les Blues and Dominant Red Barred. Notably, undesirable odors or unusual aromas were not detected by the inclusion of dietary EO. These results suggest that, from a sensory perspective, the use of experimental diets, including those incorporating EO, could be suitable for poultry production.