Optimization of Solid-State Fermentation Process for Dietary Fiber in Flaxseed Meal and Analysis of Its Microstructure and Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Culture Medium Preparation
2.3. Microbial Cultivation
2.3.1. Seed Culture Preparation
2.3.2. Fermentation Broth Preparation
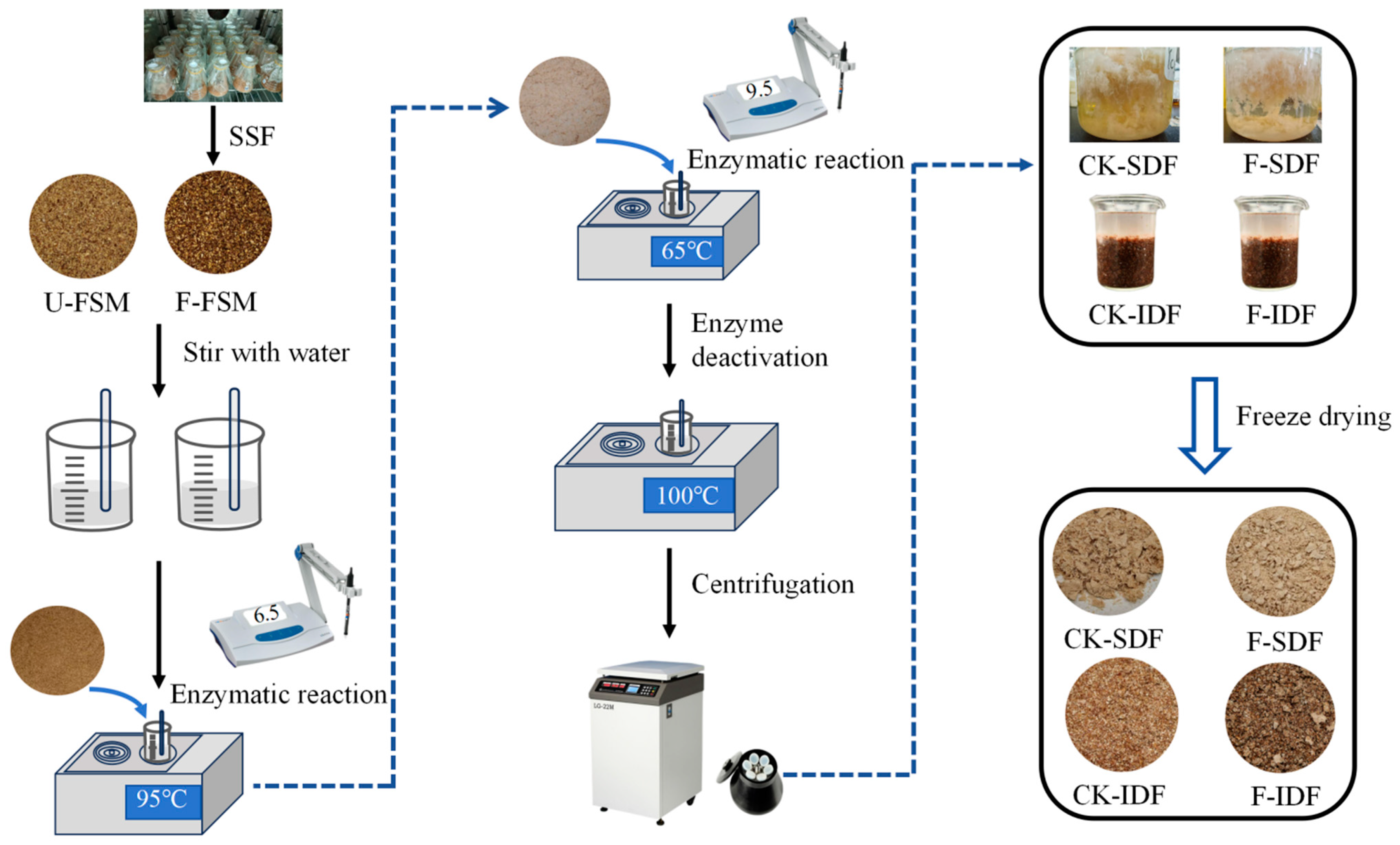
2.4. Extracting SDF
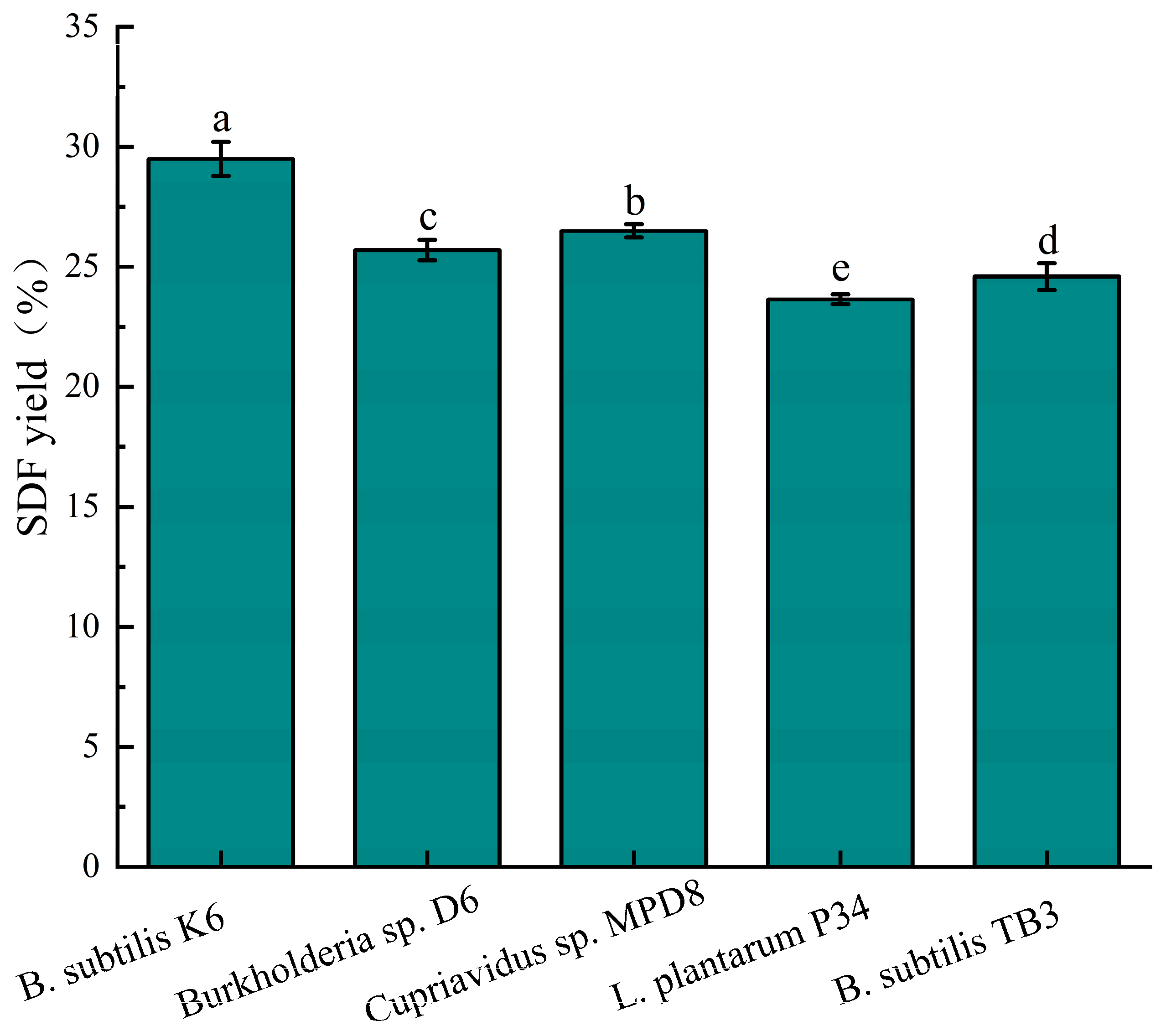
2.5. Screening of Microbial Strains for the Modification of SDF Fermentation
2.6. Optimization of the Fermentation Conditions
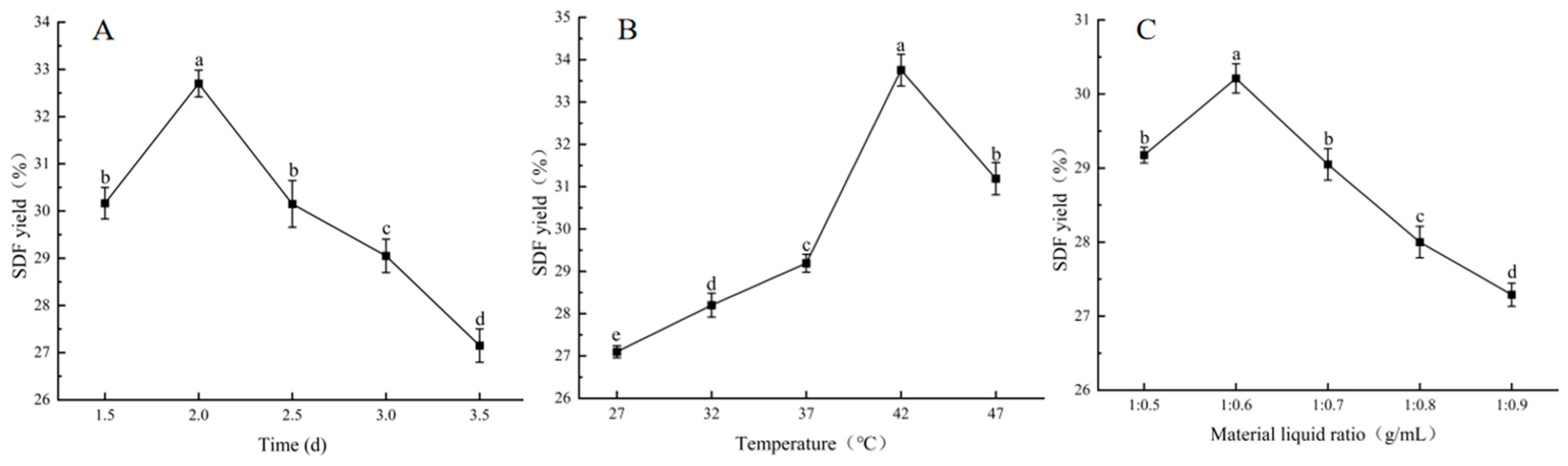
2.6.1. Single-Factor Test
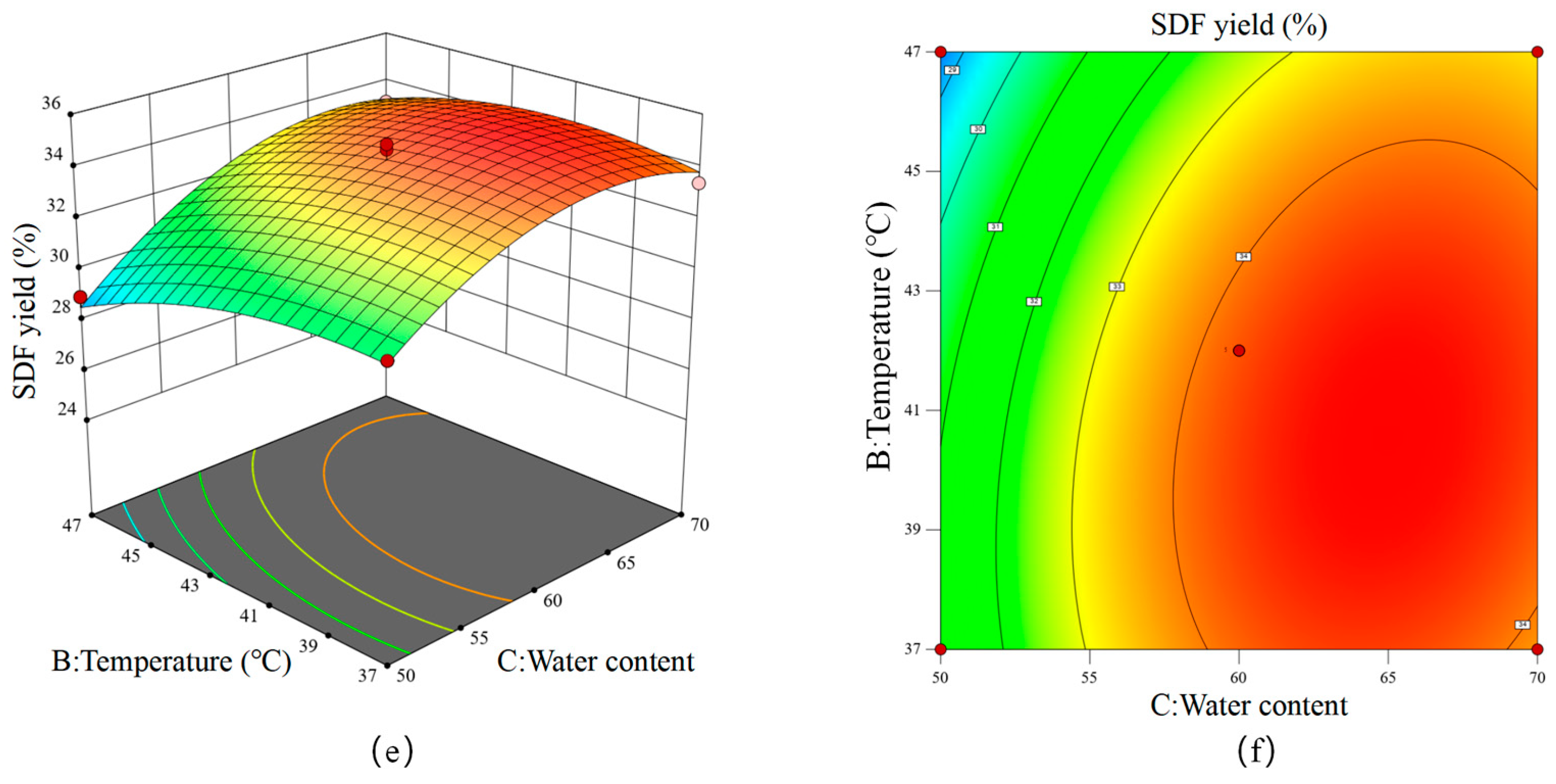
2.6.2. Response Surface Methodology (RSM)
2.7. Chemical Composition Determination
2.8. Structural Characterization
2.8.1. Scanning Electron Microscopy (SEM)
2.8.2. Confocal Laser Scanning Microscopy (CLSM)
Staining Procedure
Imaging Analysis
2.9. Functional Features
2.9.1. Water-Holding Capacity (WHC)
2.9.2. Oil-Holding Capacity (OHC)
2.9.3. Swelling Capacity (SC)
2.10. Statistical Analyses
3. Results and Discussion
3.1. Screening of Microbial Strains for SDF Fermentation Modification
3.2. Single-Factor Test
3.3. Optimization of the Fermentation Conditions
3.4. Principal Component Analysis
3.5. Analysis of the Structural Properties
3.5.1. SEM
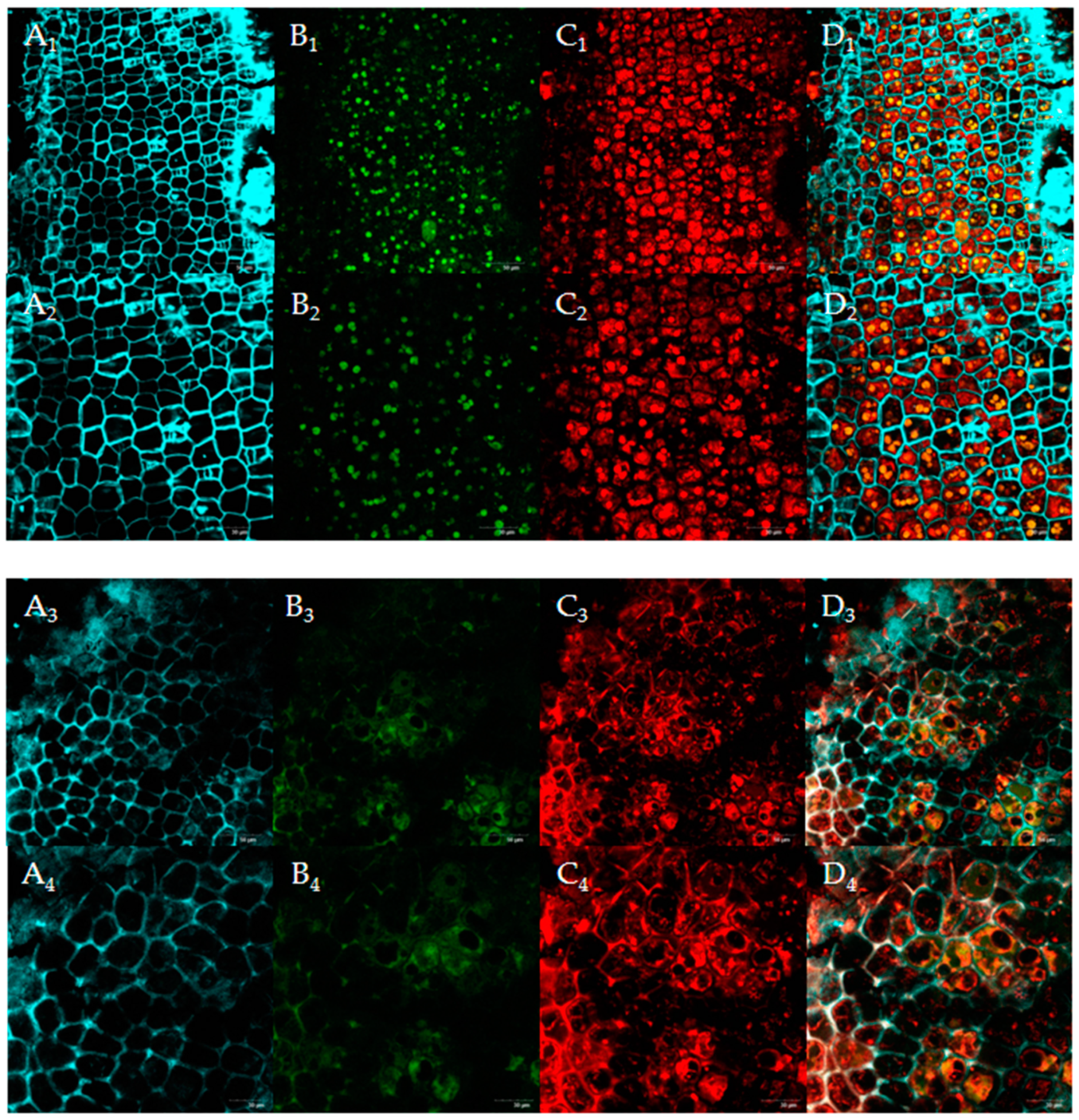
3.5.2. CLSM
3.6. Functional Features
3.6.1. WHC
3.6.2. OHC
3.6.3. SC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Sci. Technol. 2014, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; Teh, S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Lan, Y.; Ohm, J.-B.; Chen, B.; Rao, J. Physicochemical properties and aroma profiles of flaxseed proteins extracted from whole flaxseed and flaxseed meal. Food Hydrocoll. 2020, 104, 105731. [Google Scholar] [CrossRef]
- Ye, X.-P.; Xu, M.-F.; Tang, Z.-X.; Chen, H.-J.; Wu, D.-T.; Wang, Z.-Y.; Songzhen, Y.-X.; Hao, J.; Wu, L.-M.; Shi, L.-E. Flaxseed protein: Extraction, functionalities and applications. Food Sci. Technol. 2022, 42, e22021. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Cao, S.; Liu, C.; Han, Y.; Cheng, J.; Zhang, S.; Zhao, J.; Shi, Y. Let food be your medicine—Dietary fiber. Food Funct. 2024, 15, 7733–7756. [Google Scholar] [CrossRef]
- Yang, Y.-y.; Ma, S.; Wang, X.-x.; Zheng, X.-l. Modification and Application of Dietary Fiber in Foods. J. Chem. 2017, 2017, 9340427. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Miehle, E.; Haas, M.; Bader-Mittermaier, S.; Eisner, P. The role of hydration properties of soluble dietary fibers on glucose diffusion. Food Hydrocoll. 2022, 131, 107822. [Google Scholar] [CrossRef]
- Li, S.; Hu, N.; Zhu, J.; Zheng, M.; Liu, H.; Liu, J. Influence of modification methods on physicochemical and structural properties of soluble dietary fiber from corn bran. Food Chem. X 2022, 14, 100298. [Google Scholar] [CrossRef]
- Akal, C. Using dietary fiber as stabilizer in dairy products: β-glucan and inulin-type fructans. J. Food Sci. Technol. 2022, 60, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lu, L.; Zhao, L.; Peng, J.; Zhou, W. Improvement of okara noodle quality by modifying the soluble/insoluble dietary fibre ratio. Food Chem. 2025, 464, 141566. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zheng, S.; Bai, T.; Chen, D.; Qin, W.; Zhang, Q.; Lin, D.; Liu, Y.; Liu, A.; Huang, Z.; et al. The difference among structure, physicochemical and functional properties of dietary fiber extracted from triticale and hull-less barley. LWT 2022, 154, 112771. [Google Scholar] [CrossRef]
- Sang, J.; Li, L.; Wen, J.; Liu, H.; Wu, J.; Yu, Y.; Xu, Y.; Gu, Q.; Fu, M.; Lin, X. Chemical composition, structural and functional properties of insoluble dietary fiber obtained from the Shatian pomelo peel sponge layer using different modification methods. LWT 2022, 165, 113737. [Google Scholar] [CrossRef]
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Wang, J.-D.; Cai, Z.-H.; Huang, H.; Zhang, S.; Fu, L.-N.; Zhao, P.-Q.; Yan, X.-Y.; Fu, Y.-J. Improved physicochemical and functional properties of dietary fiber from Rosa roxburghii pomace fermented by Bacillus natto. Food Biosci. 2022, 50, 102030. [Google Scholar] [CrossRef]
- Chen, Q.; Mao, J.; Wang, Y.; Yin, N.; Liu, N.; Zheng, Y.; An, X.; Qi, J.; Wang, R.; Yang, Y. Fermented Wheat Bran Polysaccharides Improved Intestinal Health of Zebrafish in Terms of Intestinal Motility and Barrier Function. Fermentation 2023, 9, 293. [Google Scholar] [CrossRef]
- Shao, Y.; Kang, Q.; Zhu, J.; Zhao, C.; Hao, L.; Huang, J.; Lu, J.; Jia, S.; Yi, J. Antioxidant properties and digestion behaviors of polysaccharides from Chinese yam fermented by Saccharomyces boulardii. LWT 2022, 154, 112752. [Google Scholar] [CrossRef]
- Meng, W.; Hu, M.; Zhang, P.; Wang, J.; Yuan, Z.; Wang, F.; Li, S. Efficient conversion of insoluble dietary fiber to soluble dietary fiber by Bacillus subtilis BSNK-5 fermentation of okara and improvement of their structural and functional properties. Food Chem. 2025, 474, 143188. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xue, P.; Chen, Y.; Xie, J.; Peng, G.; Tian, S.; Chang, X.; Yu, Q. Effect of Soluble Dietary Fiber of Navel Orange Peel Prepared by Mixed Solid-State Fermentation on the Quality of Jelly. Foods 2023, 12, 1724. [Google Scholar] [CrossRef]
- Wu, L.; Tang, C.; Chen, L.; Zhao, J. Modified dietary fiber from soybean dregs by fermentation alleviated constipation in mice. Food Chem. X 2023, 19, 100810. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Factories 2020, 19, 173. [Google Scholar] [CrossRef]
- He, M.; Peng, Q.; Xu, X.; Shi, B.; Qiao, Y. Antioxidant capacities and non-volatile metabolites changes after solid-state fermentation of soybean using oyster mushroom (Pleurotus ostreatus) mycelium. Front. Nutr. 2024, 11, 1509341. [Google Scholar] [CrossRef] [PubMed]
- Ban, H.; Liu, Q.; Xiu, L.; Cai, D.; Liu, J. Effect of Solid-State Fermentation of Hericium erinaceus on the Structure and Physicochemical Properties of Soluble Dietary Fiber from Corn Husk. Foods 2024, 13, 2895. [Google Scholar] [CrossRef]
- Vong, W.C.; Au Yang, K.L.C.; Liu, S.-Q. Okara (soybean residue) biotransformation by yeast Yarrowia lipolytica. Int. J. Food Microbiol. 2016, 235, 1–9. [Google Scholar] [CrossRef]
- Liang, Z.; Li, K.; Huang, W.; Li, Z.; Xu, X.; Xu, H.; Li, S. Production, structural and functional characteristics of soluble dietary fiber from fermented okara by Penicillium expansum. Int. J. Biol. Macromol. 2023, 253, 126621. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, L.; Guo, Q.; Shi, L.; Deng, X.; Liu, X.; Xiao, C. Effects of fermentation with Lactic bacteria on the structural characteristics and physicochemical and functional properties of soluble dietary fiber from prosomillet bran. LWT 2022, 154, 112609. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Chen, Y.; Xie, J.; Song, Y.; Chang, X.; Liu, S.; Wang, Z.; Hu, X.; Yu, Q. Effects of fermentation on the structural characteristics and in vitro binding capacity of soluble dietary fiber from tea residues. LWT 2020, 131, 109818. [Google Scholar] [CrossRef]
- Yang, C.; Yao, J.; Zhang, T.; Yang, K.; Guo, j.; Pan, S. Mixed fermentation of navel orange peel by Trichoderma viride and Aspergillus niger: Effects on the structural and functional properties of soluble dietary fiber. Food Biosci. 2024, 57, 103545. [Google Scholar] [CrossRef]
- Liao, W.; Liu, S.; Dong, R.; Xie, J.; Chen, Y.; Hu, X.; Xie, J.; Xue, P.; Feng, L.; Yu, Q. Mixed solid-state fermentation for releasing bound polyphenols from insoluble dietary fiber in carrots via Trichoderma viride and Aspergillus niger. Food Funct. 2022, 13, 2044–2056. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 991.43; Total, Soluble, and Insoluble Dietary Fiber in Foods; Association of Official Analytical Chemists: Washington, DC, USA, 1996. [Google Scholar]
- Wang, L.; Xu, H.; Yuan, F.; Fan, R.; Gao, Y. Preparation and physicochemical properties of soluble dietary fiber from orange peel assisted by steam explosion and dilute acid soaking. Food Chem. 2015, 185, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Richert, A.; Kalwasińska, A.; Brzezinska, M.S.; Dąbrowska, G.B. Biodegradability of Novel Polylactide and Polycaprolactone Materials with Bacteriostatic Properties Due to Embedded Birch Tar in Different Environments. Int. J. Mol. Sci. 2021, 22, 10228. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Wang, Y.; Li, X.; Zhu, M.; Zhang, H.; Xu, M.; Yang, T.; Rao, Z. Heterologous Expression and Rational Design of l-asparaginase from Rhizomucor miehei to Improve Thermostability. Biology 2021, 10, 1346. [Google Scholar] [CrossRef]
- Bader Ul Ain, H.; Saeed, F.; Ahmed, A.; Asif Khan, M.; Niaz, B.; Tufail, T. Improving the physicochemical properties of partially enhanced soluble dietary fiber through innovative techniques: A coherent review. J. Food Process. Preserv. 2019, 43, e13917. [Google Scholar] [CrossRef]
- Sadh, P.K.; Chawla, P.; Bhandari, L.; Duhan, J.S. Bio-enrichment of functional properties of peanut oil cakes by solid state fermentation using Aspergillus oryzae. J. Food Meas. Charact. 2017, 12, 622–633. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Sun, M.; Li, D.; Hua, M.; Miao, X.; Su, Y.; Chi, Y.; Wang, J.; Niu, H. Optimization of Mixed Fermentation Conditions of Dietary Fiber from Soybean Residue and the Effect on Structure, Properties and Potential Biological Activity of Dietary Fiber from Soybean Residue. Molecules 2023, 28, 1322. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Li, N.; Wu, J.-L. Food-polysaccharide utilization via in vitro fermentation: Microbiota, structure, and function. Curr. Opin. Food Sci. 2022, 48, 100911. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids acting as emulsifying agents—How do they do it? Food Hydrocoll. 2018, 78, 2–14. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, R.; Yin, L.; Zhang, N. Novel blasting extrusion processing improved the physicochemical properties of soluble dietary fiber from soybean residue and in vivo evaluation. J. Food Eng. 2014, 120, 1–8. [Google Scholar] [CrossRef]
- Kim, H.-S.; Yu, O.-K.; Byun, M.-S.; Cha, Y.-S. Okara, a soybean by-product, prevents high fat diet-induced obesity and improves serum lipid profiles in C57BL/6J mice. Food Sci. Biotechnol. 2016, 25, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Y.; Liao, A.-M.; Thakur, K.; Huang, J.-H.; Zhang, J.-G.; Wei, Z.-J. Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution. Food Chem. 2019, 297, 124983. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, M.; Liu, Y.; Liu, J.; Zheng, T.; Li, Y.; He, S.; Jiang, M.; Wu, L.; Liu, F. Influence of fermentation with lactic bacteria on the structure, functional properties and antioxidant activity of flaxseed gum. Int. J. Biol. Macromol. 2024, 281, 136133. [Google Scholar] [CrossRef] [PubMed]


| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A—Fermentation time (d) | 1.5 | 2 | 2.5 |
| B—Fermentation temperature (°C) | 37 | 42 | 47 |
| C—Material-to-liquid ratio (g/mL) | 1:0.5 | 1:0.6 | 1:0.7 |
| Run | A | B | C | Y (%) |
|---|---|---|---|---|
| 1 | 1.5 | 47 | 60 | 31.56 ± 0.11 |
| 2 | 2.5 | 42 | 50 | 27.67 ± 0.06 |
| 3 | 2 | 47 | 70 | 33.12 ± 0.17 |
| 4 | 2 | 37 | 70 | 33.39 ± 0.20 |
| 5 | 2 | 42 | 60 | 34.87 ± 0.05 |
| 6 | 1.5 | 42 | 70 | 33.10 ± 0.22 |
| 7 | 2 | 42 | 60 | 33.79 ± 0.13 |
| 8 | 2.5 | 47 | 60 | 29.56 ± 0.35 |
| 9 | 2 | 37 | 50 | 31.19 ± 0.27 |
| 10 | 2.5 | 42 | 70 | 32.38 ± 0.21 |
| 11 | 2 | 42 | 60 | 34.66 ± 0.08 |
| 12 | 2 | 47 | 50 | 28.98 ± 0.16 |
| 13 | 1.5 | 37 | 60 | 33.28 ± 0.15 |
| 14 | 1.5 | 42 | 50 | 29.30 ± 0.31 |
| 15 | 2.5 | 37 | 60 | 31.47 ± 0.15 |
| 16 | 2 | 42 | 60 | 34.16 ± 0.07 |
| 17 | 2 | 42 | 60 | 33.91 ± 0.22 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significance a |
|---|---|---|---|---|---|---|
| Model | 73.01 | 9 | 8.11 | 29.82 | <0.0001 | ** |
| A—Time | 4.74 | 1 | 7.47 | 17.44 | 0.0042 | ** |
| B—Temperature | 4.67 | 1 | 4.67 | 17.15 | 0.0043 | ** |
| C—Material-to-liquid ratio | 27.51 | 1 | 27.57 | 101.33 | <0.0001 | ** |
| AB | 0.0090 | 1 | 0.0090 | 0.0332 | 0.8606 | |
| AC | 0.2070 | 1 | 0.2070 | 0.7610 | 0.4119 | |
| BC | 0.9409 | 1 | 0.9409 | 3.46 | 0.1052 | |
| A2 | 15.75 | 1 | 15.75 | 57.89 | 0.0001 | ** |
| B2 | 3.23 | 1 | 3.23 | 11.89 | 0.0107 | * |
| C2 | 12.62 | 1 | 12.62 | 46.40 | 0.0003 | ** |
| Resdual | 1.90 | 7 | 0.2720 | |||
| Lack of fit | 1.02 | 3 | 0.3401 | 1.54 | 0.3347 | Not significant |
| Pure error | 0.8839 | 4 | 0.2210 | |||
| Cor total | 74.92 | 16 | ||||
| R-Squared | 0.9746 | |||||
| Adj.R-squared | 0.9419 | |||||
| Adeq.precision | 16.2970 | |||||
| C.V. % | 1.62 |
| Protein (%) | Ash (%) | Fat (%) | Moisture (%) | SDF (%) | IDF (%) | |
|---|---|---|---|---|---|---|
| U-FSM | 39.37 ± 0.52 | 4.29 ± 0.1 | 4.75 ± 0.25 | 5.52 ± 0.2 | 24.43 ± 0.7 | 40.77 ± 0.2 |
| F-FSM | 46.51 ± 0.27 | 4.74 ± 0.15 | 2.5 ± 0.5 | 3.18 ± 0.14 | 33.45 ± 0.24 | 28.27 ± 0.13 |
| Item | CK-SDF | F-SDF | CK-IDF | F-IDF |
|---|---|---|---|---|
| WHC | 12.3 ± 0.14 b | 14.49 ± 0.24 a | 10.91 ± 0.2 c | 12.26 ± 0.21 b |
| OHC | 5.97 ± 0.1 c | 9.65 ± 0.09 a | 4.34 ± 0.11 d | 7.15 ± 0.13 b |
| SC | 4.3 ± 0.21 b | 5.0 ± 0.13 a | 0.67 ± 0.17 d | 1.33 ± 0.24 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, C.; Zhang, Y.; Chen, J.; Hu, J.; Yang, C.; Chen, F.; Zhu, T.; Xin, Y.; Geng, X. Optimization of Solid-State Fermentation Process for Dietary Fiber in Flaxseed Meal and Analysis of Its Microstructure and Functional Properties. Foods 2025, 14, 1722. https://doi.org/10.3390/foods14101722
Hou C, Zhang Y, Chen J, Hu J, Yang C, Chen F, Zhu T, Xin Y, Geng X. Optimization of Solid-State Fermentation Process for Dietary Fiber in Flaxseed Meal and Analysis of Its Microstructure and Functional Properties. Foods. 2025; 14(10):1722. https://doi.org/10.3390/foods14101722
Chicago/Turabian StyleHou, Chunpeng, Yiyang Zhang, Jiaxun Chen, Jianguo Hu, Chenxian Yang, Fusheng Chen, Tingwei Zhu, Ying Xin, and Xiaohui Geng. 2025. "Optimization of Solid-State Fermentation Process for Dietary Fiber in Flaxseed Meal and Analysis of Its Microstructure and Functional Properties" Foods 14, no. 10: 1722. https://doi.org/10.3390/foods14101722
APA StyleHou, C., Zhang, Y., Chen, J., Hu, J., Yang, C., Chen, F., Zhu, T., Xin, Y., & Geng, X. (2025). Optimization of Solid-State Fermentation Process for Dietary Fiber in Flaxseed Meal and Analysis of Its Microstructure and Functional Properties. Foods, 14(10), 1722. https://doi.org/10.3390/foods14101722






