Potential of Hyperthermophilic L-Asparaginase from Thermococcus sibiricus to Mitigate Dietary Acrylamide Assessed Using a Simplified Food System
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Enzyme Preparations
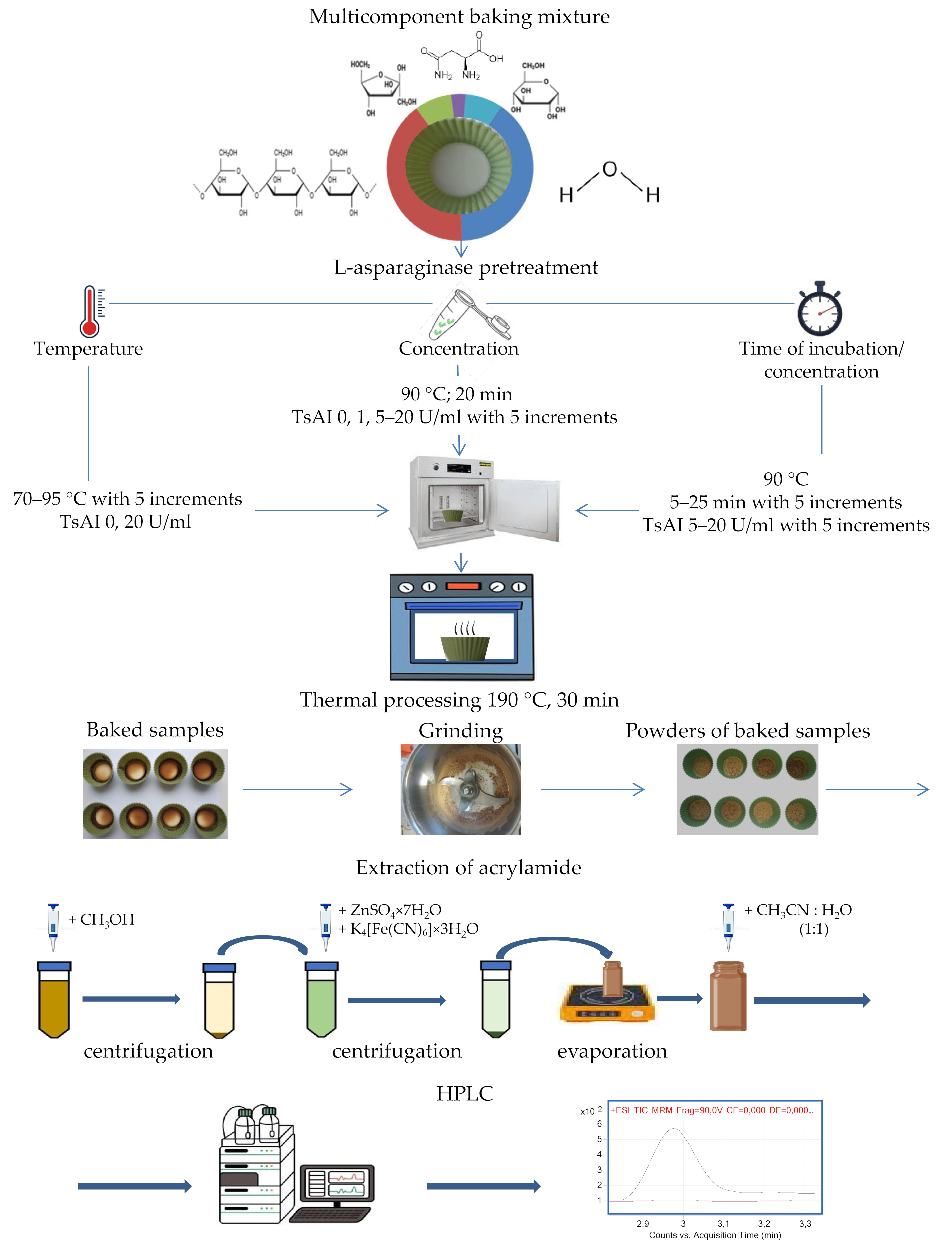
2.3. Simplified Baking Mixtures
2.4. Procedure of Enzyme Pretreatment
2.5. Thermal Processing of Samples
2.6. Preparation of Apple Juice Samples
2.7. Acrylamide Extraction
2.8. HPLC Analysis
2.9. Sensory Analysis
2.10. Evaluation of Color Differences
2.11. Statistical Analysis and Software Programs
3. Results and Discussion
3.1. Procedure for Dietary Acrylamide Formation and Detection in Thermal-Processed Food Samples
3.2. Mitigation of Dietary Acrylamide in Simplified Food System by Pretreatment with L-Asparaginase TsAI
3.2.1. Overall Procedure of Acrylamide Detection in Baked Samples Pretreated with Hyperthermophilic L-Asparaginase TsAI
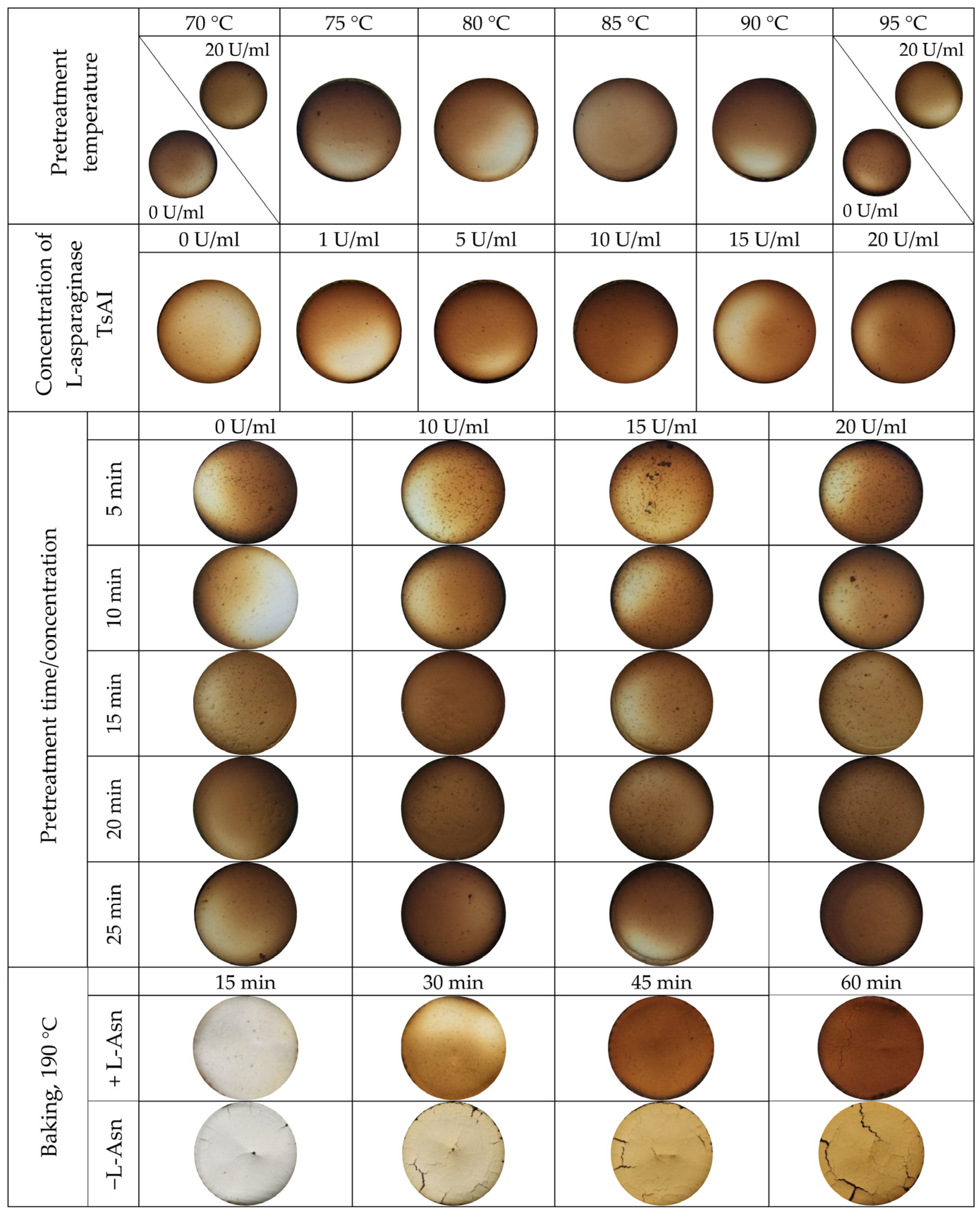
3.2.2. Sensory and Color Characteristics of Baked Samples
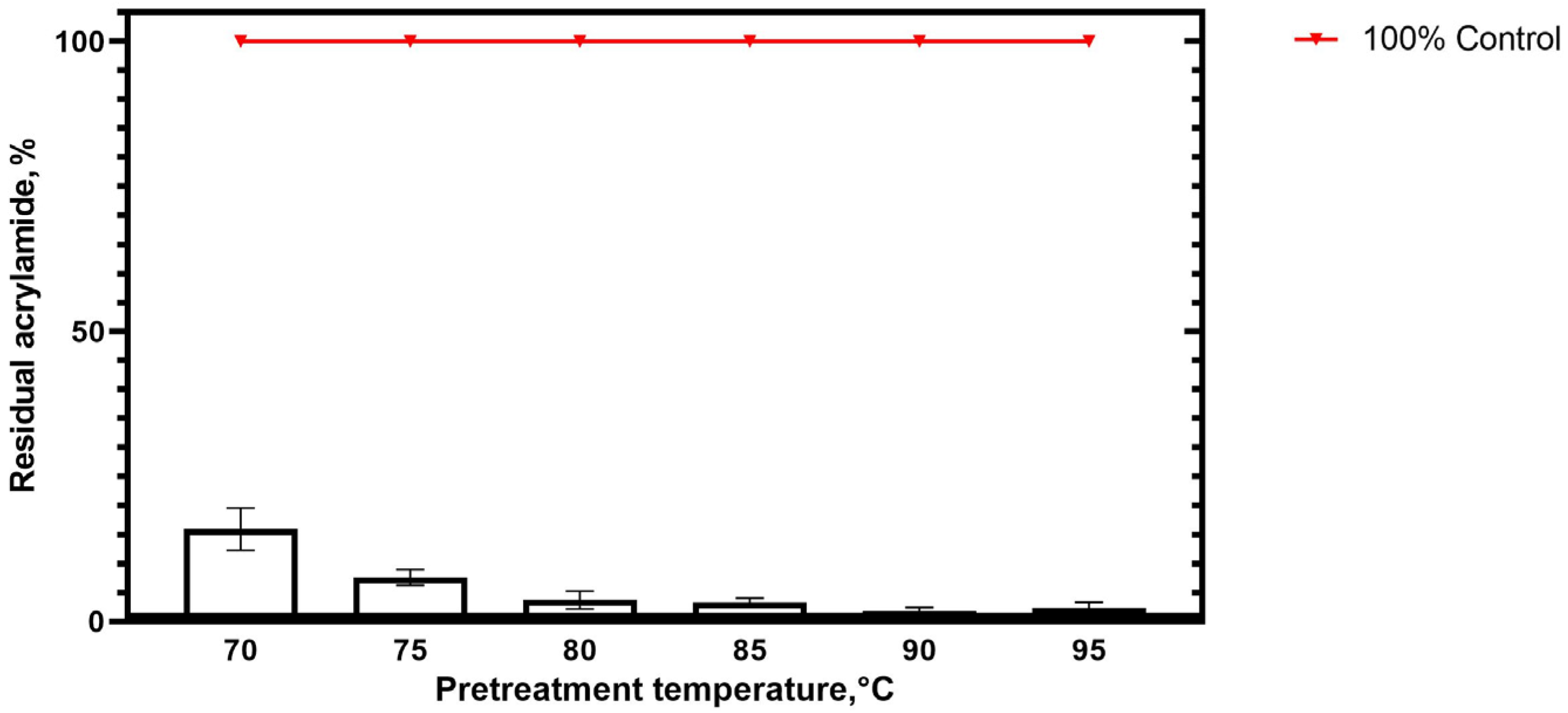
3.2.3. Acrylamide Content in Baked Samples Depending on TsAI Pretreatment Temperature
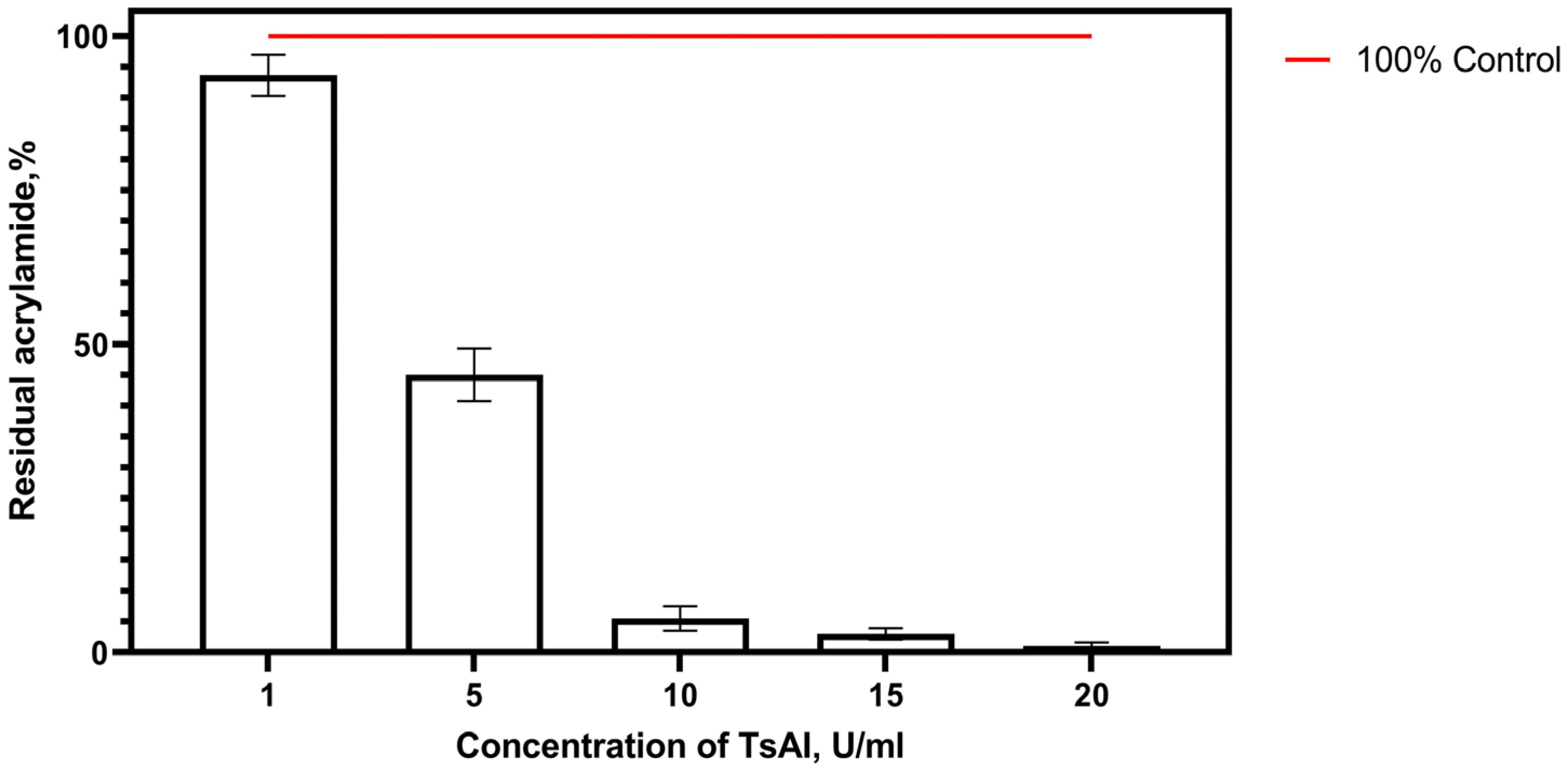
3.2.4. Effective TsAI Concentrations for Acrylamide Reduction
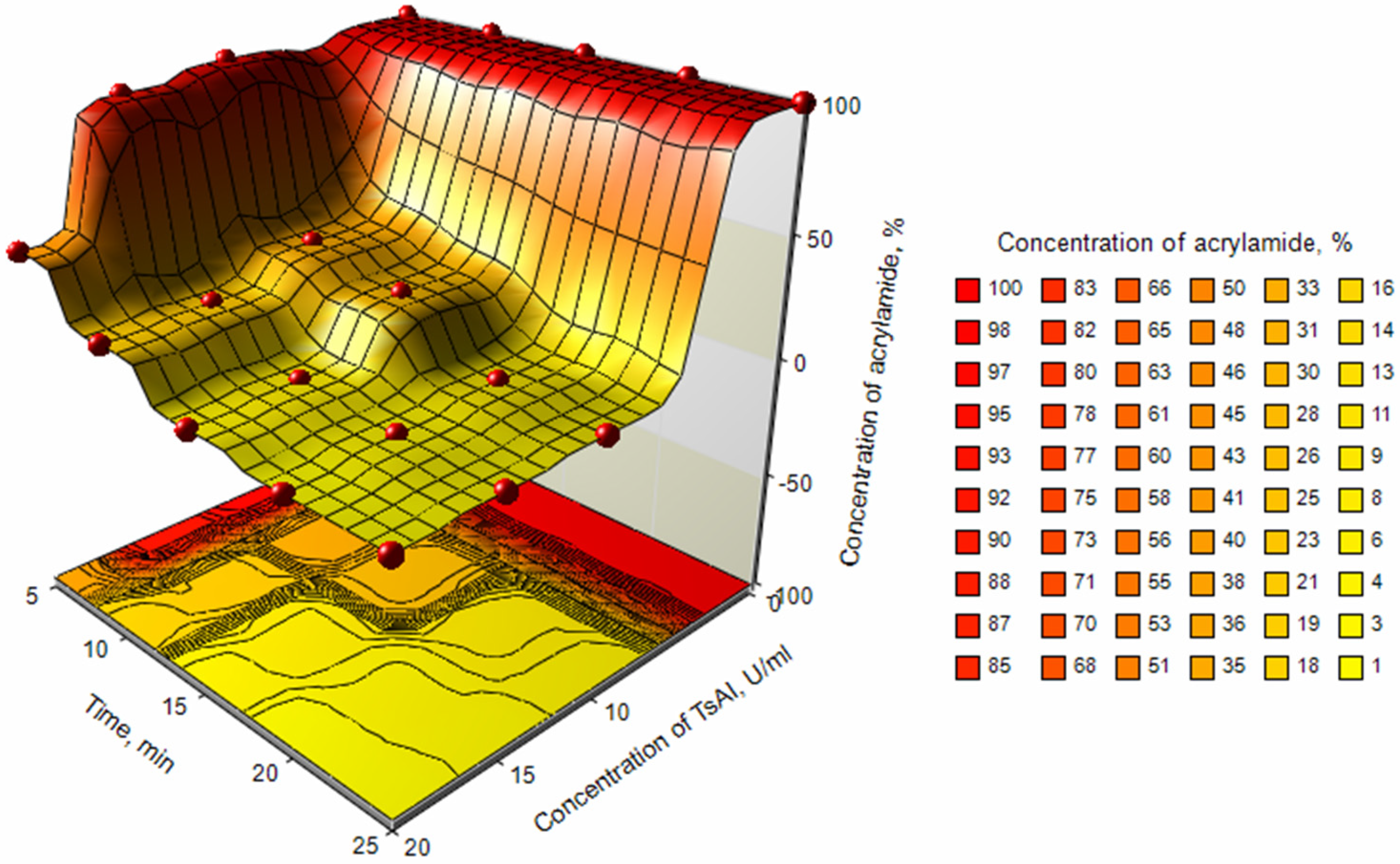
3.2.5. Determination of Effective Time/Concentration Ratio of TsAI for Acrylamide Reduction
3.3. Comparative Study of TsAI Wild-Type and Its Highly Active Mutant to Mitigate Dietary Acrylamide
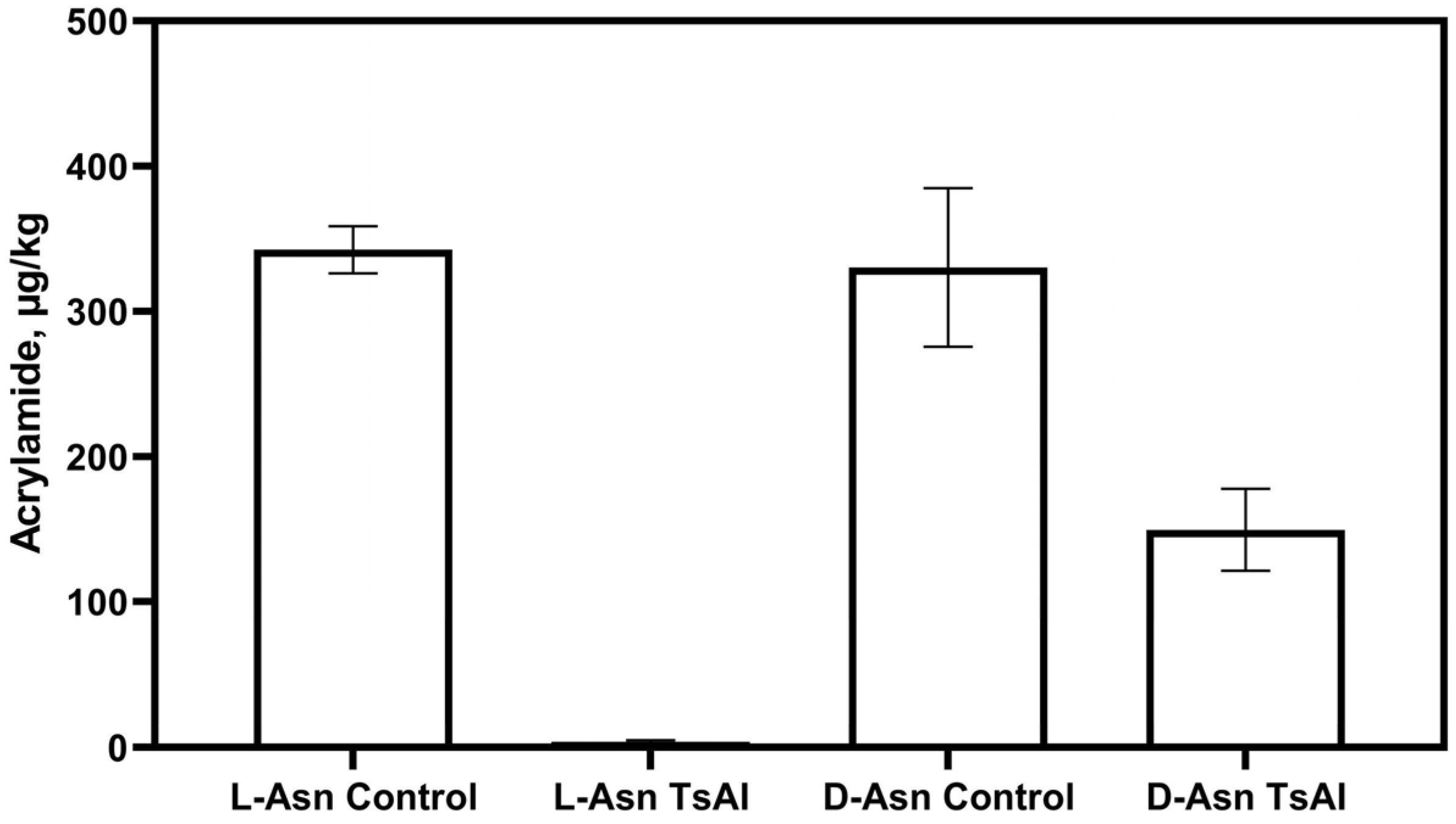
3.4. D-Enantiomer of Asparagine in Food: Acrylamide Formation and Stereospecificity of TsAI Catalytic Reaction
3.5. Reducing Acrylamide Level in Thermally Processed Apple Juice by TsAI Pretreatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acrylamide |
| RAA | Residual acrylamide |
References
- Firth, J.; Gangwisch, J.E.; Gangwisch, J.E.; Borisini, A.; Wootton, R.E.; Wootton, R.E.; Wootton, R.E.; Mayer, E.A.; Mayer, E.A. Food and Mood: How Do Diet and Nutrition Affect Mental Wellbeing? BMJ 2020, 369, m2382. [Google Scholar] [CrossRef] [PubMed]
- Ehrmantraut, L.E.; Redden, J.P.; Mann, T.; Helwig, N.E.; Vickers, Z.M. Self-Selected Diets: Exploring the Factors Driving Food Choices and Satisfaction with Dietary Variety among Independent Adults. Food Qual. Prefer. 2024, 117, 105154. [Google Scholar] [CrossRef]
- Mirza Alizadeh, A.; Mohammadi, M.; Hashempour-baltork, F.; Hosseini, H.; Shahidi, F. Process-Induced Toxicants in Food: An Overview on Structures, Formation Pathways, Sensory Properties, Safety and Health Implications. Food Prod. Process. Nutr. 2025, 7, 7. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-Processed Products Are Becoming Dominant in the Global Food System. Obes. Rev. 2013, 14 (Suppl. S2), 21–28. [Google Scholar] [CrossRef]
- Vignesh, A.; Amal, T.C.; Vasanth, K. Food Contaminants: Impact of Food Processing, Challenges and Mitigation Strategies for Food Security. Food Res. Int. 2024, 191, 114739. [Google Scholar] [CrossRef]
- Halford, N.G.; Curtis, T.Y.; Muttucumaru, N. A Classic Case of Risk and Benefit: The Maillard Reaction in Food Processing and Cooking. Biochemist 2010, 32, 10–14. [Google Scholar] [CrossRef]
- Cui, H.; Yu, J.; Zhai, Y.; Feng, L.; Chen, P.; Hayat, K.; Xu, Y.; Zhang, X.; Ho, C.T. Formation and Fate of Amadori Rearrangement Products in Maillard Reaction. Trends Food Sci. Technol. 2021, 115, 391–408. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Acrylamide: A Cooking Carcinogen? Chem. Res. Toxicol. 2000, 13, 517–522. [Google Scholar] [CrossRef]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide Formation Mechanism in Heated Foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Ma, G.; Zhang, T.; Wang, L.; Pei, H.; Li, X.; Gao, L. Insights into Flavor and Key Influencing Factors of Maillard Reaction Products: A Recent Update. Front. Nutr. 2022, 9, 973677. [Google Scholar] [CrossRef]
- Murata, M. Browning and Pigmentation in Food through the Maillard Reaction. Glycoconj. J. 2021, 38, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Cerny, C. The Aroma Side of the Maillard Reaction. In Annals of the New York Academy of Sciences; Wiley-Blackwell: Hoboken, NJ, USA, 2008; Volume 1126. [Google Scholar]
- Dumina, M.; Zhgun, A.; Pokrovskaya, M.; Aleksandrova, S.; Zhdanov, D.; Sokolov, N.; El’darov, M. A Novel L-Asparaginase from Hyperthermophilic Archaeon Thermococcus Sibiricus: Heterologous Expression and Characterization for Biotechnology Application. Int. J. Mol. Sci. 2021, 22, 9894. [Google Scholar] [CrossRef] [PubMed]
- Dumina, M.; Zhdanov, D.; Zhgun, A.; Pokrovskaya, M.; Aleksandrova, S.; Veselovsky, A.; El’darov, M. Enhancing the Catalytic Activity of Thermo-Asparaginase from Thermococcus Sibiricus by a Double Mesophilic-like Mutation in the Substrate-Binding Region. Int. J. Mol. Sci. 2023, 24, 9632. [Google Scholar] [CrossRef] [PubMed]
- Wade, H.E.; Robinson, H.K.; Phillips, B.W. Asparaginase and Glutaminase Activities of Bacteria. J. Gen. Microbiol. 1971, 69, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, O.; Kanazawa, T.; Maruyama, C.; Dozen, M. Static and Dynamic Half-Life and Lifetime Molecular Turnover of Enzymes. J. Biosci. Bioeng. 2017, 123, 28–32. [Google Scholar] [CrossRef]
- Jan, R.; Saxena, D.C.; Singh, S. Physico-Chemical, Textural, Sensory and Antioxidant Characteristics of Gluten—Free Cookies Made from Raw and Germinated Chenopodium (Chenopodium Album) Flour. LWT 2016, 71, 281–287. [Google Scholar] [CrossRef]
- Luo, M.R.; Cui, G.; Rigg, B. The Development of the CIE 2000 Colour-Difference Formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Silva-Paz, R.J.; Silva-Lizárraga, R.R.; Jamanca-Gonzales, N.C.; Eccoña-Sota, A. Evaluation of the Physicochemical and Sensory Characteristics of Gluten-Free Cookies. Front. Nutr. 2023, 10, 1304117. [Google Scholar] [CrossRef]
- Setchell, J.S. Colour Description and Communication. In Colour Design: Theories and Applications; Woodhead Publishing: Duxford, UK, 2012. [Google Scholar]
- Wojciech, M.; Maciej, T. Color Difference Delta E—A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Marcone, G.L.; Rosini, E.; Crespi, E.; Pollegioni, L. D-Amino Acids in Foods. Appl. Microbiol. Biotechnol. 2019, 104, 555–5744. [Google Scholar] [CrossRef]
- Zagon, J.; Dehne, L.I.; Bögl, K.W. D-Amino Acids in Organisms and Food. Nutr. Res. 1994, 14, 445–463. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.S. Enolization and Racemization Reactions of Glucose and Fructose on Heating with Amino-Acid Enantiomers and the Formation of Melanoidins as a Result of the Maillard Reaction. Amino Acids 2009, 36, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Pätzold, R.; Brückner, H. Gas Chromatographic Determination and Mechanism of Formation of D-Amino Acids Occurring in Fermented and Roasted Cocoa Beans, Cocoa Powder, Chocolate and Cocoa Shell. Amino Acids 2006, 31, 63–72. [Google Scholar] [CrossRef]
- Chohan, S.M.; Rashid, N. TK1656, a Thermostable l-Asparaginase from Thermococcus Kodakaraensis, Exhibiting Highest Ever Reported Enzyme Activity. J. Biosci. Bioeng. 2013, 116, 438–443. [Google Scholar] [CrossRef]
- Ran, T.; Jiao, L.; Wang, W.; Chen, J.; Chi, H.; Lu, Z.; Zhang, C.; Xu, D.; Lu, F. Structures of L-Asparaginase from Bacillus Licheniformis Reveal an Essential Residue for Its Substrate Stereoselectivity. J. Agric. Food Chem. 2021, 69, 223–231. [Google Scholar] [CrossRef]
- Citri, N.; Kitron, N.; Zyk, N. Stereospecific Features of the Conformative Response of L-Asparaginase. Biochemistry 1972, 11, 2110–2116. [Google Scholar] [CrossRef]
- Husain, I.; Sharma, A.; Kumar, S.; Malik, F. Purification and Characterization of Glutaminase Free Asparaginase from Pseudomonas Otitidis: Induce Apoptosis in Human Leukemia MOLT-4 Cells. Biochimie 2016, 121, 38–51. [Google Scholar] [CrossRef]
- Sadowska-Rociek, A.; Surma, M. A Survey on Thermal Processing Contaminants Occurrence in Dark Craft Beers. J. Food Compos. Anal. 2021, 99, 103888. [Google Scholar] [CrossRef]
- Strocchi, G.; Rubiolo, P.; Cordero, C.; Bicchi, C.; Liberto, E. Acrylamide in Coffee: What Is Known and What Still Needs to Be Explored. A Review. Food Chem. 2022, 393, 133406. [Google Scholar] [CrossRef]
- Becalski, A.; Brady, B.; Feng, S.; Gauthier, B.R.; Zhao, T. Formation of Acrylamide at Temperatures Lower than 100 °C: The Case of Prunes and a Model Study. Food Addit. Contam. Part A 2011, 28, 726–730. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, Y.; Yao, Y.; Wei, X.; Yue, T.; Meng, J. Formation of 5-Hydroxymethylfurfural in Industrial-Scale Apple Juice Concentrate Processing. Food Control 2019, 102, 56–68. [Google Scholar] [CrossRef]
- Aktağ, I.G.; Gökmen, V. Acrylamide Formation in Apple Juice Concentrates during Storage. J. Food Compos. Anal. 2023, 121, 105413. [Google Scholar] [CrossRef]
- Twaddle, N.C.; Churchwell, M.I.; McDaniel, L.P.; Doerge, D.R. Autoclave Sterilization Produces Acrylamide in Rodent Diets: Implications for Toxicity Testing. J. Agric. Food Chem. 2004, 52, 4344–4349. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Rodrigues, N.; Marx, Í.M.G.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Assessing Acrylamide Content in Sterilized Californian-Style Black Table Olives Using HPLC-MS-QQQ and a Potentiometric Electronic Tongue. LWT 2020, 129, 109605. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Fernández, A.; Mesías, M.; Martíne, M.; Díaz, M.; Martín-Tornero, E. Industrial Strategies to Reduce Acrylamide Formation in Californian-Style Green Ripe Olives. Foods 2020, 9, 1202. [Google Scholar] [CrossRef]
- Martín-Vertedor, D.; Rodrigues, N.; Marx, Í.M.G.; Veloso, A.C.A.; Peres, A.M.; Pereira, J.A. Impact of Thermal Sterilization on the Physicochemical-Sensory Characteristics of Californian-Style Black Olives and Its Assessment Using an Electronic Tongue. Food Control 2020, 117, 107369. [Google Scholar] [CrossRef]
- Dong, L.; Qiu, C.; Wei, F.; Yu, Z.; Zhang, Y.; Wang, S. The Effect of Microwave Baking Conditions on the Quality of Biscuits and the Control of Thermal Processing Hazards in the Maillard Reaction. Front. Nutr. 2020, 9, 1202. [Google Scholar] [CrossRef]
- Suman, M.; Generotti, S.; Cirlini, M.; Dall’asta, C. Acrylamide Reduction Strategy in Combination with Deoxynivalenol Mitigation in Industrial Biscuits Production. Toxins 2019, 11, 499. [Google Scholar] [CrossRef]
- Palazoğlu, T.K.; Coşkun, Y.; Tuta, S.; Mogol, B.A.; Gökmen, V. Effect of Vacuum-Combined Baking of Cookies on Acrylamide Content, Texture and Color. Eur. Food Res. Technol. 2015, 240, 243–249. [Google Scholar] [CrossRef]
- Mesías, M.; Holgado, F.; Márquez-Ruiz, G.; Morales, F.J. Effect of Sodium Replacement in Cookies on the Formation of Process Contaminants and Lipid Oxidation. LWT 2015, 62, 633–639. [Google Scholar] [CrossRef]
- Passos, C.P.; Ferreira, S.S.; Serôdio, A.; Basil, E.; Marková, L.; Kukurová, K.; Ciesarová, Z.; Coimbra, M.A. Pectic Polysaccharides as an Acrylamide Mitigation Strategy—Competition between Reducing Sugars and Sugar Acids. Food Hydrocoll. 2018, 81, 113–119. [Google Scholar] [CrossRef]
- Lo Faro, E.; Salerno, T.; Montevecchi, G.; Fava, P. Mitigation of Acrylamide Content in Biscuits through Combined Physical and Chemical Strategies. Foods 2022, 11, 2343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Duan, M.; Gao, S.; Wang, T.; Wang, Y.; Wang, X.; Zhou, Y. A Strategy for Reducing Acrylamide Content in Wheat Bread by Combining Acidification Rate and Prerequisite Substance Content of Lactobacillus and Saccharomyces Cerevisiae. Curr. Res. Food Sci. 2022, 5, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.A.; Abdelhady, M.M.; Jaafari, S.A.; Alanazi, T.M.; Mohammed, A.S. Impact of Some Enzymatic Treatments on Acrylamide Content in Biscuits. Processes 2023, 11, 1041. [Google Scholar] [CrossRef]
- Yang, H.; Li, L.; Yin, Y.; Li, B.; Zhang, X.; Jiao, W.; Liang, Y. Effect of Ground Ginger on Dough and Biscuit Characteristics and Acrylamide Content. Food Sci. Biotechnol. 2019, 28, 1359–1366. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Zhang, Y.; Lu, B.; Jin, C.; Wu, X.; Zhang, Y. Study on Mitigation of Acrylamide Formation in Cookies by 5 Antioxidants. J. Food Sci. 2012, 77, C1144–C1149. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Elghamry, H.N.; Yassin, M.A. Biochemical Characterization of Thermostable Acrylamide Amidohydrolase from Aspergillus Fumigatus with Potential Activity for Acrylamide Degradation in Various Food Products. Curr. Microbiol. 2024, 81, 1–13. [Google Scholar] [CrossRef]
- Gazi, S.; Göncüoğlu Taş, N.; Görgülü, A.; Gökmen, V. Effectiveness of Asparaginase on Reducing Acrylamide Formation in Bakery Products According to Their Dough Type and Properties. Food Chem. 2023, 402, 134224. [Google Scholar] [CrossRef]
- Zuo, S.; Zhang, T.; Jiang, B.; Mu, W. Reduction of Acrylamide Level through Blanching with Treatment by an Extremely Thermostable L-Asparaginase during French Fries Processing. Extremophiles 2015, 19, 841–851. [Google Scholar] [CrossRef]
- Kukurová, K.; Morales, F.J.; Bednáriková, A.; Ciesarová, Z. Effect of L-Asparaginase on Acrylamide Mitigation in a Fried-Dough Pastry Model. Mol. Nutr. Food Res. 2009, 53, 1532–1539. [Google Scholar] [CrossRef]
- Tuncel, N.B.; YiLmaz, N.; Sener, E. The Effect of Pea (Pisum Sativum L.)-Originated Asparaginase on Acrylamide Formation in Certain Bread Types. Int. J. Food Sci. Technol. 2010, 45, 2470–2476. [Google Scholar] [CrossRef]
- Anese, M.; Quarta, B.; Peloux, L.; Calligaris, S. Effect of Formulation on the Capacity of L-Asparaginase to Minimize Acrylamide Formation in Short Dough Biscuits. Food Res. Int. 2011, 44, 2837–2842. [Google Scholar] [CrossRef]
- Hendriksen, H.V.; Kornbrust, B.A.; Ostergaard, P.R.; Stringer, M.A. Evaluating the Potential for Enzymatic Acrylamide Mitigation in a Range of Food Products Using an Asparaginase from Aspergillus Oryzae. J. Agric. Food Chem. 2009, 57, 4168–4176. [Google Scholar] [CrossRef]
- Morales, F.; Capuano, E.; Fogliano, V. Mitigation Strategies to Reduce Acrylamide Formation in Fried Potato Products. In Annals of the New York Academy of Sciences; Wiley-Blackwell: Hoboken, NJ, USA, 2008; Volume 1126. [Google Scholar]
- FAO/WHO Expert Committee on Food Additives (JECFA). Safety Evaluation of Certain Food Additives; Renouf Pub Co Ltd.: Oxford, UK, 2009. [Google Scholar]
- FAO/WHO Expert Committee on Food Additives (JECFA). Safety Evaluation of Certain Food Additives; Renouf Pub Co Ltd.: Oxford, UK, 2008. [Google Scholar]
- Aguilar, F.; Autrup, H.; Barlow, S.; Castle, L.; Crebelli, R.; Engel, K.; Gontard, N.; Gott, D.; Grilli, S.; Gürtler, R.; et al. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food Adopted on 7 March 2008. EFSA J. 2008. [Google Scholar] [CrossRef]
- Roney, N.; Llados, F.; Little, S.; Knaebel, D. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Ammonia. Fed. Regist. 2004, 1–269. [Google Scholar]
- Govindaraju, I.; Sana, M.; Chakraborty, I.; Rahman, M.H.; Biswas, R.; Mazumder, N. Dietary Acrylamide: A Detailed Review on Formation, Detection, Mitigation, and Its Health Impacts. Foods 2024, 13, 556. [Google Scholar] [CrossRef]
- Casado, F.J.; Montaño, A.; Spitzner, D.; Carle, R. Investigations into Acrylamide Precursors in Sterilized Table Olives: Evidence of a Peptic Fraction Being Responsible for Acrylamide Formation. Food Chem. 2013, 141, 1158–1165. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Moawad, H.; El-Shweihy, N.M.; El-Ewasy, S.M.; Elsehemy, I.A.; Abdelwahed, N.A.M. Process Development for Scale-up Production of a Therapeutic L-Asparaginase by Streptomyces Brollosae NEAE-115 from Shake Flasks to Bioreactor. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Vimal, A.; Kumar, A. Biotechnological Production and Practical Application of L-Asparaginase Enzyme. Biotechnol. Genet. Eng. Rev. 2017, 33, 40–61. [Google Scholar] [CrossRef]
- Rabelo, N.G.; Simões, L.A.; Fernandes, N.d.A.T.; Souza, A.C.; Fernandes, M.L.P.; Veríssimo, L.A.A.; Schwan, R.F.; Dias, D.R. Optimization of L-Asparaginase Production from Aspergillus Caespitosus: Solid-State and Submerged Fermentation Using Low-Cost Substrates and Partial Purification. Appl. Microbiol. 2025, 5, 19. [Google Scholar] [CrossRef]

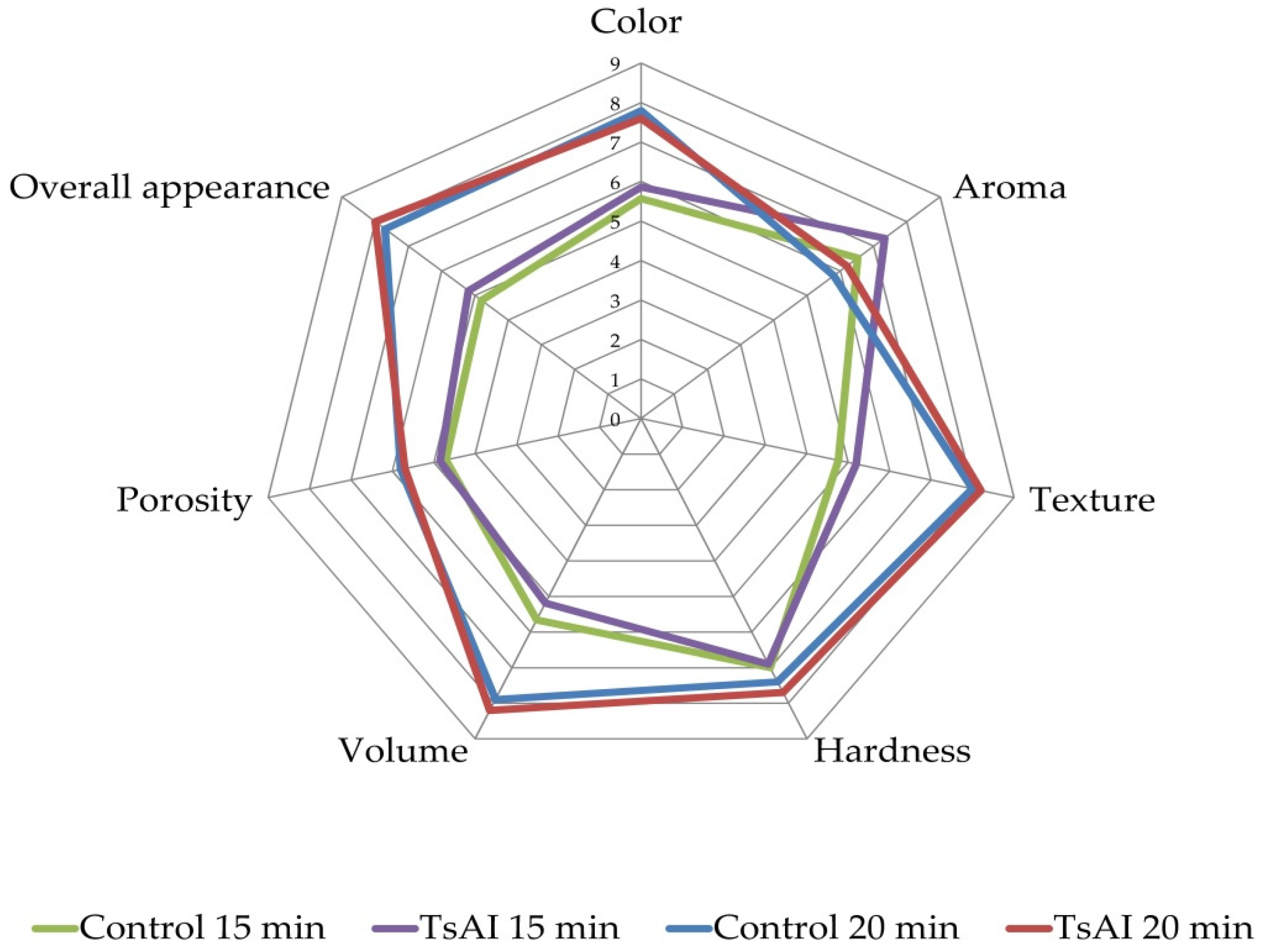
| Sample | Color | Aroma | Texture | Hardness | Volume | Porosity | Overall Appearance |
|---|---|---|---|---|---|---|---|
| 15 Min Pretreatment | |||||||
| Control | 5.57 ± 1.18 | 6.52 ± 2.15 | 4.76 ± 1.65 | 7.00 ± 1.77 | 5.66 ± 1.50 | 4.71 ± 1.71 | 4.80 ± 1.33 |
| TsAI | 5.86 ± 1.33 | 7.33 ± 2.08 | 5.19 ± 1.74 | 6.90 ± 1.90 | 5.19 ± 1.22 | 4.85 ± 1.70 | 5.19 ± 1.45 |
| p | >0.05 | <0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
| 20 Min Pretreatment | |||||||
| Control | 7.80 ± 1.48 | 5.80 ± 2.49 | 7.95 ± 1.39 | 7.42 ± 2.98 | 7.90 ± 1.55 | 5.81 ± 2.11 | 7.71 ± 1.41 |
| TsAI | 7.57 ± 1.65 | 6.19 ± 2.33 | 8.23 ± 1.09 | 7.71 ± 2.87 | 8.23 ± 1.65 | 5.71 ± 2.51 | 8.04 ± 1.45 |
| p | > 0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 | >0.05 |
| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Curcumin | 71.53 | −14.36 | 72.89 | |
| 15 Min Pretreatment | ||||
| Control | 63.15 | 5.34 | 61.97 | 3.62 |
| TsAI | 66.00 | 1.05 | 62.09 | |
| 20 Min Pretreatment | ||||
| Control | 64.83 | 7.39 | 67.40 | 1.42 |
| TsAI | 65.04 | 5.09 | 65.98 | |
| Independent Parameters | Range | ANOVA for RAA (Response) | |||||
|---|---|---|---|---|---|---|---|
| SS | DF | MS | F Value | p | Significance | ||
| Concentration | 10–20 U/mL | 66,767.46 | 3 | 22,255.82 | 43,667.95 | <0.0001 | Significant |
| Incubation | 5–25 min | 25,541.42 | 4 | 6385.35 | 12,528.65 | <0.0001 | Significant |
| Concentration/incubation | 10–20 U/mL/ 5–25 min | 12,269.75 | 12 | 1022.48 | 2006.19 | <0.0001 | Significant |
| Method | Advantages | Disadvantages | Example | Reference | ||
|---|---|---|---|---|---|---|
| Food Matrix | Reduction in AA 1, % | |||||
| Physical | Microwaves | Simplicity in technological application | Controversial results | Biscuit | 30.9 | [39] |
| Reduction in heat treatment | Ease of application | Impact on organoleptic characteristics | Biscuit | 60.0 | [40] | |
| Vacuum | Reducing heat treatment duration | Affect product texture | Cookies | 53.0 | [41] | |
| Chemical | CaCl2 | Precise regulation of concentration and composition of chemical components | Influence on the organoleptic characteristics | Cookies | 58.0 | [42] |
| Pectin | Biscuit | 67.0 | [43] | |||
| Tartaric acid | Biscuit | 52.0 | [43] | |||
| Replacing ammonium bicarbonate | Biscuit | 87.2 | [44] | |||
| Biological | Fermentation | |||||
| Lactic acid bacteria | Ease of use, availability of components | Strict conditions and process control for desirable effects | Wheat bread | 48.7 | [45] | |
| Baker’s yeast | Sourdough bread | 40.1 | [45] | |||
| Biscuit | 75.2 | [46] | ||||
| Herbal Ingredients | ||||||
| Green tea extract | Natural components | Unstable effects | Biscuit | 73.3 | [46] | |
| Ground ginger | Biscuit | 23.7 | [47] | |||
| Antioxidants of bamboo leaves | Cookie | 63.9 | [48] | |||
| Tea polyphenols | Cookie | 43.0 | [48] | |||
| Vitamin E | Cookie | 71.2 | [48] | |||
| Enzymatic | ||||||
| Glucose oxidase | High efficiency | High cost | Biscuit | 63.9 | [46] | |
| Acrylamide amidohydrolase | Cookie | 95.0 | [49] | |||
| Asparaginase | Cookie | 54.0 | [50] | |||
| Cracker | 80.0 | [50] | ||||
| Biscuit | 96.0 | [50] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumina, M.; Kalinin, S.; Zhdanov, D. Potential of Hyperthermophilic L-Asparaginase from Thermococcus sibiricus to Mitigate Dietary Acrylamide Assessed Using a Simplified Food System. Foods 2025, 14, 1720. https://doi.org/10.3390/foods14101720
Dumina M, Kalinin S, Zhdanov D. Potential of Hyperthermophilic L-Asparaginase from Thermococcus sibiricus to Mitigate Dietary Acrylamide Assessed Using a Simplified Food System. Foods. 2025; 14(10):1720. https://doi.org/10.3390/foods14101720
Chicago/Turabian StyleDumina, Maria, Stanislav Kalinin, and Dmitry Zhdanov. 2025. "Potential of Hyperthermophilic L-Asparaginase from Thermococcus sibiricus to Mitigate Dietary Acrylamide Assessed Using a Simplified Food System" Foods 14, no. 10: 1720. https://doi.org/10.3390/foods14101720
APA StyleDumina, M., Kalinin, S., & Zhdanov, D. (2025). Potential of Hyperthermophilic L-Asparaginase from Thermococcus sibiricus to Mitigate Dietary Acrylamide Assessed Using a Simplified Food System. Foods, 14(10), 1720. https://doi.org/10.3390/foods14101720






