Research on Aseptic Milk Extraction Technology and Mechanism of Slightly Acidic Electrolytic Water Coupled with Ultrasound Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Reagents
2.2. SAEW and US Preparation
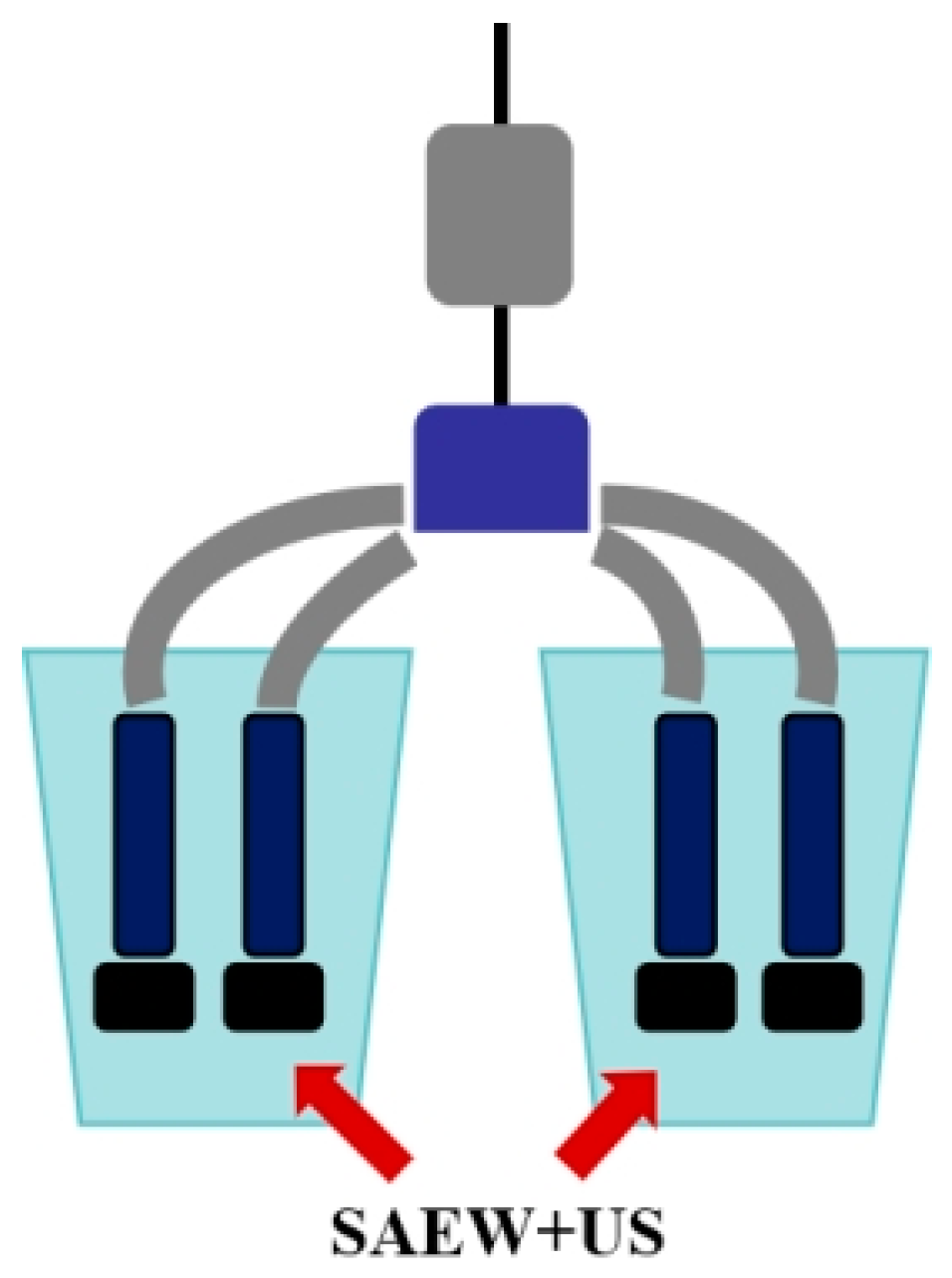
2.3. Application Effect on Milk-Pressing Cups
2.4. Determination of Minimum Inhibitory Concentration (MIC)
2.5. Study on the Effect of Combined Sterilization
2.6. Scanning Electron Microscope (SEM) Analysis
2.7. Determination of Nucleic Acid and Soluble Protein Release
2.8. Determination of Reactive Oxygen Species (ROS)
2.9. Flow Cytometry Test
2.10. Examination of Cell Membrane Permeability
2.11. Laser Scanning Confocal Microscopy (LSCM) Analysis
2.12. Statistical Analysis
3. Results
3.1. Milking Cups Simulate the Antibacterial Effect
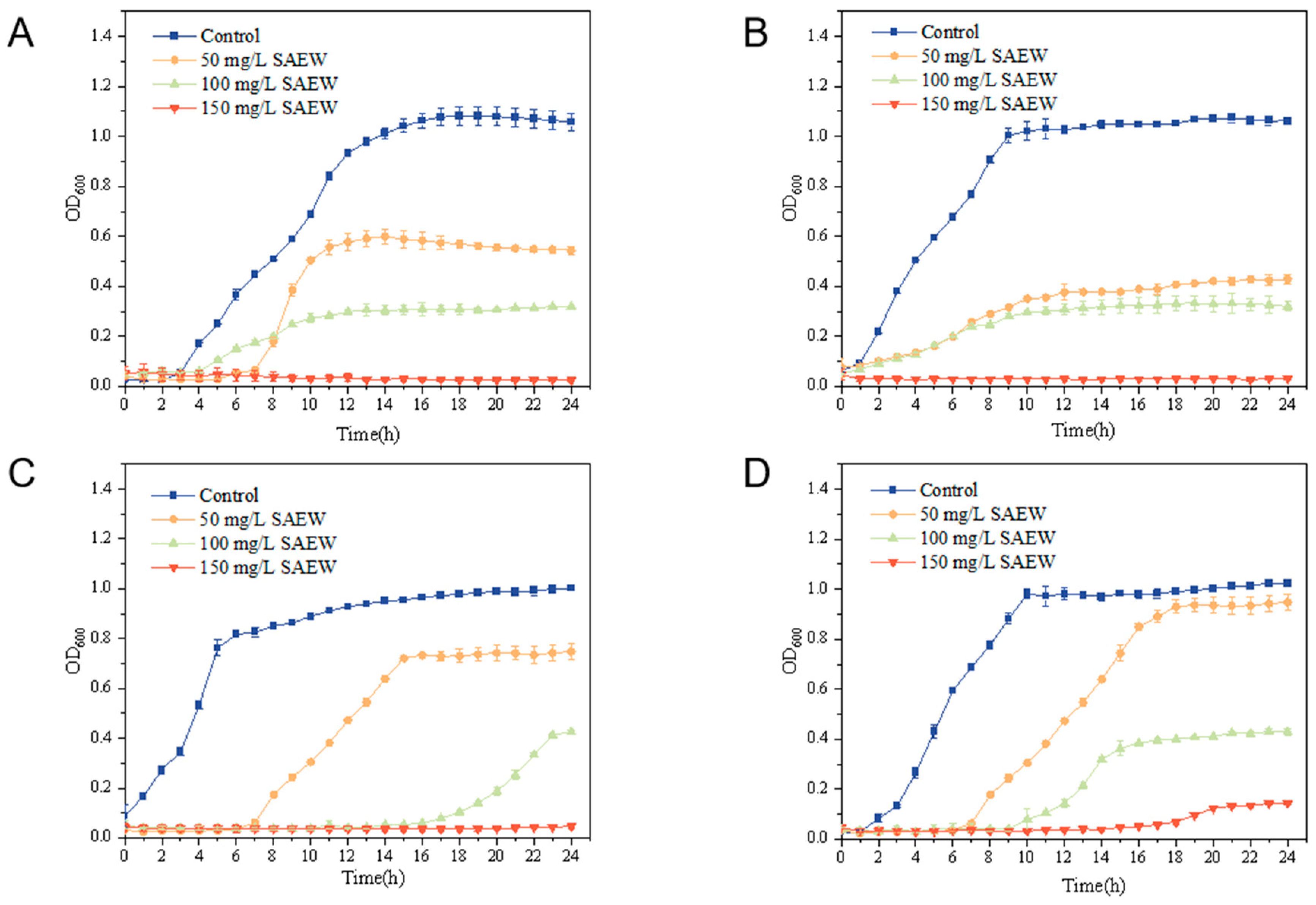
3.2. Minimum Inhibitory Concentration (MIC) Measurements for SAEW
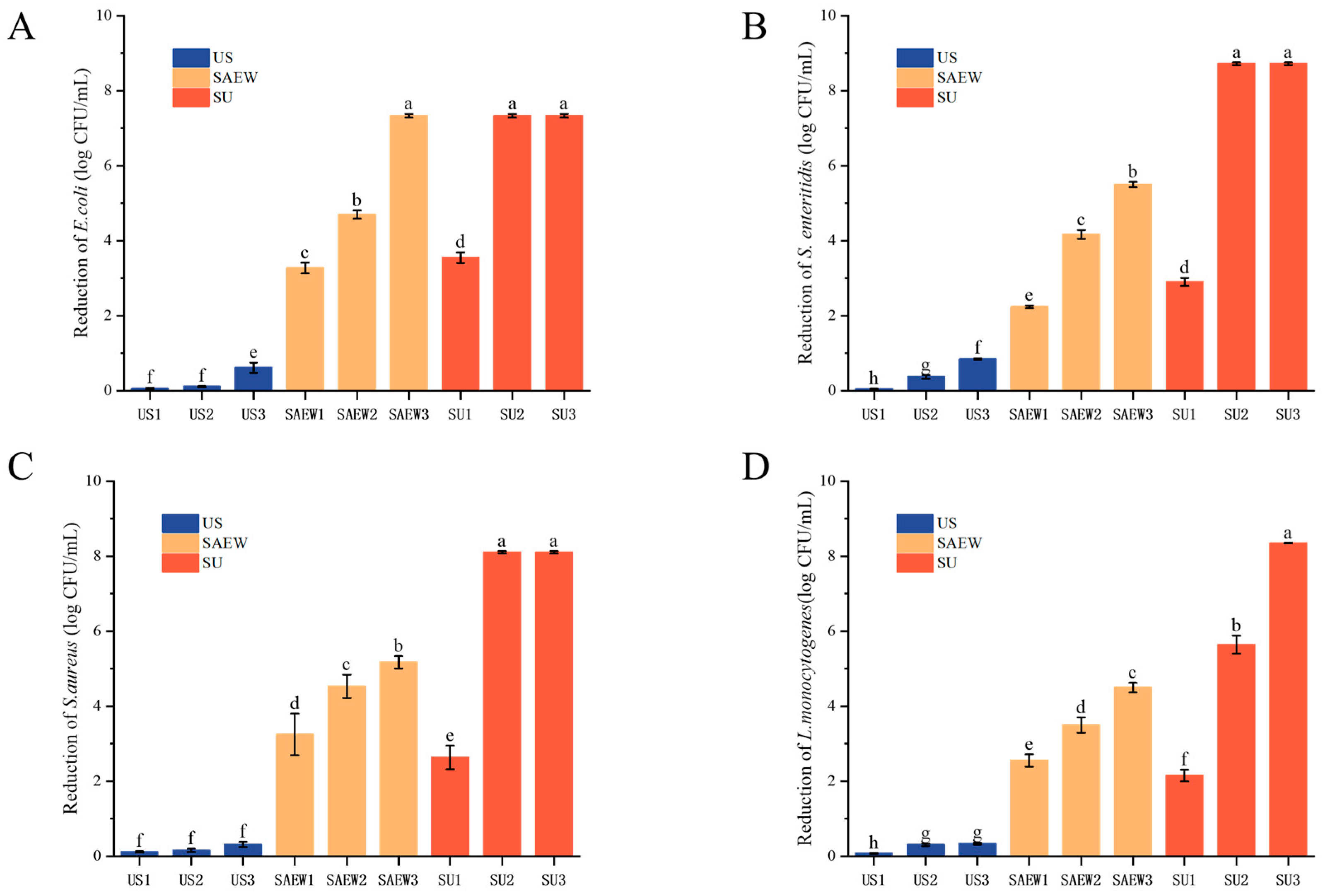
3.3. Bactericidal Efficacy of SAEW, US, and SAEW + US on the Tested Strains
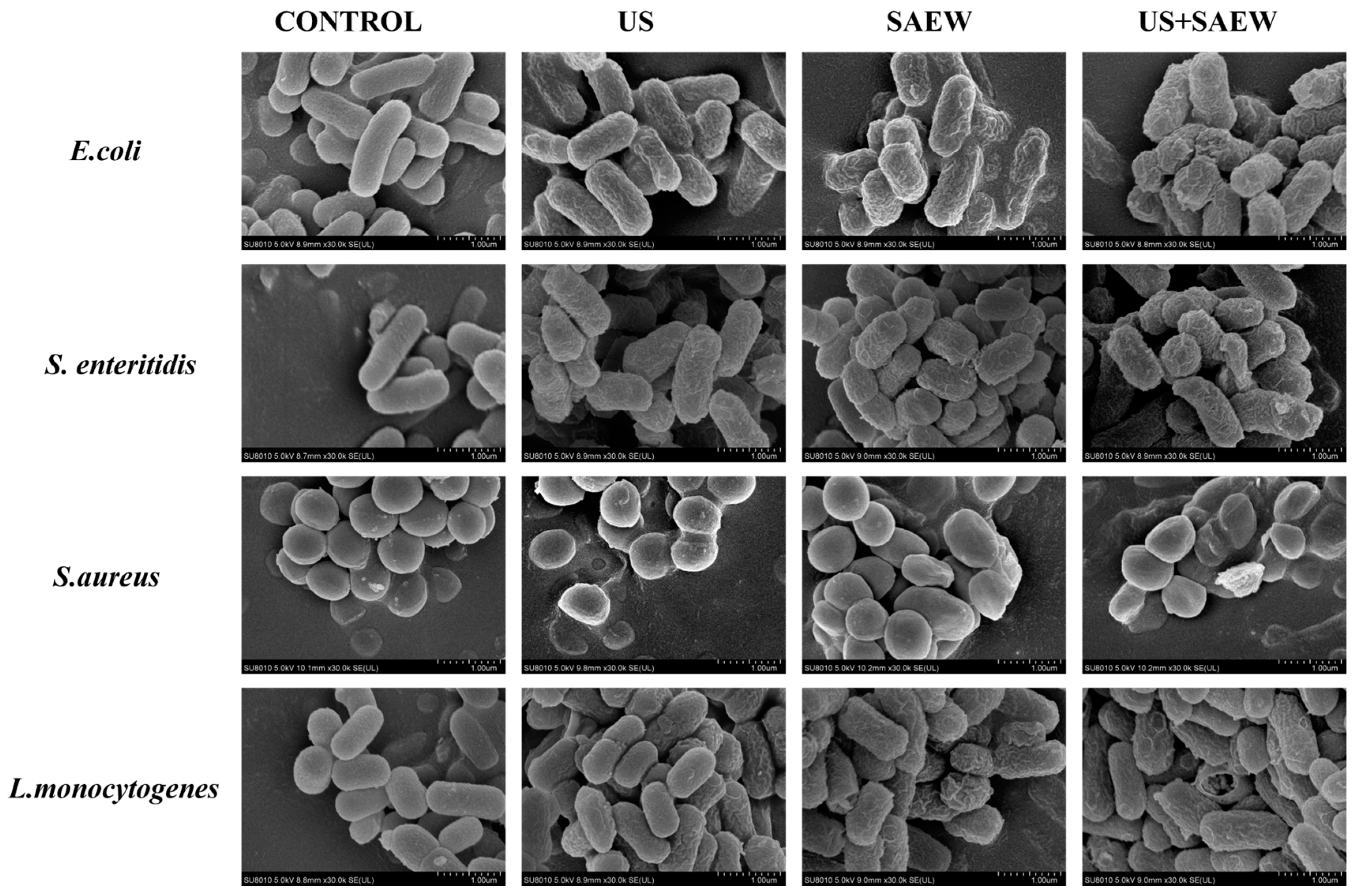
3.4. Morphological Changes in Cells
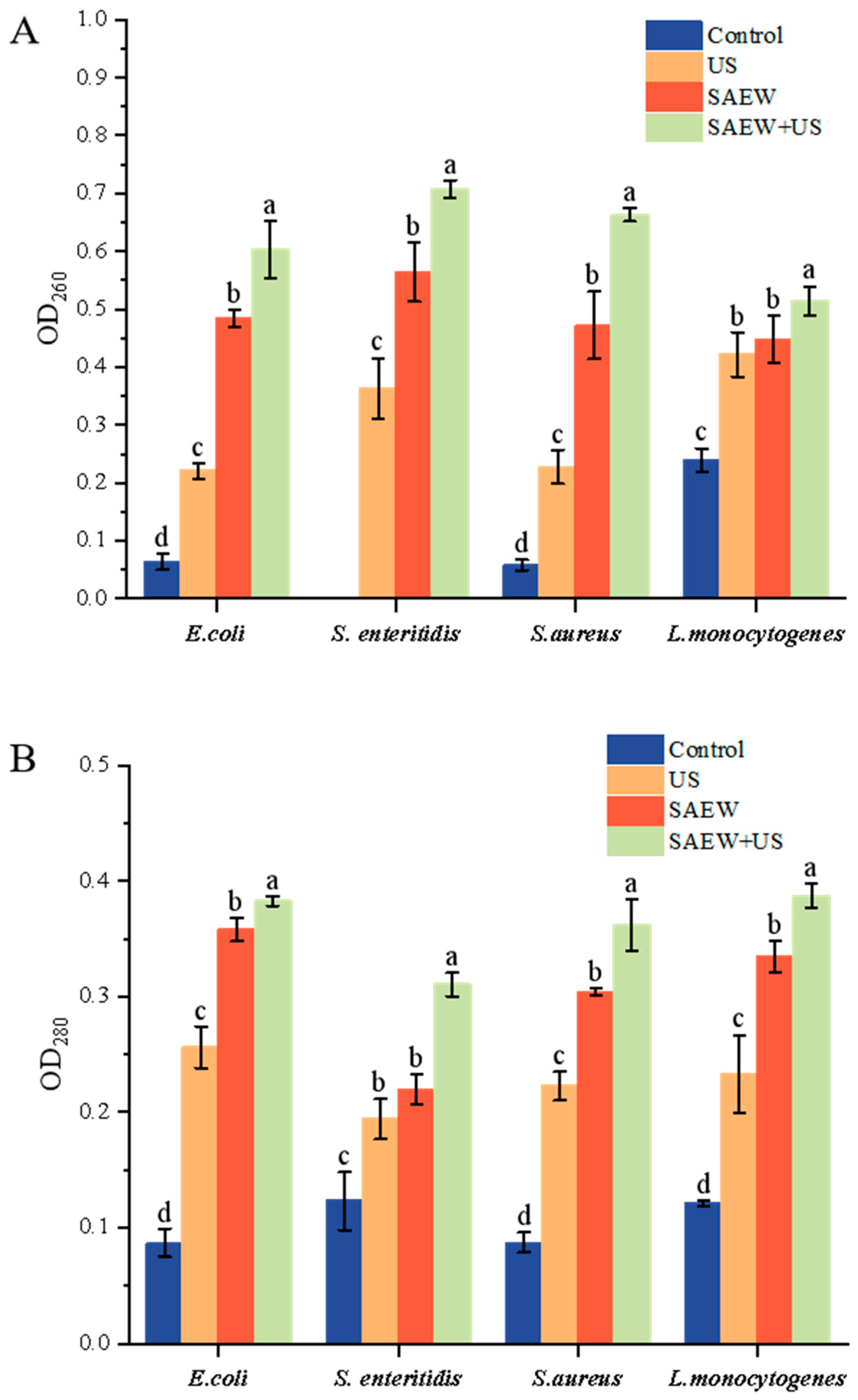
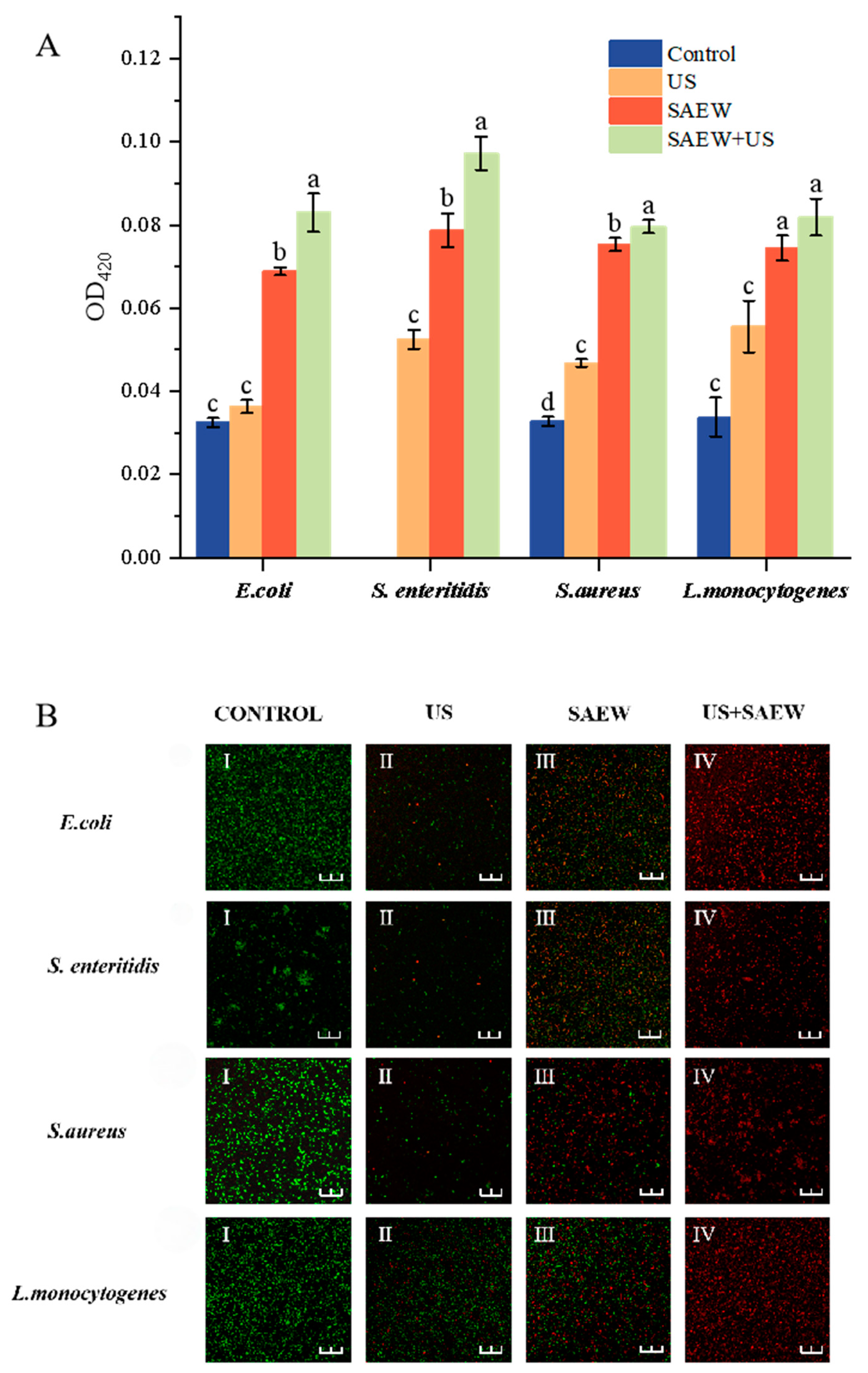
3.5. Determination of Nucleic Acid and Soluble Protein Release
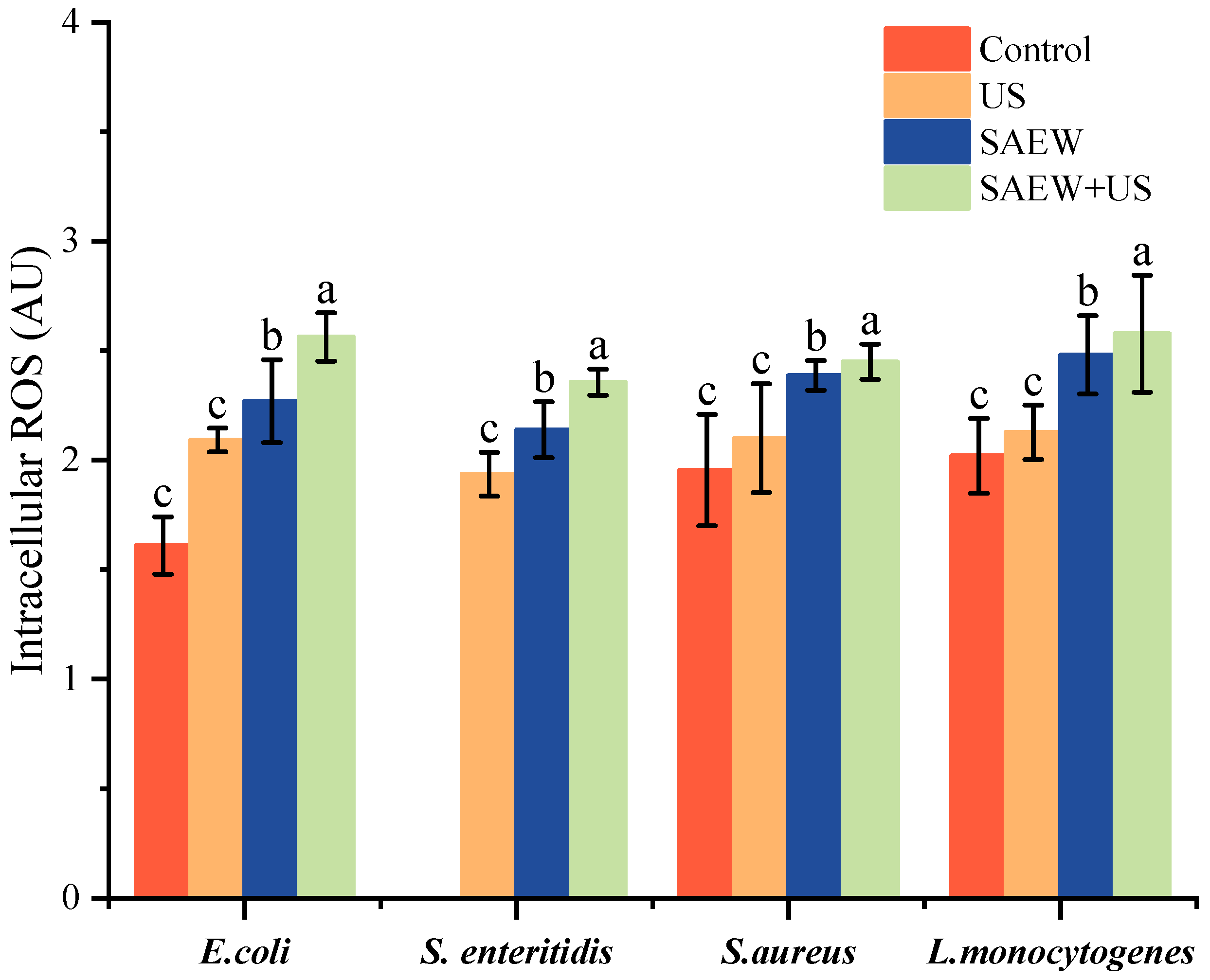
3.6. Changes in Cellular ROS Levels
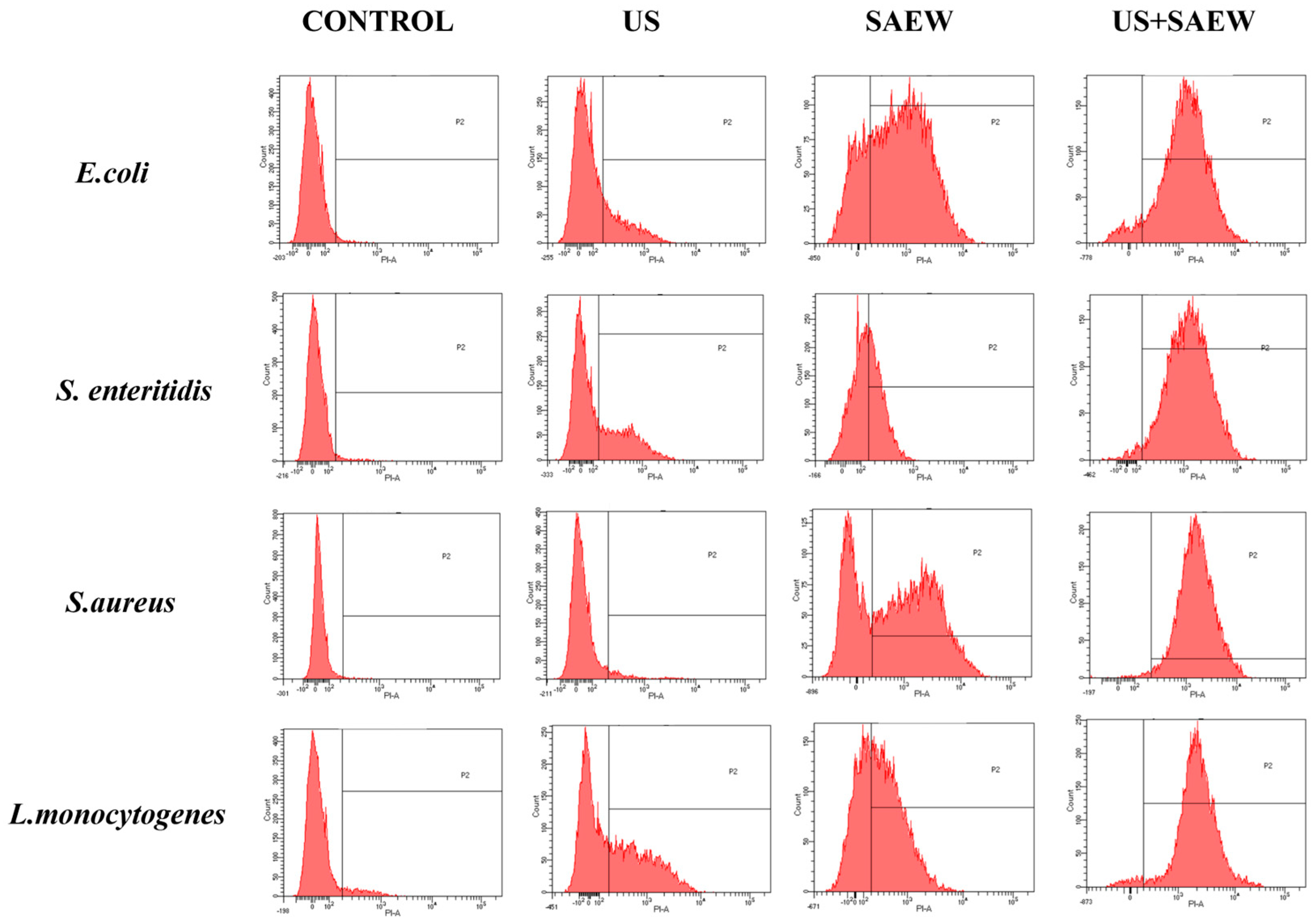
3.7. Analysis of Cell Membrane Integrity
3.8. Analysis of Cell Membrane Permeability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vargova, M.; Vyrostkova, J.; Veszelits Lakticova, K.; Zigo, F. Effectiveness of sanitation regime in a milking parlour to control microbial contamination of teats and surfaces teat cups’. Ann. Agric. Environ. Med. 2023, 30, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Escobedo, L.; Cadenas-Jimenez, I.; Olmos, R.; Carrera-Salinas, A.; Berbel, D.; Camara, J.; Tubau, F.; Dominguez, M.A.; Ardanuy, C.; Gonzalez-Diaz, A.; et al. Detection of bla(CTX-M-15) in an integrative and conjugative element in four extensively drug-resistant Haemophilus parainfluenzae strains causing urethritis. Int. J. Antimicrob. Agents 2023, 62, 106991. [Google Scholar] [CrossRef]
- Qiu, X.; Yin, H.; Xing, Q. Research Progress on Fatigue Life of Rubber Materials. Polymers 2022, 14, 4592. [Google Scholar] [CrossRef]
- Kable, M.E.; Srisengfa, Y.; Laird, M.; Zaragoza, J.; McLeod, J.; Heidenreich, J.; Marco, M.L. The core and seasonal microbiota of raw bovine milk in tanker trucks and the impact of transfer to a milk processing facility. MBio 2016, 7, e00836-16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Farid, M. A review on recent development in non-conventional food sterilization technologies. J. Food Eng. 2016, 182, 33–45. [Google Scholar] [CrossRef]
- Flachowsky, G.; Franke, K.; Meyer, U.; Leiterer, M.; Schöne, F. Influencing factors on iodine content of cow milk. Eur. J. Nutr. 2013, 53, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Borucki Castro, S.I.; Berthiaume, R.; Robichaud, A.; Lacasse, P. Effects of iodine intake and teat-dipping practices on milk iodine concentrations in dairy cows. J. Dairy Sci. 2012, 95, 213–220. [Google Scholar] [CrossRef]
- Hou, W.; Ma, X.; Yu, Z.; Bari, L.; Jiang, H.; Du, Q.; Fan, R.; Wang, J.; Yang, Y.; Han, R. Impact of ultrasonic and heat treatments on the physicochemical properties and rennet-induced coagulation characteristics of milk from various species. Ultrason. Sonochem. 2024, 111, 107084. [Google Scholar] [CrossRef]
- Dash, K.K.; Fayaz, U.; Dar, A.H.; Shams, R.; Manzoor, S.; Sundarsingh, A.; Deka, P.; Khan, S.A. A comprehensive review on heat treatments and related impact on the quality and microbial safety of milk and milk-based products. Food Chem. Adv. 2022, 1, 100041. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Wang, N.; Sun, X.; Han, X.; Yin, R.; Pei, X.; Liu, C.; Pang, X.; Huang, F.; et al. Comparative study on microbiological, physicochemical and nutritional properties of whole cow milk by thermal and non-thermal processing technologies. Food Biosci. 2024, 59, 104012. [Google Scholar] [CrossRef]
- Padonou, S.W.; Houngbédji, M.; Hounhouigan, M.H.; Chadare, F.J.; Hounhouigan, D.J. B-vitamins and heat processed fermented starchy and vegetable foods in sub-Saharan Africa: A review. J. Food Sci. 2023, 88, 3155–3188. [Google Scholar] [CrossRef]
- Yu, M.; Jiang, C.; Meng, Y.; Wang, F.; Qian, J.; Fei, F.; Yin, Z.; Zhao, W.; Zhao, Y.; Liu, H. Effect of low temperature on the resistance of Listeria monocytogenes and Escherichia coli O157:H7 to acid electrolyzed water. Food Res. Int. 2023, 168, 112776. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tian, Q.; Li, G.; Yi, J.; Hu, X.; Jiang, Y. Advanced application of slightly acidic electrolyzed water for fresh-cut fruits and vegetables preservation. Food Res. Int. 2024, 195, 114996. [Google Scholar] [CrossRef]
- Zhang, B.; Zang, Y.; Mo, Q.; Sun, L.; Tu, M.; Shu, D.; Li, Y.; Xue, F.; Wu, G.; Zhao, X. Antibacterial activity and mechanism of slightly acidic electrolyzed water (SAEW) combined with ultraviolet light against Staphylococcus aureus. LWT 2023, 182, 114746. [Google Scholar] [CrossRef]
- Naka, A.; Yakubo, M.; Nakamura, K.; Kurahashi, M. Effectiveness of slightly acidic electrolyzed water on bacteria reduction: In vitro and spray evaluation. PeerJ 2020, 8, e8593. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Zhang, R.; Zhang, X.; Shi, H. Sublethal injury and recovery of Listeria monocytogenes and Escherichia coli O157:H7 after exposure to slightly acidic electrolyzed water. Food Control 2019, 106, 106746. [Google Scholar] [CrossRef]
- Xuan, X.-T.; Ding, T.; Li, J.; Ahn, J.-H.; Zhao, Y.; Chen, S.-G.; Ye, X.-Q.; Liu, D.-H. Estimation of growth parameters of Listeria monocytogenes after sublethal heat and slightly acidic electrolyzed water (SAEW) treatment. Food Control 2017, 71, 17–25. [Google Scholar] [CrossRef]
- Li, F.; Zhong, Q.; Kong, B.; Pan, N.; Xia, X.; Bao, Y. Synergistic effect and disinfection mechanism of combined treatment with ultrasound and slightly acidic electrolyzed water and associated preservation of mirror carp (Cyprinus carpio L.) during refrigeration storage. Food Chem. 2022, 386, 132858. [Google Scholar] [CrossRef]
- Yoon, S.-R.; Lee, J.Y.; Yang, J.-S.; Ha, J.-H. Bactericidal effects of diluted slightly acidic electrolyzed water in quantitative suspension and cabbage tests. LWT 2021, 152, 112291. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Shi, Z.; Li, B. Optimization and modeling of slightly acidic electrolyzed water for the clean-in-place process in milking systems. Foods 2020, 9, 1685. [Google Scholar] [CrossRef]
- Wang, F.; Lin, Y.-N.; Xu, Y.; Ba, Y.-B.; Zhang, Z.-H.; Zhao, L.; Lam, W.; Guan, F.-L.; Zhao, Y.; Xu, C.-H. Mechanisms of acidic electrolyzed water killing bacteria. Food Control 2023, 147, 109609. [Google Scholar] [CrossRef]
- Xuan, X.; Zhang, Z.; Shang, H.; Sheng, Z.; Cui, Y.; Lin, X.; Chen, S.; Zhu, L. Microbial diversity and antibacterial mechanism of slightly acidic electrolyzed water against Pseudomonas fluorescens in razor clam during storage. Food Res. Int. 2025, 204, 115929. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Suo, K.; Zhang, Y.; Yang, Z.; Zhou, C.; Shi, L.; Chen, W.; Wang, J.; Wang, C.; Zheng, Y. Ultrasound synergistic slightly acidic electrolyzed water treatment of grapes: Impacts on microbial loads, wettability, and postharvest storage quality. Ultrason. Sonochem. 2024, 103, 106751. [Google Scholar] [CrossRef]
- Iram, A.; Wang, X.; Demirci, A. Electrolyzed Oxidizing Water and Its Applications as Sanitation and Cleaning Agent. Food Eng. Rev. 2021, 13, 411–427. [Google Scholar] [CrossRef]
- Costello, K.M.; Velliou, E.; Gutierrez-Merino, J.; Smet, C.; Kadri, H.E.; Impe, J.F.V.; Bussemaker, M. The effect of ultrasound treatment in combination with nisin on the inactivation of Listeria innocua and Escherichia coli. Ultrason. Sonochemistry 2021, 79, 105776. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Tobar, D.; Perez-Won, M.; Jara-Quijada, E.; Gonzalez-Cavieres, L.; Tabilo-Munizaga, G.; Lemus-Mondaca, R. Principles of ultrasonic agglomeration and its effect on physicochemical and macro- and microstructural properties of foods. Food Chem. 2025, 463, 141309. [Google Scholar] [CrossRef]
- Pang, L.; Chen, C.; Liu, M.; Huang, Z.; Zhang, W.; Shi, J.; Yang, X.; Jiang, Y. A comprehensive review of effects of ultrasound pretreatment on processing technologies for food allergens: Allergenicity, nutritional value, and technofunctional properties and safety assessment. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70100. [Google Scholar] [CrossRef]
- Fan, K.; Wu, J.; Chen, L. Ultrasound and its combined application in the improvement of microbial and physicochemical quality of fruits and vegetables: A review. Ultrason. Sonochem. 2021, 80, 105838. [Google Scholar] [CrossRef]
- Hong, C.; Zhou, H.-C.; Zhao, Y.-M.; Ma, H. Ultrasonic washing as an abiotic elicitor to induce the accumulation of phenolics of fresh-cut red cabbages: Effects on storage quality and microbial safety. Front. Nutr. 2022, 9, 1006440. [Google Scholar] [CrossRef]
- Amiri, A.; Ramezanian, A.; Mortazavi, S.M.H.; Hosseini, S.M.H. Ultrasonic potential in maintaining the quality and reducing the microbial load of minimally processed pomegranate. Ultrason. Sonochem. 2021, 70, 105302. [Google Scholar] [CrossRef]
- Li, L.; Sun, H.-N.; Zhang, M.; Mu, T.-H. Effects of ultrasound with slightly acid electrolytic water on storage of sweet potato: Physiological, nutritional, sensory and microstructural characteristics. Food Control 2025, 167, 110830. [Google Scholar] [CrossRef]
- Luo, K.; Kim, S.Y.; Wang, J.; Oh, D.-H. A combined hurdle approach of slightly acidic electrolyzed water simultaneous with ultrasound to inactivate Bacillus cereus on potato. LWT 2016, 73, 615–621. [Google Scholar] [CrossRef]
- Khalid, N.I.; Sulaiman, N.S.; Ab Aziz, N.; Taip, F.S.; Nor-Khaizura, M.A.R.; Sobri, S.; Abd Rahim, M.H. Assessing the efficacy of electrolyzed water for sanitizing contaminated stainless-steel surfaces in the meat industry. J. Food Eng. 2024, 382, 112199. [Google Scholar] [CrossRef]
- Yan, P.; Chelliah, R.; Jo, K.h.; Oh, D.H. Research Trends on the Application of Electrolyzed Water in Food Preservation and Sanitation. Processes 2021, 9, 2240. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Jiang, W. Application of electrolyzed water in postharvest fruits and vegetables storage: A review. Trends Food Sci. Technol. 2021, 114, 599–607. [Google Scholar] [CrossRef]
- Al Bsoul, A.; Magnin, J.-P.; Commenges-Bernole, N.; Gondrexon, N.; Willison, J.; Petrier, C. Effectiveness of ultrasound for the destruction of Mycobacterium sp. strain (6PY1). Ultrason. Sonochem. 2010, 17, 106–110. [Google Scholar] [CrossRef]
- Gao, S.; Hemar, Y.; Lewis, G.D.; Ashokkumar, M. Inactivation of Enterobacter aerogenes in reconstituted skim milk by high- and low-frequency ultrasound. Ultrason. Sonochem. 2014, 21, 2099–2106. [Google Scholar] [CrossRef]
- Ning, Y.; Yan, A.; Yang, K.; Wang, Z.; Li, X.; Jia, Y. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017, 228, 533–540. [Google Scholar] [CrossRef]
- Cichoski, A.J.; Flores, D.R.M.; De Menezes, C.R.; Jacob-Lopes, E.; Zepka, L.Q.; Wagner, R.; Barin, J.S.; de Moraes Flores, E.M.; da Cruz Fernandes, M.; Campagnol, P.C.B. Ultrasound and slightly acid electrolyzed water application: An efficient combination to reduce the bacterial counts of chicken breast during pre-chilling. Int. J. Food Microbiol. 2019, 301, 27–33. [Google Scholar] [CrossRef]
- Lou, X.; Shu, W.; Wang, Y.; Guo, C.; Liu, H.; Yang, H. Effect of slightly acidic electrolysed water against Shewanella baltica in phosphate-buffered saline and on golden pomfret sticks. Food Control 2024, 156, 110131. [Google Scholar] [CrossRef]
- Li, H.; Liang, D.; Huang, J.; Cui, C.; Rao, H.; Zhao, D.; Hao, J. The Bactericidal Efficacy and the Mechanism of Action of Slightly Acidic Electrolyzed Water on Listeria monocytogenes’ Survival. Foods 2021, 10, 2671. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, Y.; Mo, Q.; Zhang, B.; Shu, D.; Sun, L.; Zhao, X.; Zhang, R.; Zheng, J.; Jia, Y.; et al. Antibacterial activity and mechanism of slightly acidic electrolyzed water combined with ultraviolet light against Salmonella enteritidis. Food Control 2023, 148, 109681. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Sansonetty, F.; Rodrigues, A.G.; Costa-Oliveira, S.; Tavares, C.; Martinez-de-Oliveira, J. Cytometric approach for a rapid evaluation of susceptibility of Candida strains to antifungals. Clin. Microbiol. Infect. 2001, 7, 609–618. [Google Scholar] [CrossRef]
- Mansur, A.R.; Oh, D.H. Combined effects of thermosonication and slightly acidic electrolyzed water on the microbial quality and shelf life extension of fresh-cut kale during refrigeration storage. Food Microbiol. 2015, 51, 154–162. [Google Scholar] [CrossRef]
- Guo, L.; Sun, Y.; Zhu, Y.; Wang, B.; Xu, L.; Huang, M.; Li, Y.; Sun, J. The antibacterial mechanism of ultrasound in combination with sodium hypochlorite in the control of Escherichia coli. Food Res. Int. 2020, 129, 108887. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Li, Y.; Wang, C.; Kang, S.; Hu, X.; Zhang, L.; Li, X.; Al-Asmari, F.; Sameeh, M.Y.; Yang, B.; et al. Synergistic bactericidal effect of ultrasound combined with oregano essential oil nanoemulsion on Listeria monocytogenes and its application in blueberry preservation. Food Control 2024, 165, 110619. [Google Scholar] [CrossRef]
- Ruhal, R.; Kataria, R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef]
- Liu, L.; Lan, W.; Wang, Y.; Xie, J. Antibacterial activity and mechanism of slightly acidic electrolyzed water against Shewanella putrefaciens and Staphylococcus saprophytic. Biochem. Biophys. Res. Commun. 2022, 592, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Memisi, N.; Moracanin, S.V.; Milijasevic, M.; Babic, J.; Djukic, D. CIP cleaning processes in the dairy industry. Procedia Food Sci. 2015, 5, 184–186. [Google Scholar] [CrossRef]
- Bava, L.; Zucali, M.; Sandrucci, A.; Brasca, M.; Vanoni, L.; Zanini, L.; Tamburini, A. Effect of cleaning procedure and hygienic condition of milking equipment on bacterial count of bulk tank milk. J. Dairy Res. 2011, 78, 211–219. [Google Scholar] [CrossRef]
- Paliy, A.; Aliiev, E.; Paliy, A.; Kotko, Y.; Kolinchuk, R.; Livoschenko, E.; Chekan, O.; Nazarenko, S.; Livoschenko, L.; Uskova, L. Determining the effective mode of operation for the system of washing the milking machine milk supply line. East. Eur. J. Enterp. Technol. 2022, 5, 74–81. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xie, Q.; Cui, D.; Ren, J.; Zhao, W.; Xu, X. Research on Aseptic Milk Extraction Technology and Mechanism of Slightly Acidic Electrolytic Water Coupled with Ultrasound Treatment. Foods 2025, 14, 1711. https://doi.org/10.3390/foods14101711
Liu Y, Xie Q, Cui D, Ren J, Zhao W, Xu X. Research on Aseptic Milk Extraction Technology and Mechanism of Slightly Acidic Electrolytic Water Coupled with Ultrasound Treatment. Foods. 2025; 14(10):1711. https://doi.org/10.3390/foods14101711
Chicago/Turabian StyleLiu, Ye, Qinggang Xie, Dongying Cui, Jiaqi Ren, Wanyu Zhao, and Xiaoxi Xu. 2025. "Research on Aseptic Milk Extraction Technology and Mechanism of Slightly Acidic Electrolytic Water Coupled with Ultrasound Treatment" Foods 14, no. 10: 1711. https://doi.org/10.3390/foods14101711
APA StyleLiu, Y., Xie, Q., Cui, D., Ren, J., Zhao, W., & Xu, X. (2025). Research on Aseptic Milk Extraction Technology and Mechanism of Slightly Acidic Electrolytic Water Coupled with Ultrasound Treatment. Foods, 14(10), 1711. https://doi.org/10.3390/foods14101711





