Stepwise Sous-Vide Cooking as a Novel Approach to Enhance the Water-Holding Capacity and Tenderness of Chicken Breast
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sous-Vide Cooking Procedure
2.3. Analysis of Sous-Vide Cooked Chicken Breast
2.3.1. Cooking Loss
2.3.2. Shear Force
2.3.3. Moisture Content
2.3.4. pH Measurement
2.3.5. SURFACE Color Characteristic
2.3.6. Protein Solubility
2.3.7. Total Collagen Content
2.3.8. Myofibrillar Fragmentation Index (MFI)
2.3.9. Lipid Oxidation
2.3.10. Protein Oxidation
2.4. Statistical Analysis
3. Results and Discussion
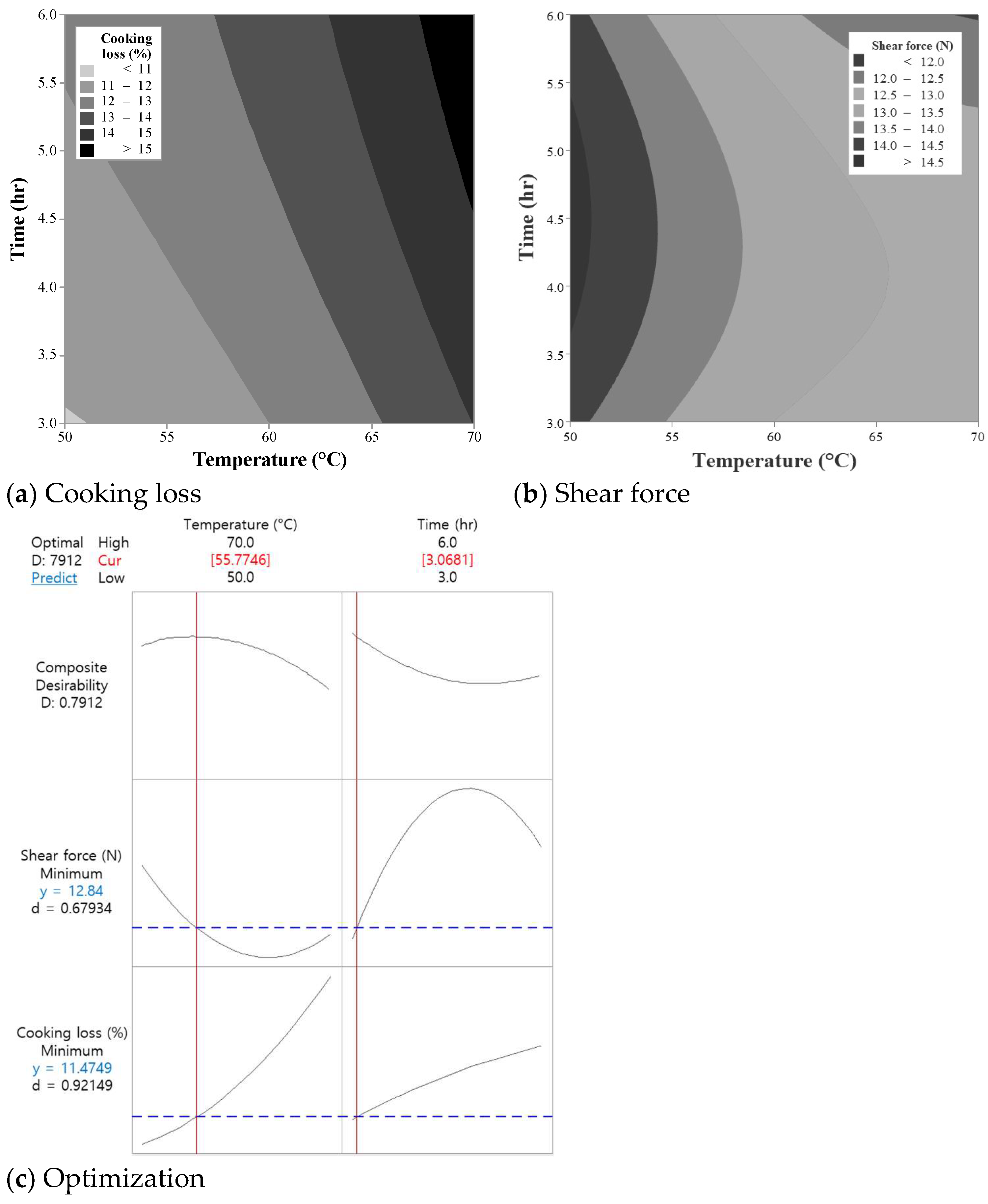
3.1. Optimization of Sous-Vide Cooking Conditions (Experiment I)
3.1.1. Effects on Cooking Loss
3.1.2. Effects on Shear Force
3.1.3. Optimization and Validation
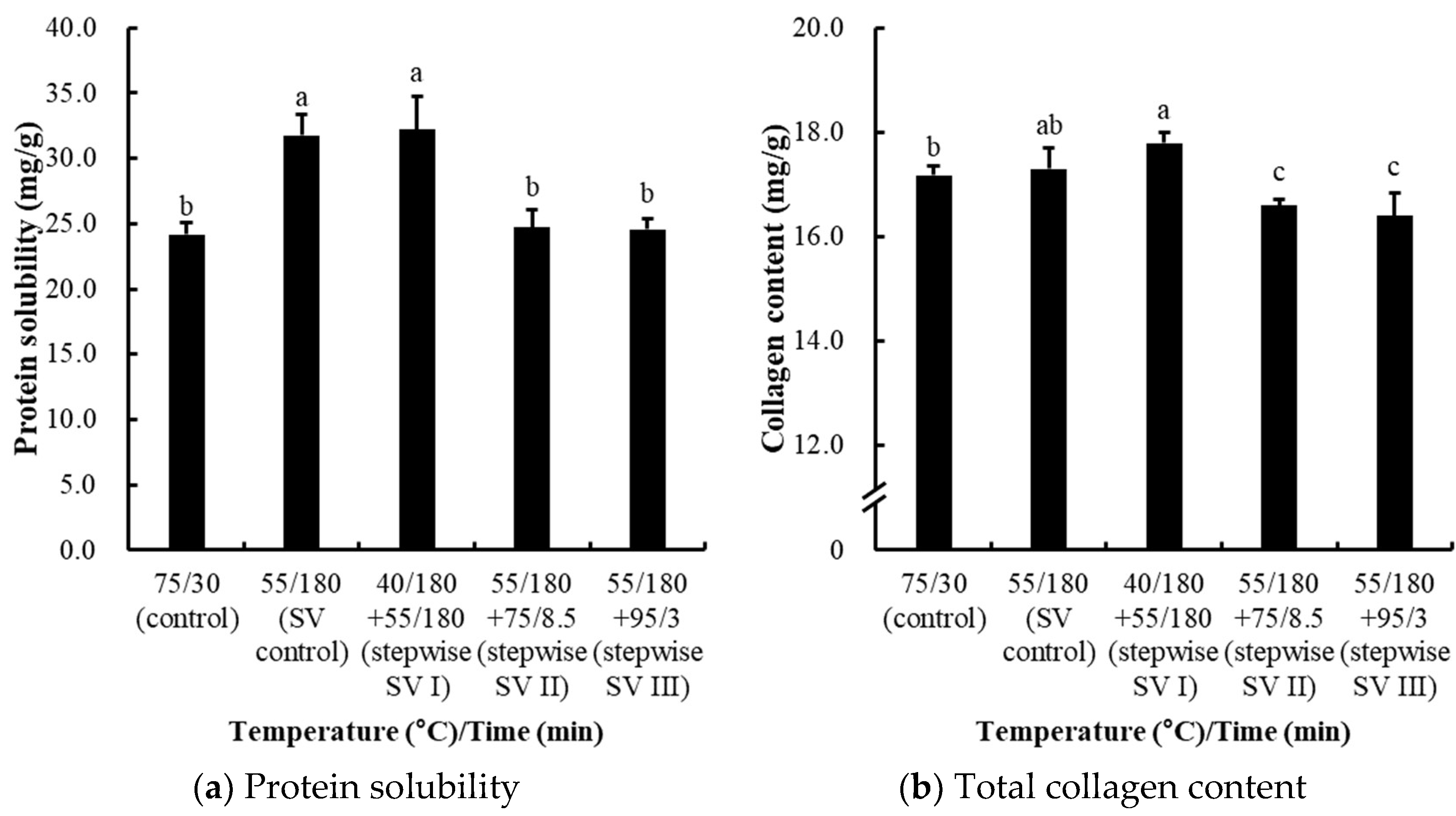
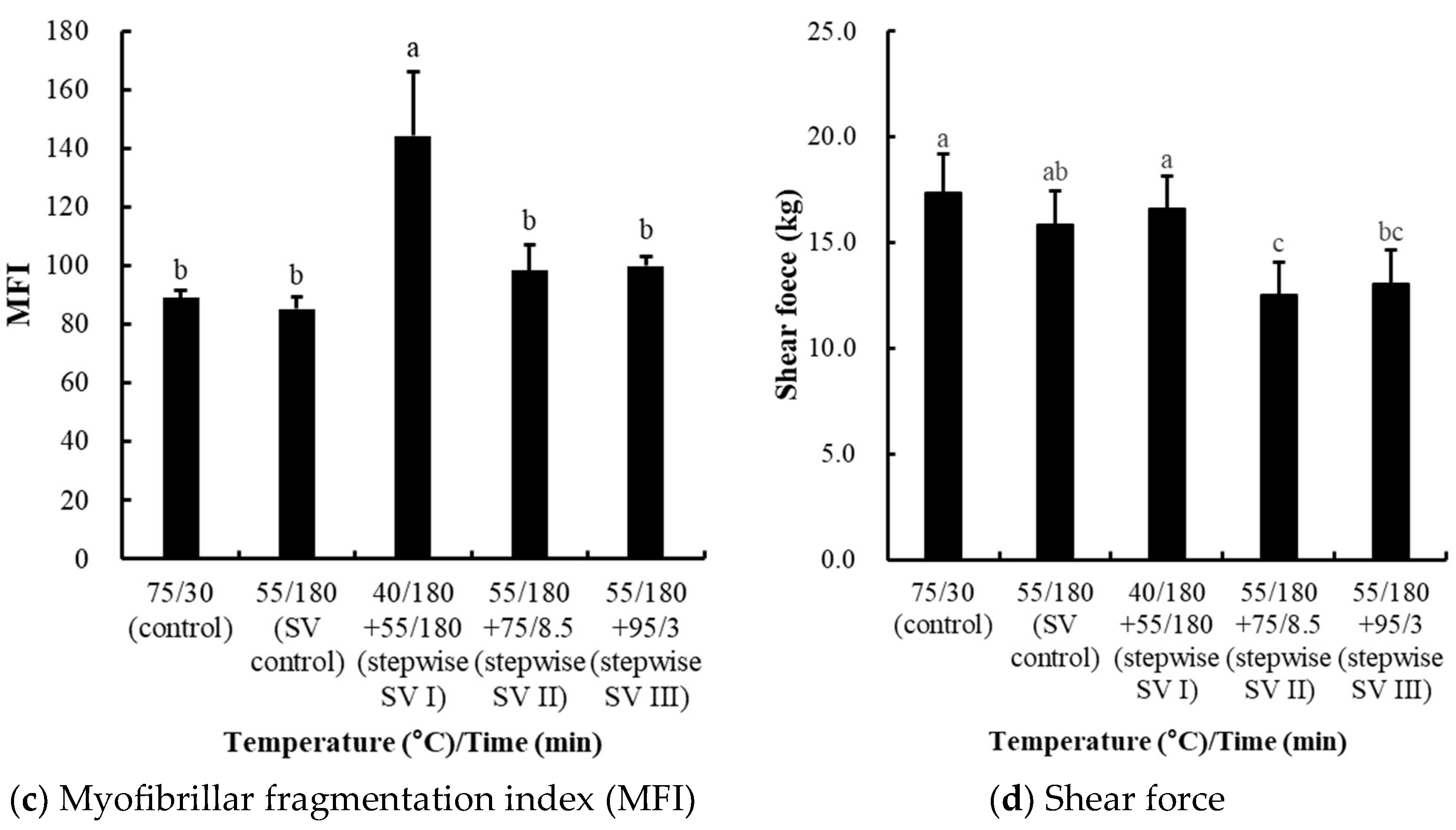
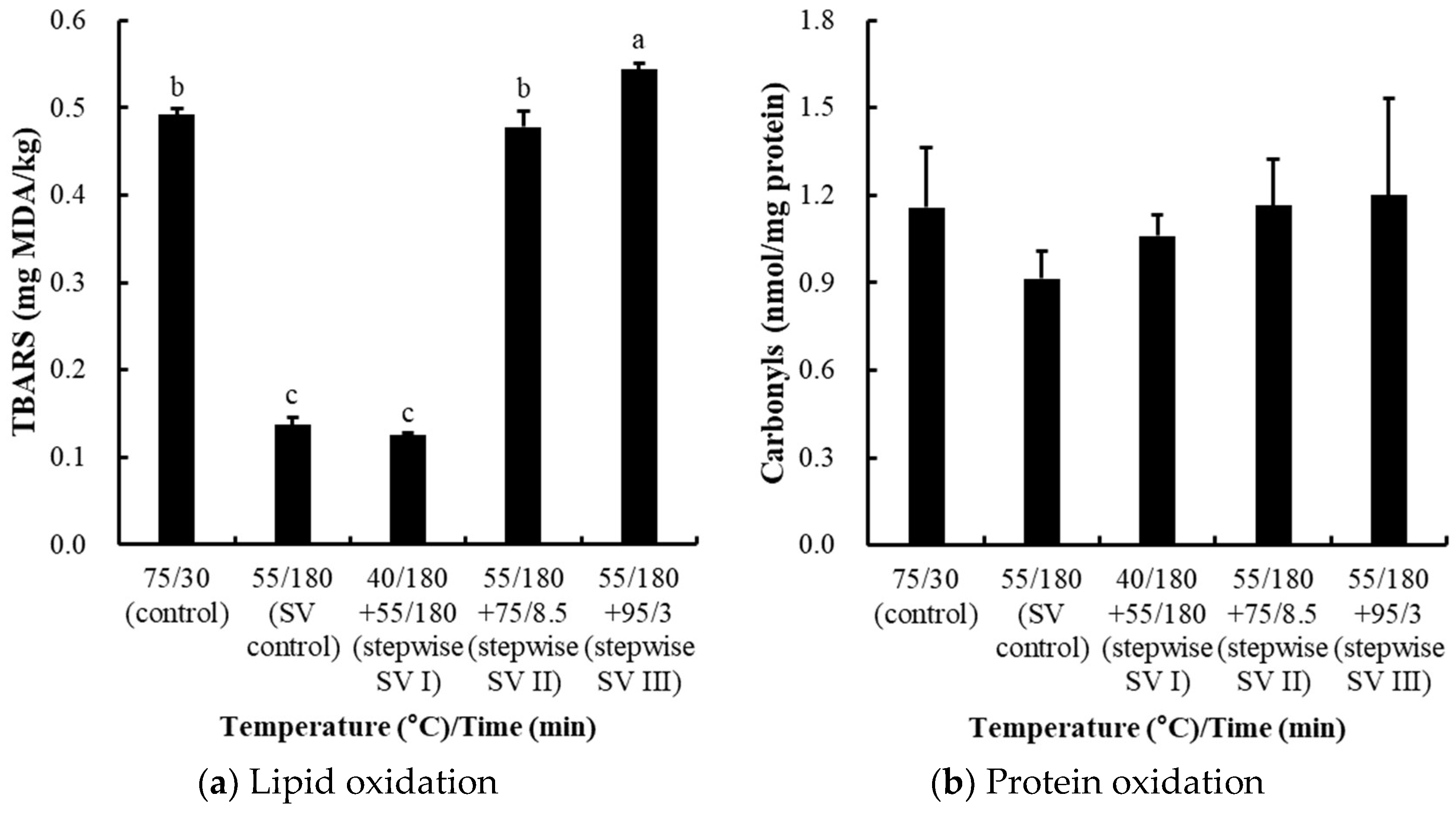
3.2. Comparisons of Stepwise Sous-Vide Cooking Conditions (Experiment II)
3.2.1. Effects on pH and Surface Color Characteristics
3.2.2. Effects on Water-Holding Capacity (Cooking Loss and Moisture Content)
3.2.3. Effects on Protein Solubility, Collagen Content, Myofibrillar Fragmentation Index (MFI), and Shear Force
3.2.4. Effects on Oxidation Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latoch, A.; Gluchowski, A.; Czarniecka-Skubina, E. Sous-vide as an alternative method of cooking to improve the quality of meat: A review. Foods 2023, 12, 3110. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, B.; Oh, E.; Kim, Y.S.; Choi, Y.M. Combined effects of sous-vide cooking conditions on meat and sensory quality characteristics of chicken breast meat. Poult. Sci. 2020, 99, 3286–3291. [Google Scholar] [CrossRef]
- Haghighi, H.; Belmonte, A.M.; Masino, F.; Minelli, G.; Di Paolo Lio Fiego, D.; Pulvirenti, A. Effect of time and temperature on physicochemical and microbiological properties of sous vide chicken breast fillets. Foods 2021, 11, 3189. [Google Scholar] [CrossRef]
- Song, D.H.; Yang, N.E.; Seomoon, K.M.; Jang, I.S.; Chin, K.B.; Kim, H.W. Sous-vide cooking as a practical strategy to improve quality attributes and shelf stability of reduced-salt chicken breast ham. Poult. Sci. 2023, 102, 102444. [Google Scholar] [CrossRef]
- Barido, F.H.; Jang, A.; Park, J.I.; Kim, Y.J.; Lee, S.K. Combined effects of processing method and black garlic extract on quality characteristics, antioxidative, and fatty acid profile of chicken breast. Poult. Sci. 2022, 101, 101723. [Google Scholar] [CrossRef] [PubMed]
- Bircan, C.; Barringer, S.A. Determination of protein denaturation of muscle foods using the dielectric properties. J. Food Sci. 2002, 67, 202–205. [Google Scholar] [CrossRef]
- Noh, S.W.; Song, D.H.; Ham, Y.K.; Yang, N.E.; Kim, H.W. Physicochemical properties of chicken breast and thigh as affected by sous-vide cooking conditions. Foods 2023, 12, 2592. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Interventions of two-stage thermal sous-vide cooking on the toughness of beef semitendinosus. Meat Sci. 2019, 157, 107882. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Comparison of single and double combination of temperature-time in sous vide treated semitendinosus muscle from cattle and goat. Food Sci. Anim. Resour. 2019, 39, 45–53. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- AMSA. Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012. [Google Scholar]
- Warner, R.D.; Kauffman, R.G.; Greaser, M.L. Muscle protein changes post mortem in relation to pork quality traits. Meat Sci. 1997, 45, 339–352. [Google Scholar] [CrossRef]
- Starkey, C.P.; Geesink, G.H.; Oddy, V.H.; Hopkins, D.L. Explaining the variation in lamb longissimus shear force across and within ageing periods using protein degradation, sarcomere length and collagen characteristics. Meat Sci. 2015, 105, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Hunt, M.C.; Sørheim, O.; Slinde, E. Color and heat denaturation of myoglobin forms in ground beef. J. Food Sci. 1999, 64, 847–851. [Google Scholar] [CrossRef]
- Baldwin, D.E. Sous vide cooking: A review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Ježek, F.; Kameník, J.; Macharáčková, B.; Bogdanovičová, K.; Bednář, J. Cooking of meat: Effect on texture, cooking loss and microbiological quality—A review. Acta Vet. Brno 2019, 88, 487–496. [Google Scholar] [CrossRef]
- Purslow, P.P. Contribution of collagen and connective tissue to cooked meat toughness; some paradigms reviewed. Meat Sci. 2018, 144, 127–134. [Google Scholar] [CrossRef]
- Dominguez-Hernandez, E.; Salaseviciene, A.; Ertbjerg, P. Low-temperature long-time cooking of meat: Eating quality and underlying mechanisms. Meat Sci. 2018, 143, 104–113. [Google Scholar] [CrossRef]
- Zayas, J.F. Solubility of proteins. In Functionality of Proteins in Food; Zayas, J.F., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 6–75. [Google Scholar] [CrossRef]
- Tornberg, E.V.A. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Xu, W.; Gao, F.; Zhou, G. Effect of final cooked temperature on tenderness, protein solubility and microstructure of duck breast muscle. LWT 2013, 51, 266–274. [Google Scholar] [CrossRef]
- Ayub, H.; Ahmad, A. Physicochemical changes in sous-vide and conventionally cooked meat. Int. J. Gastron. Food Sci. 2019, 17, 100145. [Google Scholar] [CrossRef]
- Martens, H.; Stabursvik, E.; Martens, M. Texture and colour changes in meat during cooking related to thermal denaturation of muscle proteins. J. Texture Stud. 1982, 13, 291–309. [Google Scholar] [CrossRef]
- Purslow, P.P.; Oiseth, S.; Hughes, J.; Warner, R.D. The structural basis of cooking loss in beef: Variations with temperature and ageing. Food Res. Int. 2016, 89, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dong, H.; Zhang, M.; Liu, F.; Bian, H.; Zhu, Y.; Xu, W. Changes in actomyosin dissociation and endogenous enzyme activities during heating and their relationship with duck meat tenderness. Food Chem. 2013, 141, 675–679. [Google Scholar] [CrossRef]
- Okitani, A.; Ichinose, N.; Itoh, J.; Tsuji, Y.; Oneda, Y.; Hatae, K.; Migita, K.; Matsuishi, M. Liberation of actin from actomyosin in meats heated to 65 °C. Meat Sci. 2009, 81, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, D.; Pasquini, M. Influence of cooking conditions on cooking loss and tenderness of raw and marinated chicken breast meat. LWT 2005, 38, 895–901. [Google Scholar] [CrossRef]
- Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K.; Sałek, P.; Pakuła, K. Effect of heat treatment by the sous-vide method on the quality of poultry meat. Foods 2021, 10, 1610. [Google Scholar] [CrossRef]
- Mei, L.; Crum, A.D.; Decker, E.A. Development of lipid oxidation and inactivation of antioxidant enzymes in cooked pork and beef. J. Food Lipids 1994, 1, 273–283. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, M.; Wang, T.; Wang, D.; Sun, C.; Bian, H.; Li, P.; Zou, Y.; Xu, W. Lipid oxidation induced by heating in chicken meat and the relationship with oxidants and antioxidant enzymes activities. Poult. Sci. 2020, 99, 1761–1767. [Google Scholar] [CrossRef]


| Run | Coded Levels | Factor Levels | Response Variables | |||
|---|---|---|---|---|---|---|
| X1 | X2 | X1 | X2 | Cooking Loss (%) | Shear Force (N) | |
| 1 | −1 | −1 | 50 | 3.0 | 11.13 | 14.12 |
| 2 | 0 | 0 | 60 | 4.5 | 13.64 | 13.42 |
| 3 | 0 | 0 | 60 | 4.5 | 13.56 | 13.61 |
| 4 | −1 | 0 | 50 | 4.5 | 11.88 | 14.94 |
| 5 | 0 | 0 | 60 | 4.5 | 13.82 | 13.11 |
| 6 | 0 | 1 | 60 | 6.0 | 14.07 | 13.03 |
| 7 | 0 | 0 | 60 | 4.5 | 11.25 | 13.66 |
| 8 | 1 | 0 | 70 | 4.5 | 14.78 | 12.93 |
| 9 | 1 | 1 | 70 | 6.0 | 15.48 | 11.81 |
| 10 | 1 | −1 | 70 | 3.0 | 14.43 | 12.78 |
| 11 | 0 | −1 | 60 | 3.0 | 11.39 | 12.94 |
| 12 | −1 | 1 | 50 | 6.0 | 11.72 | 13.93 |
| 13 | 0 | 0 | 60 | 4.5 | 11.96 | 12.55 |
| Source | Cooking Loss (%) | Shear Force (N) | ||||
|---|---|---|---|---|---|---|
| Sum of Squares | Mean Square | p Value | Sum of Squares | Mean Square | p Value | |
| Model | 20.2900 | 4.0580 | 0.043 | 6.2516 | 1.2503 | 0.010 |
| Linear | 19.6026 | 9.8013 | 0.008 | 5.1776 | 2.5888 | 0.003 |
| X1 | 16.4783 | 16.4783 | 0.004 | 4.9868 | 4.9868 | 0.001 |
| X2 | 3.1243 | 3.1243 | 0.114 | 0.1908 | 0.1908 | 0.322 |
| Quadratic | 0.6348 | 0.3174 | 0.728 | 0.9218 | 0.4609 | 0.132 |
| X12 | 0.6240 | 0.6240 | 0.446 | 0.5070 | 0.5070 | 0.126 |
| X22 | 0.0419 | 0.0419 | 0.840 | 0.7513 | 0.7513 | 0.072 |
| Interaction | 0.0525 | 0.0525 | 0.821 | 0.1521 | 0.1521 | 0.373 |
| X1X2 | 0.0525 | 0.0525 | 0.821 | 0.1521 | 0.1521 | 0.373 |
| Error | 6.6982 | 0.9569 | 1.1756 | 0.1679 | ||
| Lack of fit | 1.2675 | 0.4225 | 0.818 | 0.3414 | 0.1138 | 0.677 |
| Pure error | 5.4307 | 1.3577 | 0.8342 | 0.2086 | ||
| Total | 26.9881 | 7.4271 | ||||
| Model summary | ||||||
| R-squared | 0.7518 | 0.8417 | ||||
| RMSE | 0.9782 | 0.4098 | ||||
| Traits | Temperature (°C)/Time (min) | |||||
|---|---|---|---|---|---|---|
| Conventional Control (75/30) | Sous-Vide Control (55/180) | Stepwise SV I (40/180 + 55/180) | Stepwise SV II (55/180 + 75/8.5) | Stepwise SV III (55/180 + 95/3) | Significance of p Value | |
| pH value | 6.23 ± 0.02 | 6.34 ± 0.09 | 6.26 ± 0.13 | 6.29 ± 0.09 | 6.32 ± 0.08 | NS (1) |
| CIE L* (lightness) | 83.69 ± 0.65 a | 80.34 ± 1.28 c | 80.56 ± 0.96 c | 82.54 ± 0.55 b | 82.62 ± 0.85 b | 0.042 |
| CIE a* (redness) | 3.15 ± 0.46 b | 4.48 ± 0.38 a | 4.39 ± 0.44 a | 3.44 ± 0.51 b | 3.56 ± 0.54 b | 0.023 |
| CIE b* (yellowness) | 13.30 ± 0.33 ab | 13.67 ± 1.08 b | 13.52 ± 1.52 b | 13.95 ± 0.94 a | 13.78 ± 1.51 a | 0.021 |
| Hue (°) | 76.31 ± 1.68 | 72.25 ± 0.59 | 72.25 ± 3.23 | 75.07 ± 1.24 | 74.04 ± 3.36 | NS |
| Chroma | 13.35 ± 0.41 | 14.33 ± 1.13 | 14.20 ± 1.37 | 14.44 ± 1.03 | 14.33 ± 1.37 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, S.-W.; Song, D.-H.; Kim, H.-W. Stepwise Sous-Vide Cooking as a Novel Approach to Enhance the Water-Holding Capacity and Tenderness of Chicken Breast. Foods 2025, 14, 1708. https://doi.org/10.3390/foods14101708
Noh S-W, Song D-H, Kim H-W. Stepwise Sous-Vide Cooking as a Novel Approach to Enhance the Water-Holding Capacity and Tenderness of Chicken Breast. Foods. 2025; 14(10):1708. https://doi.org/10.3390/foods14101708
Chicago/Turabian StyleNoh, Sin-Woo, Dong-Heon Song, and Hyun-Wook Kim. 2025. "Stepwise Sous-Vide Cooking as a Novel Approach to Enhance the Water-Holding Capacity and Tenderness of Chicken Breast" Foods 14, no. 10: 1708. https://doi.org/10.3390/foods14101708
APA StyleNoh, S.-W., Song, D.-H., & Kim, H.-W. (2025). Stepwise Sous-Vide Cooking as a Novel Approach to Enhance the Water-Holding Capacity and Tenderness of Chicken Breast. Foods, 14(10), 1708. https://doi.org/10.3390/foods14101708






