Population Dynamics of Listeria monocytogenes and Yeast and Mold Levels on Different Pear Varieties During Simulated Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. L. monocytogenes Strains and Culture Preparation
2.2. Pear Inoculation
2.3. Storage Treatment of Inoculated Pears
2.4. Microbiological Analysis
2.5. Statistical Analysis
3. Results
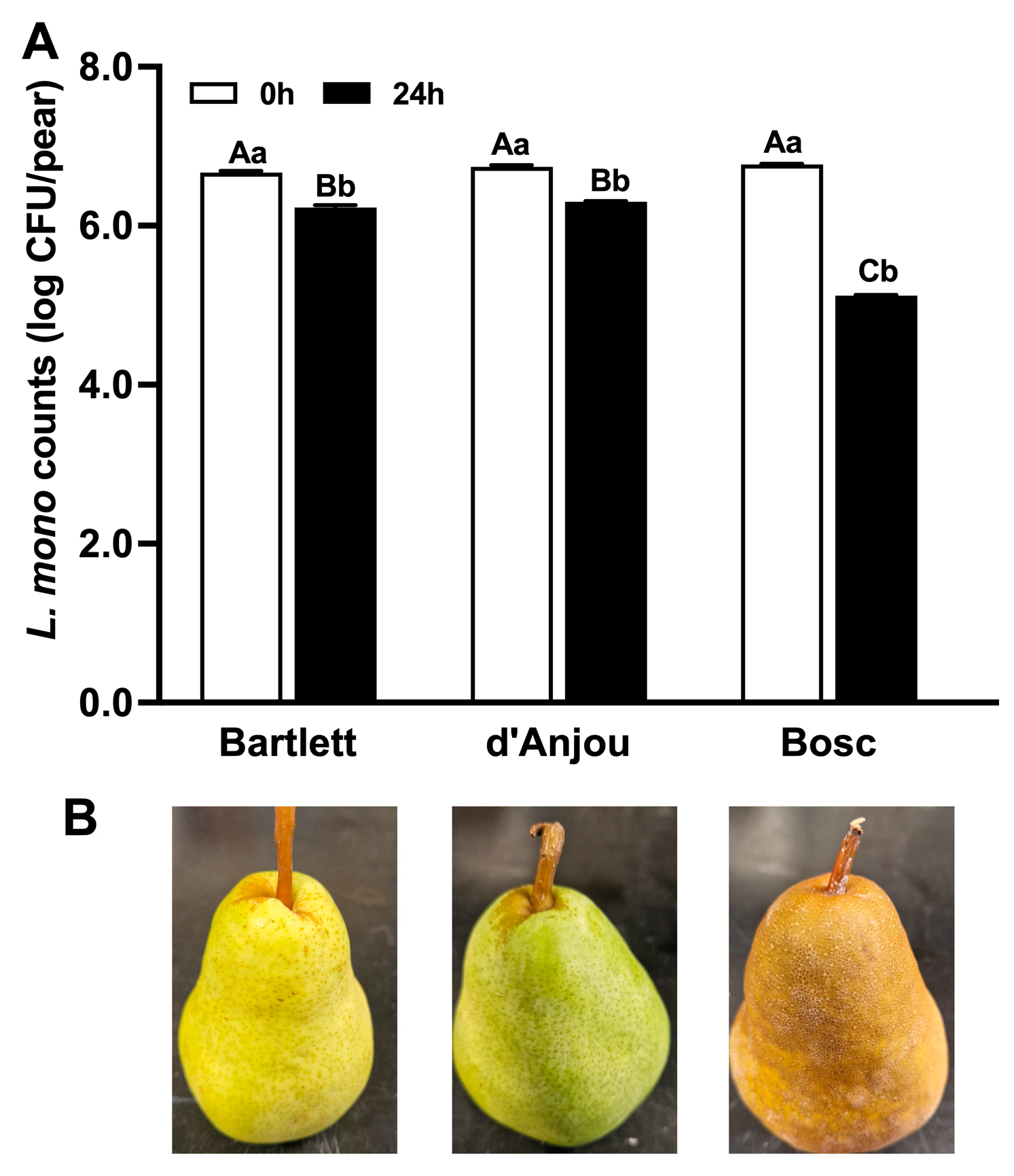
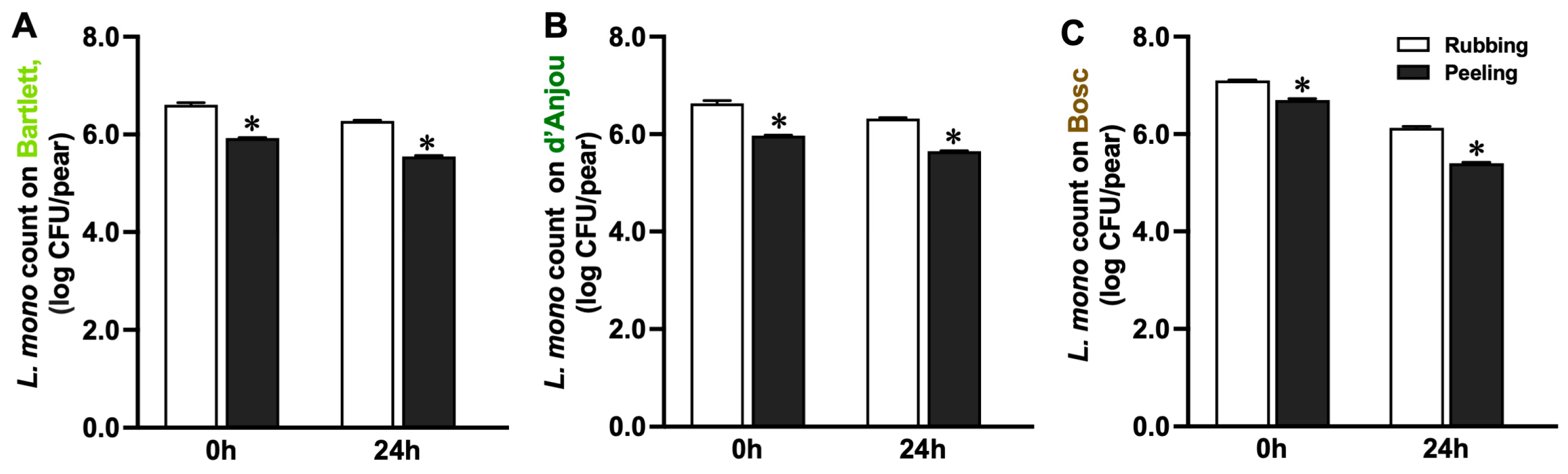
3.1. Attachment Behavior of L. monocytogenes on Pear Surfaces
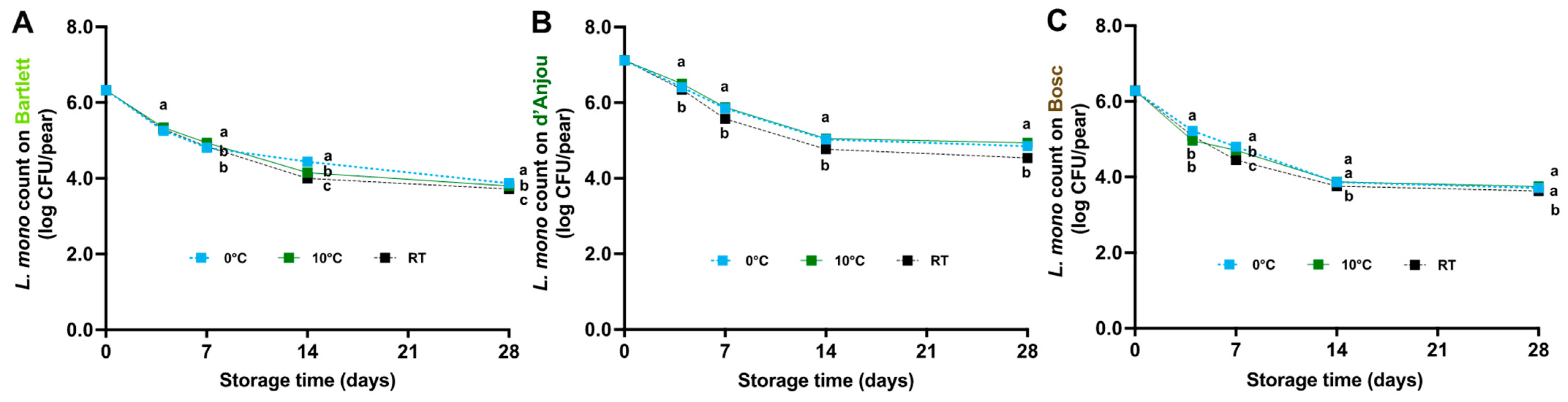
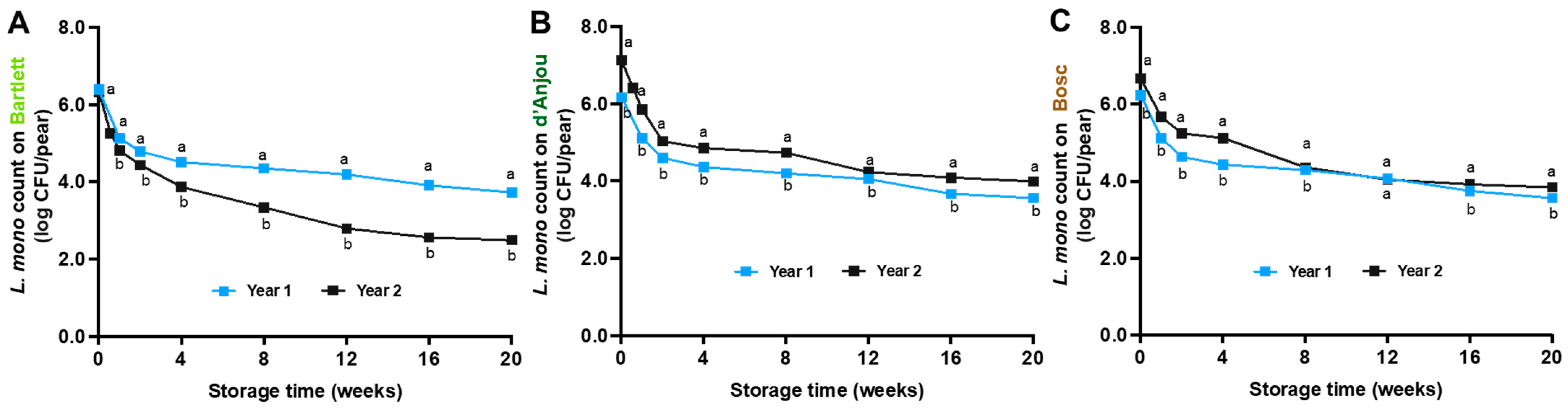
3.2. L. monocytogenes Survival on Pears Under Different Storage Temperatures
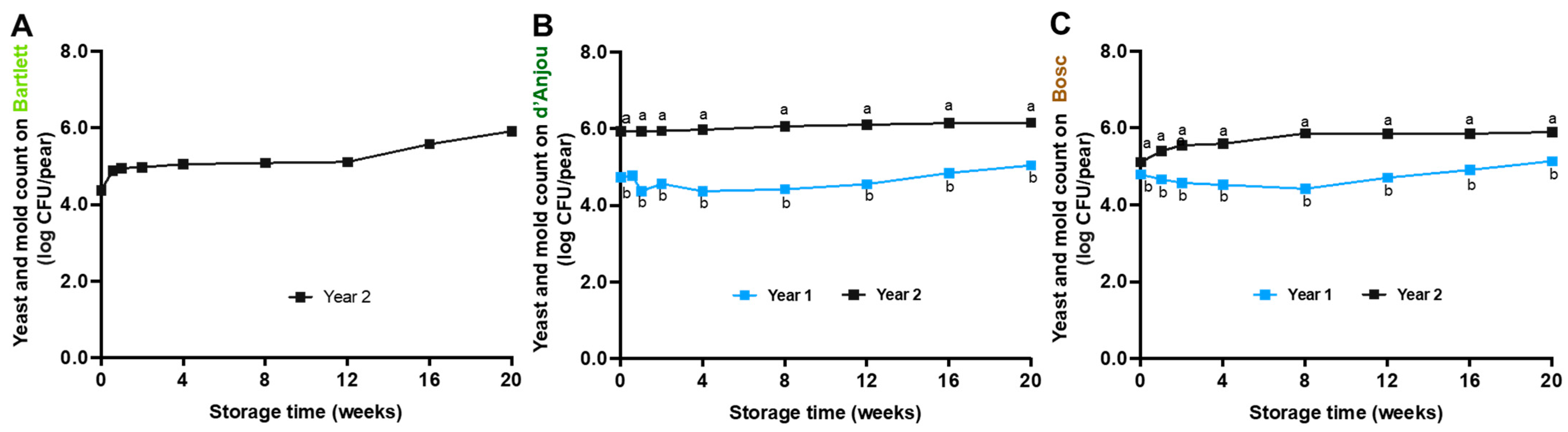
3.3. Fate of Yeasts and Molds on Pears During Storage
4. Discussion
4.1. Attachment Behavior of L. monocytogenes on Pears
4.2. Fate of L. monocytogenes on Pears During Storage
4.3. Levels of Resident Yeast and Mold During Pear Storage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.; Mahon, B.; Jones, T.; Griffin, P. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol. Infect. 2015, 143, 2795–2804. [Google Scholar] [CrossRef]
- Su, Y.; Liu, A.; Zhu, M.-J. Mapping the landscape of listeriosis outbreaks (1998–2023): Trends, challenges, and regulatory responses in the United States. Trends Food Sci. Technol. 2024, 154, 104750. [Google Scholar] [CrossRef]
- Angelo, K.M.; Conrad, A.R.; Saupe, A.; Dragoo, H.; West, N.; Sorenson, A.; Barnes, A.; Doyle, M.; Beal, J.; Jackson, K.A. Multistate outbreak of Listeria monocytogenes infections linked to whole apples used in commercially produced, prepackaged caramel apples: United States, 2014–2015. Epidemiol. Infect. 2017, 145, 848–856. [Google Scholar] [CrossRef] [PubMed]
- FDA; Northstar Produce Inc. Recalls Granny Smith Size 175 Apples Because of Possible Health Risk. 2015. Available online: https://fdarecall.wordpress.com/2015/10/30/northstar-produce-inc-recalls-granny-smith-size-175-apples-because-of-possible-health-risk/ (accessed on 8 January 2025).
- FDA; Jack Brown Produce, Inc. Recalls Gala, Fuji, Honeycrisp and Golden Delicious Apples Due to Possible Health Risk. 2017. Available online: https://www.fda.gov/Safety/Recalls/ucm589722.htm (accessed on 8 January 2025).
- FDA; Fresh Pak Inc. Recalls Lot Specific Sliced Apple Products Because of Possible Health Risk. 2017. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/fresh-pak-inc-recalls-lot-specific-sliced-apple-products-because-possible-health-risk (accessed on 9 January 2025).
- FDA. North Bay Produce Voluntarily Recalls Fresh Apples Because of Possible Health Risk. 2019. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/north-bay-produce-voluntarily-recalls-fresh-apples-because-possible-health-risk (accessed on 9 January 2025).
- FDA. Country Fresh Expands Voluntary Recall. 2020. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/country-fresh-expands-voluntary-recall (accessed on 9 January 2025).
- Poimenidou, S.V.; Chatzithoma, D.N.; Nychas, G.J.; Skandamis, P.N. Adaptive response of Listeria monocytogenes to heat, salinity and low pH, after habituation on cherry tomatoes and lettuce leaves. PLoS ONE 2016, 11, e0165746. [Google Scholar] [CrossRef]
- Salazar, J.K.; Carstens, C.K.; Bathija, V.M.; Narula, S.S.; Parish, M.; Tortorello, M.L. Fate of Listeria monocytogenes in fresh apples and caramel apples. J. Food Prot. 2016, 79, 696–702. [Google Scholar] [CrossRef]
- Sheng, L.; Edwards, K.; Tsai, H.C.; Hanrahan, I.; Zhu, M.J. Fate of Listeria monocytogenes on fresh apples under different storage temperatures. Front. Microbiol. 2017, 8, 1396. [Google Scholar] [CrossRef]
- Sheng, L.; Hanrahan, I.; Sun, X.; Taylor, M.H.; Mendoza, M.; Zhu, M.-J. Survival of Listeria innocua on Fuji apples under commercial cold storage with or without low dose continuous ozone gaseous. Food Microbiol. 2018, 76, 21–28. [Google Scholar] [CrossRef]
- Ruiz-Llacsahuanga, B.; Hamilton, A.; Zaches, R.; Hanrahan, I.; Critzer, F. Prevalence of Listeria species on food contact surfaces in Washington State apple packinghouses. Appl. Environ. Microbiol. 2021, 87, e02932. [Google Scholar] [CrossRef]
- Saquet, A.A. Storage of pears. Sci. Hortic. 2019, 246, 1009–1016. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, X.; Su, Y.; Xue, Y.; Gao, H.; Mendoza, M.; Green, T.; Hanrahan, I.; Zhu, M.-J. Effects of 1-methylcyclopropene and gaseous ozone on Listeria innocua survival and fruit quality of Granny Smith apples during long-term commercial cold storage. Food Microbiol. 2022, 102, 103922. [Google Scholar] [CrossRef] [PubMed]
- Freed, C.; Stearns, R.; Freshour, N.; Luo, Y.; Shen, C. Survival of Listeria monocytogenes on organic Honeycrisp and Fuji apples during storage at 5, 12 and 22.5°C. J. Agric. Food Res. 2022, 10, 100455. [Google Scholar] [CrossRef]
- Glass, K.A.; Golden, M.C.; Wanless, B.J.; Bedale, W.; Czuprynski, C. Growth of Listeria monocytogenes within a caramel-coated apple microenvironment. mBio 2015, 6, e01232. [Google Scholar] [CrossRef] [PubMed]
- Macarisin, D.; Sheth, I.; Hur, M.; Wooten, A.; Kwon, H.J.; Gao, Z.; De Jesus, A.; Jurick, W.; Chen, Y. Survival of outbreak, food, and environmental strains of Listeria monocytogenes on whole apples as affected by cultivar and wax coating. Sci. Rep. 2019, 9, 12170. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Su, Y.; Hua, Z.; Zhu, H.; Ünlü, G.; Ross, C.; Mendoza, M.; Hanrahan, I.; Tang, J.; Zhu, M.-J. Listeria monocytogenes cross-contamination during apple waxing and subsequent survival under different storage conditions. Food Microbiol. 2023, 110, 104166. [Google Scholar] [CrossRef]
- Shen, X.; Su, Y.; Hua, Z.; Chiu, T.; Wang, Y.; Mendoza, M.; Hanrahan, I.; Zhu, M.-J. Evaluating serotype-specific survival of Listeria monocytogenes and Listeria innocua on wax-coated Granny Smith apples during storage. Int. J. Food Microbiol. 2025, 427, 110964. [Google Scholar] [CrossRef]
- Silva, G.J.; Souza, T.M.; Barbieri, R.L.; Costa de Oliveira, A. Origin, domestication, and dispersing of Pear (Pyrus spp.). Adv. Agric. 2014, 2014, 541097. [Google Scholar] [CrossRef]
- AMRC. Pears. Available online: https://www.agmrc.org/commodities-products/fruits/pears#:~:text=Introduction,year%20(NASS%2C%202022) (accessed on 15 July 2023).
- NASS. State Agricultural Overview Washington. Available online: https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=WASHINGTON (accessed on 20 July 2023).
- Prange, R.K.; Wright, A.H. A review of storage temperature recommendations for apples and pears. Foods 2023, 12, 466. [Google Scholar] [CrossRef]
- Wu, X.; Yin, H.; Shi, Z.; Chen, Y.; Qi, K.; Qiao, X.; Wang, G.; Cao, P.; Zhang, S. Chemical composition and crystal morphology of epicuticular wax in mature fruits of 35 Pear (Pyrus spp.) cultivars. Front. Plant Sci. 2018, 9, 679. [Google Scholar] [CrossRef]
- Morice, I.M.; Shorland, F.B. Composition of the surface waxes of apple fruits and changes during storage. J. Sci. Food Agric. 1973, 24, 1331–1339. [Google Scholar] [CrossRef]
- Wenneker, M.; Thomma, B.P. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Blanckenberg, A.; Fawole, O.A.; Opara, U.L. Postharvest losses in quantity and quality of pear (cv. Packham’s Triumph) along the supply chain and associated economic, environmental and resource impacts. Sustainability 2022, 14, 603. [Google Scholar] [CrossRef]
- Shen, X.; Su, Y.; Hua, Z.; Sheng, L.; Mendoza, M.; He, Y.; Green, T.; Hanrahan, I.; Blakey, R.; Zhu, M.J. Effectiveness of low-dose continuous gaseous ozone in controlling Listeria innocua on Red Delicious apples during 9-month commercial cold storage. Front. Microbiol. 2021, 12, 712757. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Hang, M.; Su, Y.; de Avila, J.M.; Zhu, M.-J. Evaluating chlorine sanitization at practical concentrations for controlling Listeria monocytogenes and Salmonella on fresh peaches. Foods 2024, 13, 3344. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-C.; Shen, C.; Farnham, M.W.; Ku, K.-M. Three-dimensional epicuticular wax on plant surface reduces attachment and survival rate of Salmonella during storage. Postharvest Biol. Technol. 2020, 166, 111197. [Google Scholar] [CrossRef]
- Ku, K.-M.; Chiu, Y.-C.; Shen, C.; Jenks, M. Leaf cuticular waxes of lettuce are associated with reduced attachment of the foodborne pathogen Salmonella spp. at harvest and after postharvest storage. LWT-Food Sci. Technol. 2020, 117, 108657. [Google Scholar] [CrossRef]
- Beuchat, L.R. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 2002, 4, 413–423. [Google Scholar] [CrossRef]
- Thomas, G.A.; Paradell Gil, T.; Muller, C.T.; Rogers, H.J.; Berger, C.N. From field to plate: How do bacterial enteric pathogens interact with ready-to-eat fruit and vegetables, causing disease outbreaks? Food Microbiol. 2024, 117, 104389. [Google Scholar] [CrossRef]
- Kovacs, E.; Kovacs, J.; Zackel, E.; Genova, J. Structural and chemical changes on pear skin. Acta Hortic. 1994, 368, 243–250. [Google Scholar] [CrossRef]
- Amarante, C.; Banks, N.H.; Ganesh, S. Relationship between character of skin cover of coated pears and permeance to water vapour and gases. Postharvest Biol. Technol. 2001, 21, 291–301. [Google Scholar] [CrossRef]
- Kjelleberg, S.; Humphrey, b.; Kevin, M. Initial phases of starvation and activity of bacteria at surfaces. Appl. Environ. Microbiol. 1983, 46, 978–984. [Google Scholar] [CrossRef]
- Jayeola, V.; Farber, J.M.; Kathariou, S. Induction of the viable-but-nonculturable state in Salmonella contaminating dried fruit. Appl. Environ. Microbiol. 2022, 88, e01733-21. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Aviat, F.; Michel, V.; Le Bayon, I.; Gay-Perret, P.; Kutnik, M.; Federighi, M. Methods for recovering microorganisms from solid surfaces used in the food industry: A review of the literature. Int. J. Environ. Res. Public Health 2013, 10, 6169–6183. [Google Scholar] [CrossRef]
- Kroft, B.; Gu, G.; Bolten, S.; Micallef, S.A.; Luo, Y.; Millner, P.; Nou, X. Effects of temperature abuse on the growth and survival of Listeria monocytogenes on a wide variety of whole and fresh-cut fruits and vegetables during storage. Food Control 2022, 137, 108919. [Google Scholar] [CrossRef]
- Moreira, J.; Mera, E.; Singh Chhetri, V.; King, J.M.; Gentimis, T.; Adhikari, A. Effect of storage temperature and produce type on the survival or growth of Listeria monocytogenes on peeled rinds and fresh-cut produce. Front. Microbiol. 2023, 14, 1151819. [Google Scholar] [CrossRef]
- Colás-Medà, P.; Abadias, M.; Oliveira, M.; Usall, J.; Viñas, I. Influence of fruit matrix and storage temperature on the survival of Listeria monocytogenes in a gastrointestinal simulation. Food Control 2017, 73, 1045–1052. [Google Scholar] [CrossRef]
- Colas-Meda, P.; Vinas, I.; Oliveira, M.; Anguera, M.; Serrano, J.C.E.; Abadias, M. Exposure to minimally processed pear and melon during shelf life could modify the pathogenic potential of Listeria monocytogenes. Food Microbiol. 2017, 62, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Qadri, O.S.; Yousuf, B.; Srivastava, A.K.; Yildiz, F. Fresh-cut fruits and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks—A review. Cogent Food Agric. 2015, 1, 1121606. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Eifert, J.; Williams, R.; Marcy, J.; Welbaum, G. Survival of Listeria monocytogenes on fresh blueberries (Vaccinium corymbosum) stored under controlled atmosphere and ozone. J. Food Prot. 2014, 77, 832–836. [Google Scholar] [CrossRef]
- Han, Y.; Linton, R.; Nielsen, S.; Nelson, P. Reduction of Listeria monocytogenes on green peppers (Capsicum annuum L.) by gaseous and aqueous chlorine dioxide and water washing and its growth at 7 °C. J. Food Prot. 2001, 64, 1730–1738. [Google Scholar] [CrossRef]
- Wenneker, M.; Köhl, J. Postharvest decay of apples and pears in the Netherlands. Acta Hortic. 2014, 1053, 107–112. [Google Scholar] [CrossRef]
- Mari, M.; Guizzardi, M.; Pratella, G.C. Biological control of gray mold in pears by antagonistic bacteria. BioControl 1996, 7, 30–37. [Google Scholar] [CrossRef]
- Michailides, T.J.; Spotts, R.A. Postharvest diseases of pome and stone fruits caused by Mucor piriformis in the Pacific Northwest and California. Plan. Disease 1990, 74, 537–543. [Google Scholar] [CrossRef]
- Beuchat, L.R.; Brackett, R.E. Inhibitory Effects of Raw Carrots on Listeria monocytogenes. Appl. Environ. Microbiol. 1990, 56, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Tewari, A. Effect of Environmental Factors, Edaphic Factors and Nutrition on Plant Disease Development. 2019. Available online: https://www.researchgate.net/profile/Vinaykumar-Rachappanavar/publication/338330640_Identification_and_management_of_seed-borne_Diseases_by_conventional_and_modern_techniques/links/5e0cd8394585159aa4ab4e82/Identification-and-management-of-seed-borne-Diseases-by-conventional-and-modern-techniques.pdf#page=115 (accessed on 25 February 2025).
- Sardella, D.; Muscat, A.; Brincat, J.-P.; Gatt, R.; Decelis, S.; Valdramidis, V. A comprehensive review of the pear fungal diseases. Int. J. Fruit Sci. 2016, 16, 351–377. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, X.; Gao, Z.; Chen, T.; Zhang, L. Relationships between fungal diversity and fruit quality of Yuluxiang pear during low temperature storage. Front. Microbiol. 2023, 14, 1132271. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.; Li, Y.; Bi, Y.; Li, R.; Yuan, J.; Xu, W.; Prusky, D. AaHog1 regulates infective structural differentiation mediated by physicochemical signals from pear fruit cuticular wax, stress response, and Alternaria alternata pathogenicity. J. Fungi 2022, 8, 266. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Y.; Li, H.; Gao, Q.; Cheng, Y.; Ogunyemi, S.O.; Guan, J. Fruit bagging reduces the postharvest decay and alters the diversity of fruit surface fungal community in ‘Yali’ pear. BMC Microbiol. 2022, 22, 239. [Google Scholar] [CrossRef]
- Martinez-Alonso, M.; Escolano, J.; Montesinos, E.; Gaju, N. Diversity of the bacterial community in the surface soil of a pear orchard based on 16S rRNA gene analysis. Int. Microbiol. 2010, 13, 123–134. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, M.; Afari, E.L.; Shen, X.; Su, Y.; Mendoza, M.; Hanrahan, I.; Zhu, M.-J. Population Dynamics of Listeria monocytogenes and Yeast and Mold Levels on Different Pear Varieties During Simulated Storage. Foods 2025, 14, 1701. https://doi.org/10.3390/foods14101701
Hang M, Afari EL, Shen X, Su Y, Mendoza M, Hanrahan I, Zhu M-J. Population Dynamics of Listeria monocytogenes and Yeast and Mold Levels on Different Pear Varieties During Simulated Storage. Foods. 2025; 14(10):1701. https://doi.org/10.3390/foods14101701
Chicago/Turabian StyleHang, Mengqian, Edmund Larbi Afari, Xiaoye Shen, Yuan Su, Manoella Mendoza, Ines Hanrahan, and Mei-Jun Zhu. 2025. "Population Dynamics of Listeria monocytogenes and Yeast and Mold Levels on Different Pear Varieties During Simulated Storage" Foods 14, no. 10: 1701. https://doi.org/10.3390/foods14101701
APA StyleHang, M., Afari, E. L., Shen, X., Su, Y., Mendoza, M., Hanrahan, I., & Zhu, M.-J. (2025). Population Dynamics of Listeria monocytogenes and Yeast and Mold Levels on Different Pear Varieties During Simulated Storage. Foods, 14(10), 1701. https://doi.org/10.3390/foods14101701






