Investigation of Co-Assembly of Peanut Protein and Rice Protein: Effects on Protein Conformation and Immunoglobulin E Binding Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PP–RP Complexes
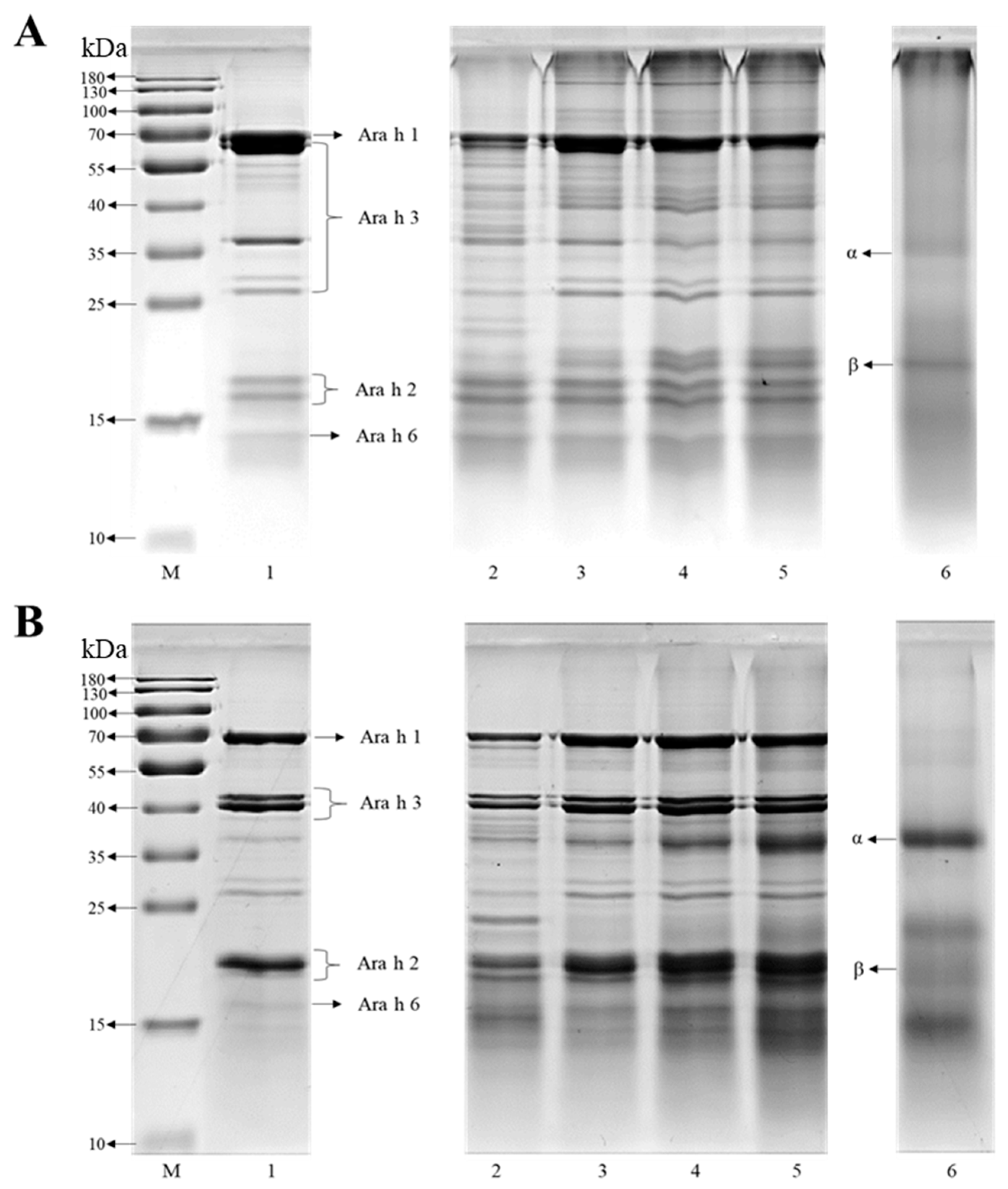
2.3. The Characterization of SDS-PAGE
2.4. Atomic Force Microscopy Analysis
2.5. Particle Size and Zeta Potential Analyses
2.6. Surface Hydrophobicity Analysis
2.7. Fluorescence Spectroscopy Analysis
2.8. Ultraviolet-Visible (UV) Spectroscopy Analysis
2.9. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.10. Differential Scanning Calorimetry (DSC) Analysis
2.11. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
2.12. Digestibility Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Co-Assembled Structures
3.2. Morphological Analysis
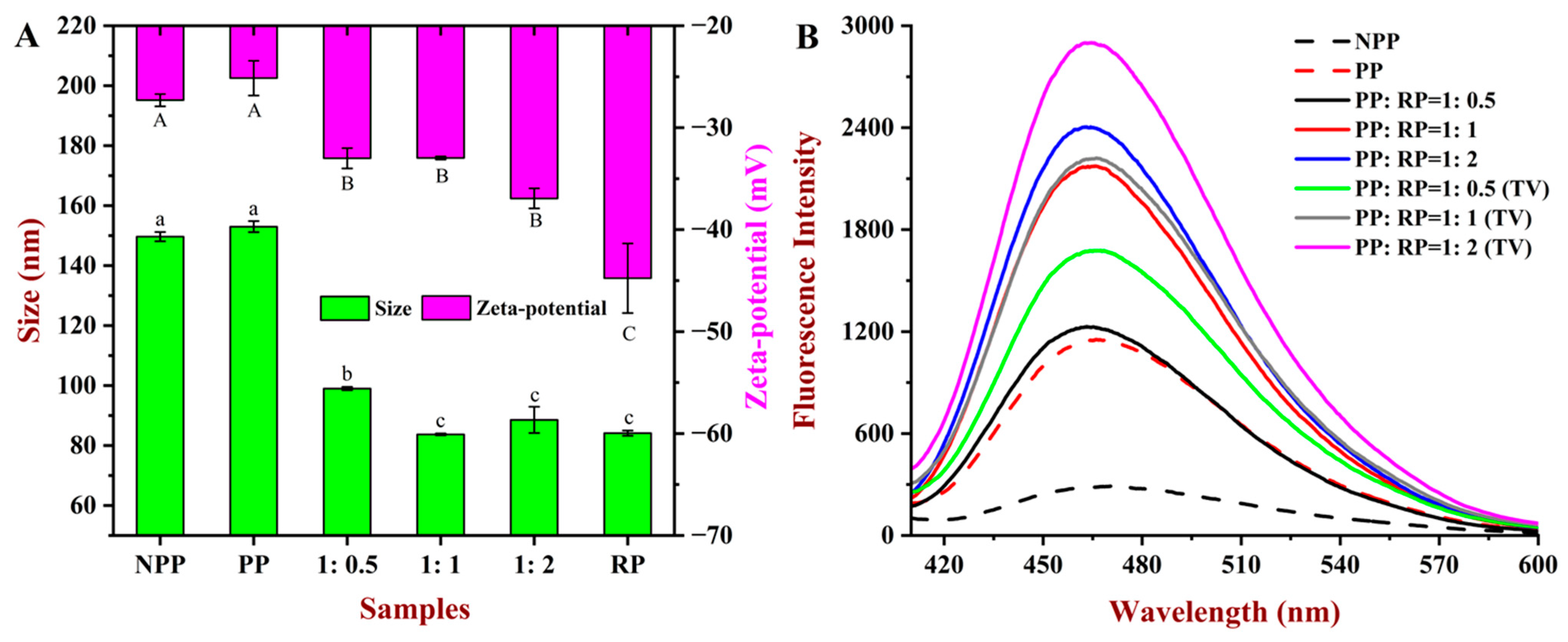
3.3. Particle Size and Zeta Potential
3.4. Surface Hydrophobicity
3.5. Spectroscopy Analysis
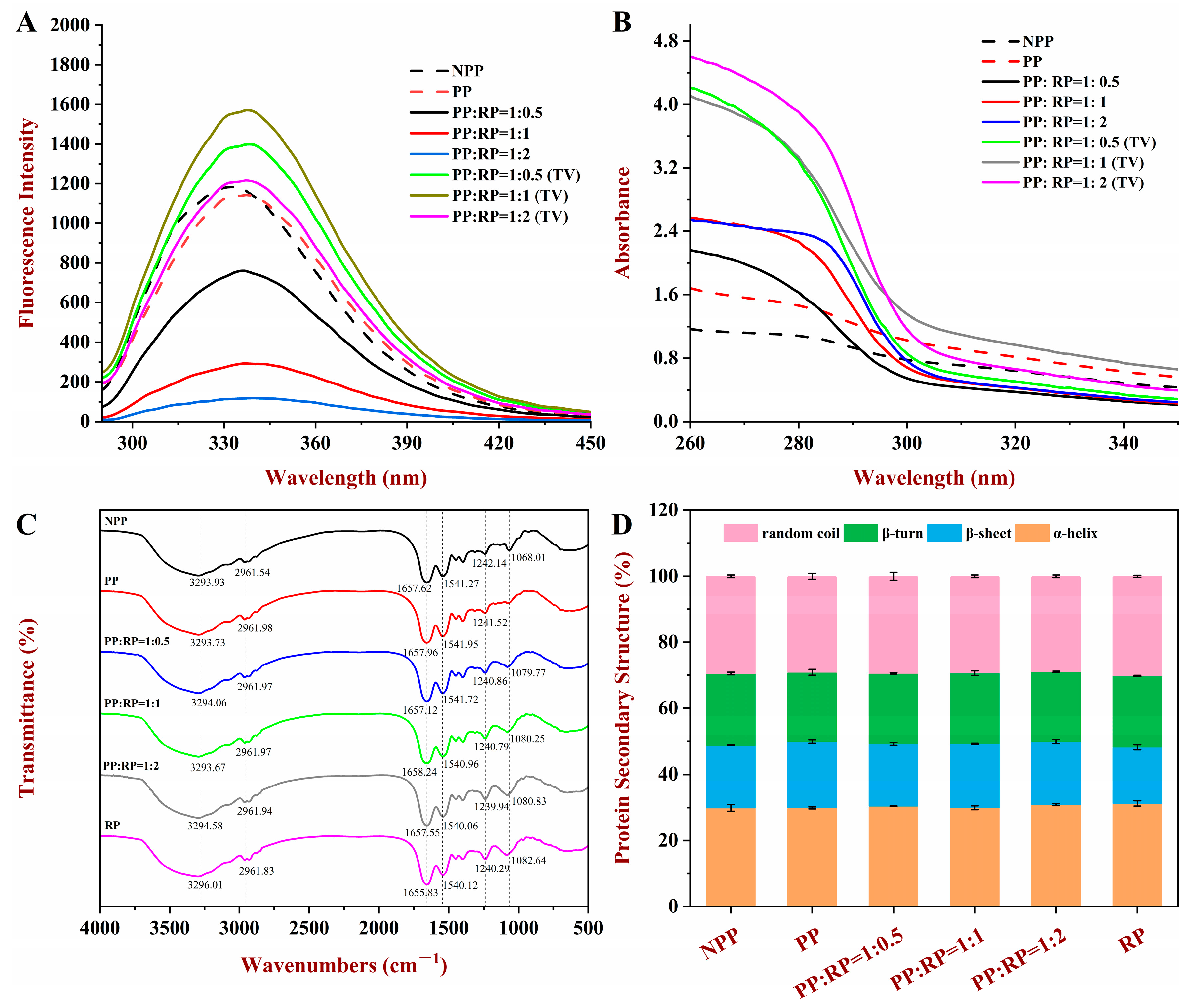
3.5.1. Fluorescence Spectroscopy
3.5.2. UV Spectroscopy
3.5.3. FTIR Spectroscopy
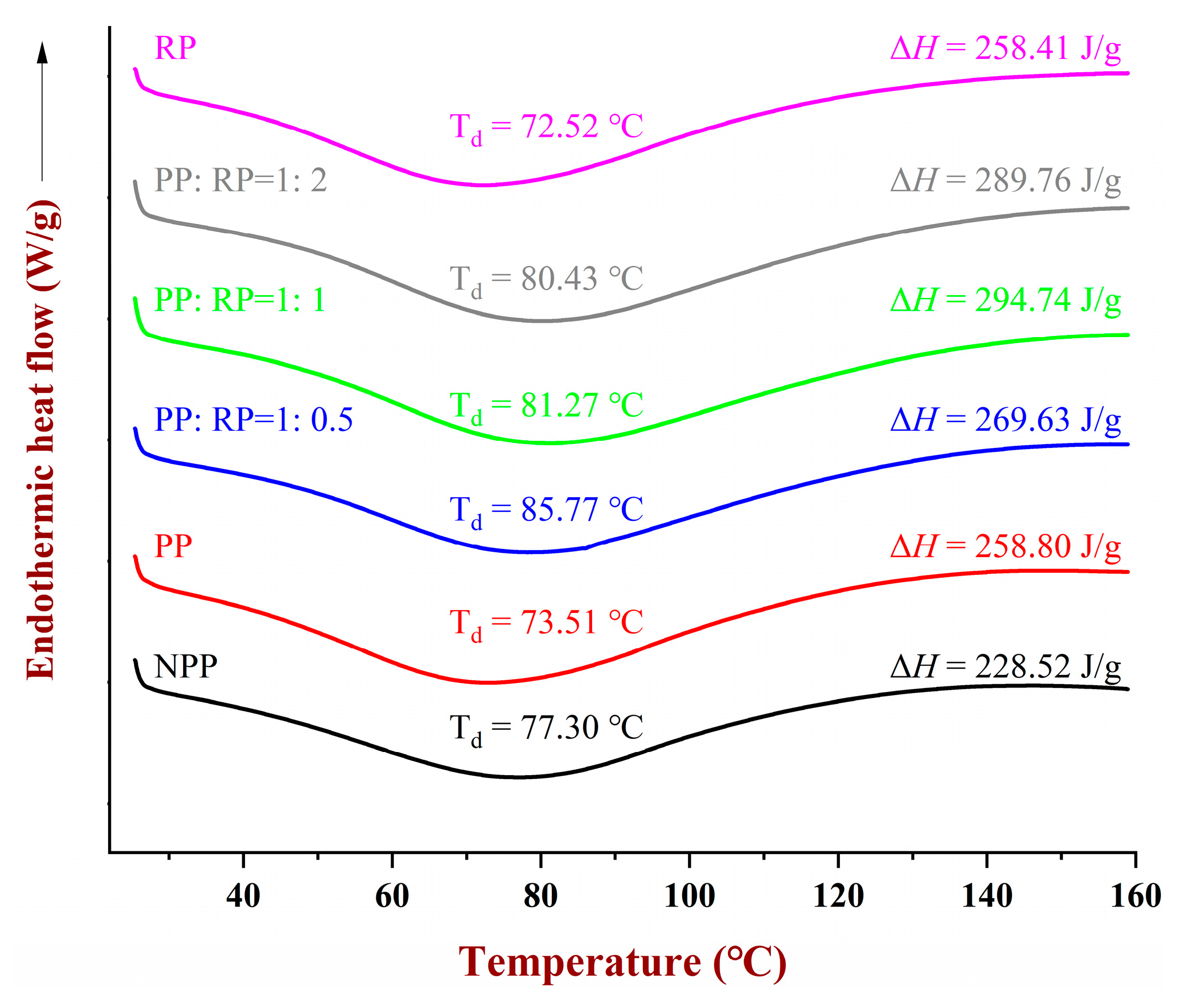
3.6. DSC Analysis
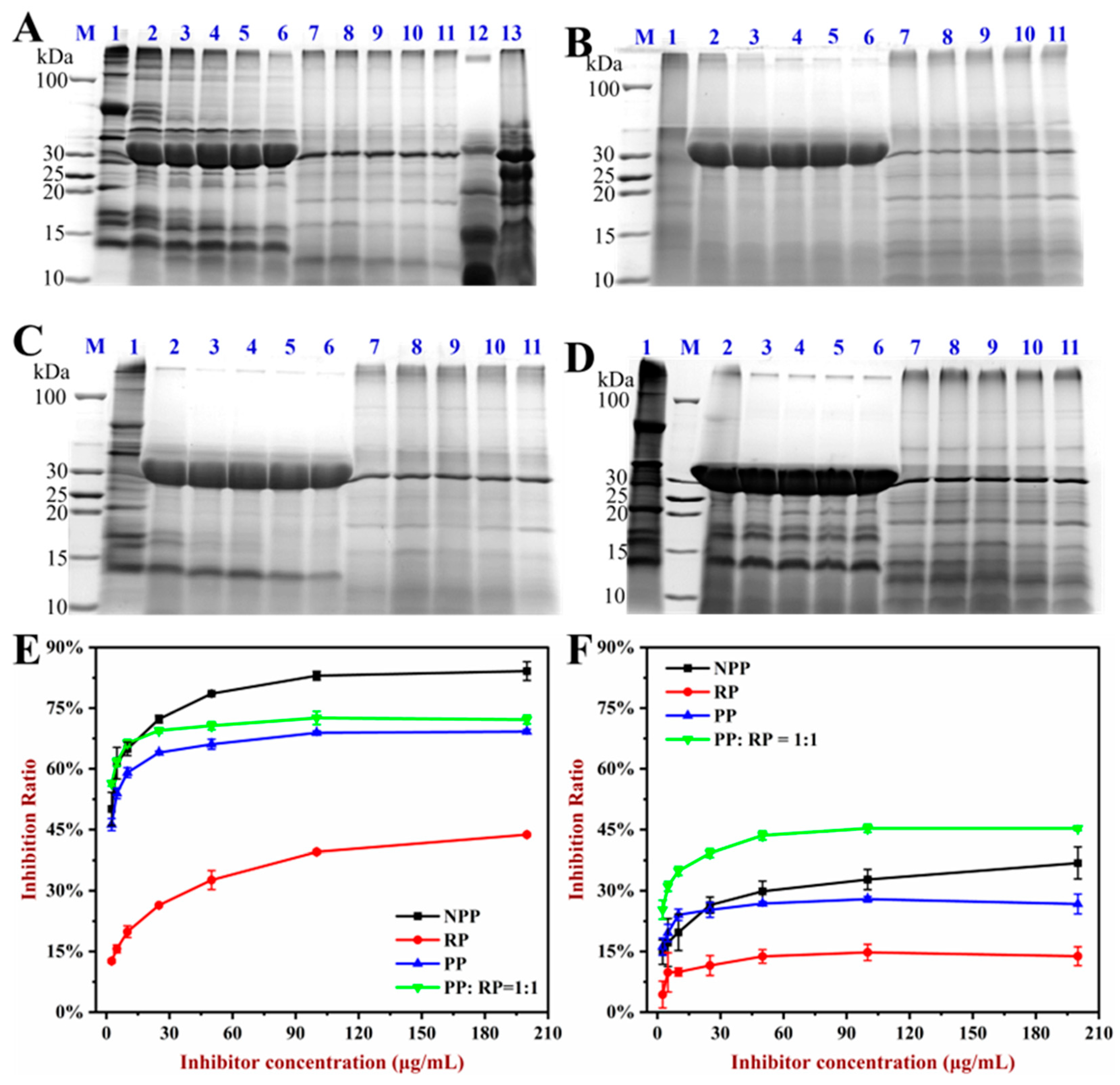
3.7. In Vitro Allergenicity Assessment
3.8. Digestibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peters, R.L.; Krawiec, M.; Koplin, J.J.; Santos, A.F. Update on food allergy. Pediatr. Allergy Immunol. 2021, 32, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Hossny, E.; Ebisawa, M.; El-Gamal, Y.; Arasi, S.; Dahdah, L.; El-Owaidy, R.; Galvan, C.A.; Lee, B.W.; Levin, M.; Martinez, S.; et al. Challenges of managing food allergy in the developing world. World Allergy Organ. J. 2019, 12, 100089. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Summary report of the Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens. Part 1: Review and Validation of Codex Priority Allergen List Through Risk Assessment; FAO/WHO: Rome, Italy, 2022. [Google Scholar]
- Shah, F.; Shi, A.M.; Ashley, J.; Kronfel, C.; Wang, Q.; Maleki, S.J.; Adhikari, B.; Zhang, J.C. Peanut Allergy: Characteristics and Approaches for Mitigation. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1361–1387. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Zhang, Y.; Song, M.; Zhou, X.Y.; Tang, Y.; Wu, Z.H.; Chen, H.B. Allergenicity of peanut allergens and its dependence on the structure. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1058–1081. [Google Scholar] [CrossRef]
- Chen, X.; Negi, S.S.; Liao, S.; Gao, V.; Braun, W.; Dreskin, S.C. Conformational IgE epitopes of peanut allergens Ara h 2 and Ara h 6. Clin. Exp. Allergy 2016, 46, 1120–1128. [Google Scholar] [CrossRef]
- Sun, X.B.; He, S.D.; Ye, Y.K.; Cao, X.D.; Liu, H.Y.; Wu, Z.Y.; Yue, J.Y.; Jin, R.S.; Sun, H.J. Combined effects of pH and thermal treatments on IgE-binding capacity and conformational structures of lectin from black kidney bean (Phaseolus vulgaris L.). Food Chem. 2020, 329, 127183. [Google Scholar] [CrossRef]
- de Jongh, H.H.J.; de Jong, G.A.H.; Apostolovic, D.; Taylor, S.L.; Baumert, J.L.; Koppelman, S.J. Effect of heat treatment on the conformational stability of intact and cleaved forms of the peanut allergen Ara h 6 in relation to its IgE-binding potency. Food Chem. 2020, 326, 127027. [Google Scholar] [CrossRef]
- Yu, J.M.; Mikiashvili, N. Effectiveness of different proteases in reducing allergen content and IgE-binding of raw peanuts. Food Chem. 2020, 307, 125565. [Google Scholar] [CrossRef]
- Cuadrado, C.; Sanchiz, A.; Arribas, C.; Pedrosa, M.M.; Gamboa, P.; Betancor, D.; Blanco, C.; Cabanillas, B.; Linacero, R. Mitigation of peanut allergenic reactivity by combined processing: Pressured heating and enzymatic hydrolysis. Innov. Food Sci. Emerg. Technol. 2023, 86, 103383. [Google Scholar] [CrossRef]
- Ahmed, I.; Chen, H.; Li, J.L.; Wang, B.; Li, Z.X.; Huang, G.H. Enzymatic crosslinking and food allergenicity: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5856–5879. [Google Scholar] [CrossRef]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.C.; Qian, L.H.; Zhang, Y.H.; Gong, P.X.; Liu, W.; Li, H.J. Fabrication of foxtail millet prolamin/caseinate/chitosan hydrochloride composite nanoparticles using antisolvent and pH-driven methods for curcumin delivery. Food Chem. 2023, 404, 134604. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yue, M.; Xu, P.C.; Wang, R.; Chen, Z.X. Toward water-solvation of rice proteins via backbone hybridization by casein. Food Chem. 2018, 258, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, L.L.; Feng, W.; Wang, T. Fabrication of hydrophilic composites by bridging the secondary structures between rice proteins and pea proteins toward enhanced nutritional properties. Food Funct. 2020, 11, 7446–7455. [Google Scholar] [CrossRef]
- Geng, Q.; Zhang, Y.; McClements, D.J.; Zhou, W.L.; Dai, T.T.; Wu, Z.H.; Chen, H.B. Investigation of peanut allergen-procyanidin non-covalent interactions: Impact on protein structure and in vitro allergenicity. Int. J. Biol. Macromol. 2024, 258, 128340. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Tech. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.H.; Li, K.; Li, X.; Yang, A.S.; Tong, P.; Chen, H.B. Allergenicity assessment on thermally processed peanut influenced by extraction and assessment methods. Food Chem. 2019, 281, 130–139. [Google Scholar] [CrossRef]
- McAllister, C.; Karymov, M.A.; Kawano, Y.; Lushnikov, A.Y.; Mikheikin, A.; Uversky, V.N.; Lyubchenko, Y.L. Protein interactions and misfolding analyzed by AFM force spectroscopy. J. Mol. Biol. 2005, 354, 1028–1042. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.B. Structural and functional properties of self-assembled peanut protein nanoparticles prepared by ultrasonic treatment: Effects of ultrasound intensity and protein concentration. Food Chem. 2023, 413, 135626. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.M.; Liu, J.Y.; Zhang, H.J.; Gong, L.X.; Li, Z.F.; Liu, H.Z.; Wang, Z.Y. Effect of non-covalently bound polyphenols on the structural and functional properties of wheat germ protein. Food Hydrocolloid 2024, 149, 109534. [Google Scholar] [CrossRef]
- Cao, M.M.; Liao, L.; Zhang, X.C.; Chen, X.; Peng, S.F.; Zou, L.Q.; Liang, R.H.; Liu, W. Electric field-driven fabrication of anisotropic hydrogels from plant proteins: Microstructure, gel performance and formation mechanism. Food Hydrocoll. 2023, 136, 108297. [Google Scholar] [CrossRef]
- Valenzuela-Lagarda, J.L.; Pacheco-Aguilar, R.; Gutiérrez-Dorado, R.; Mendoza, J.L.; López-Valenzuela, J.A.; Mazorra-Manzano, M.A.; Muy-Rangel, M.D. Interaction of Squid (Dosidicus giga) Mantle Protein with a Mixtures of Potato and Corn Starch in an Extruded Snack, as Characterized by FTIR and DSC. Molecules 2021, 26, 2103. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, S.; Xu, H.; Zhang, T.; Lin, X.; Wu, X. Effect of Covalent Interaction with Chlorogenic Acid on the Allergenic Capacity of Ovalbumin. J. Agric. Food Chem. 2018, 66, 9794–9800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, M.; Xu, J.Y.; Li, X.; Yang, A.S.; Tong, P.; Wu, Z.H.; Chen, H.B. IgE recognition and structural analysis of disulfide bond rearrangement and chemical modifications in allergen aggregations in roasted peanuts. J. Agr. Food Chem. 2023, 71, 9110–9119. [Google Scholar] [CrossRef]
- He, S.D.; Zhao, J.L.; Cao, X.D.; Ye, Y.K.; Wu, Z.Y.; Yue, J.Y.; Yang, L.; Jin, R.S.; Sun, H.J. Low pH-shifting treatment would improve functional properties of black turtle bean (Phaseolus vulgaris L.) protein isolate with immunoreactivity reduction. Food Chem. 2020, 330, 127217. [Google Scholar] [CrossRef]
- Wang, T.; Xu, P.C.; Chen, Z.X.; Zhou, X.; Wang, R. Alteration of the structure of rice proteins by their interaction with soy protein isolates to design novel protein composites. Food Funct. 2018, 9, 4282–4291. [Google Scholar] [CrossRef]
- Tang, C.H. Nano-architectural assembly of soy proteins: A promising strategy to fabricate nutraceutical nanovehicles. Adv. Colloid Interface Sci. 2021, 291, 102402. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, R.; Feng, W.; Zhou, X.; Chen, Z.X.; Wang, T. Carboxymethylcellulose/pectin inhibiting structural folding of rice proteins via trinary structural interplays. Int. J. Biol. Macromol. 2019, 133, 93–100. [Google Scholar] [CrossRef]
- Zhong, Y.J.; Zhao, J.C.; Dai, T.T.; McClements, D.J.; Liu, C.M. The effect of whey protein-puerarin interactions on the formation and performance of protein hydrogels. Food Hydrocoll. 2021, 113, 106444. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; He, Y.; Wen, L.; Nan, H.; Zheng, F.; Liu, H.; Lu, S.; Wu, M.; Zhang, H. A review of the interaction between anthocyanins and proteins. Food Sci. Technol. Int. 2021, 27, 470–482. [Google Scholar] [CrossRef]
- Wang, L.; Wen, J.; Wang, L.; Jiang, L.; Zhang, Y.; Sui, X. Characterization of the extreme pH-induced molten globule state of soy protein isolate and its influence on functional properties. Food Hydrocoll. 2023, 144, 109040. [Google Scholar] [CrossRef]
- Asaithambi, N.; Singha, P.; Singh, S.K. Influence of hydrodynamic cavitation on functional, nutritional, and structural characteristics of egg-white protein hydrolysates. Food Hydrocoll. Health 2023, 4, 100153. [Google Scholar] [CrossRef]
- Apostolovic, D.; Stanic-Vucinic, D.; de Jongh, H.H.J.; de Jong, G.A.H.; Mihailovic, J.; Radosavljevic, J.; Radibratovic, M.; Nordlee, J.A.; Baumert, J.L.; Milcic, M.; et al. Conformational stability of digestion-resistant peptides of peanut conglutins reveals the molecular basis of their allergenicity. Sci. Rep. 2016, 6, 29249. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, Q.; McClements, D.J.; Dai, T.; Li, C.; Wu, Z.; Chen, H. Investigation of Co-Assembly of Peanut Protein and Rice Protein: Effects on Protein Conformation and Immunoglobulin E Binding Capacity. Foods 2025, 14, 1699. https://doi.org/10.3390/foods14101699
Geng Q, McClements DJ, Dai T, Li C, Wu Z, Chen H. Investigation of Co-Assembly of Peanut Protein and Rice Protein: Effects on Protein Conformation and Immunoglobulin E Binding Capacity. Foods. 2025; 14(10):1699. https://doi.org/10.3390/foods14101699
Chicago/Turabian StyleGeng, Qin, David Julian McClements, Taotao Dai, Changhong Li, Zhihua Wu, and Hongbing Chen. 2025. "Investigation of Co-Assembly of Peanut Protein and Rice Protein: Effects on Protein Conformation and Immunoglobulin E Binding Capacity" Foods 14, no. 10: 1699. https://doi.org/10.3390/foods14101699
APA StyleGeng, Q., McClements, D. J., Dai, T., Li, C., Wu, Z., & Chen, H. (2025). Investigation of Co-Assembly of Peanut Protein and Rice Protein: Effects on Protein Conformation and Immunoglobulin E Binding Capacity. Foods, 14(10), 1699. https://doi.org/10.3390/foods14101699






