Functionalities of Octenyl Succinic Anhydride Wheat Starch and Its Effect on the Quality of Model Dough and Noodles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wheat Starch Extraction and Preparation of Modified Starch
- (1)
- Wheat starch extraction
- (2)
- Preparation of modified starches
2.3. Determination of Degree of Substitution (DS)
2.4. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5. X-Ray Diffraction (XRD)
2.6. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
2.7. Scanning Electron Microscopy (SEM)
2.8. Determination of Solubility and Swelling Degree
2.9. Determination of Pasting Properties
2.10. Determination of Dynamic Rheological Properties
2.11. Mixolab Behavior of Model Flour
2.12. Cooking Characteristics Measurement
2.13. Statistical Analysis
3. Results and Discussion
3.1. Degree of Substitution
3.2. FT-IR Spectroscopy Analysis
3.3. XRD Analysis
3.4. 1H NMR Spectroscopy
3.5. EM Analysis
3.6. Solubility and Swelling Characteristics
3.7. Pasting Properties
3.8. Mixolab Parameters of Mixed Flour
3.9. Rheological Properties
3.9.1. Dynamic Oscillation Frequency Sweep
3.9.2. Dynamic Oscillation Temperature Sweep
3.10. Cooking Characteristics of Noodles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, S.; Wang, Z.; Liu, H.; Li, L.; Zheng, X.; Tian, X.; Sun, B.; Wang, X. Supplementation of wheat flour products with wheat bran dietary fiber: Purpose, mechanisms, and challenges. Trends Food Sci. Technol. 2022, 123, 281–289. [Google Scholar] [CrossRef]
- Tebben, L.; Shen, Y.; Li, Y. Improvers and functional ingredients in whole wheat bread: A review of their effects on dough properties and bread quality. Trends Food Sci. Technol. 2018, 81, 10–24. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Feng, X. Effect of hydrocolloids on gluten proteins, dough, and flour products: A review. Food Res. Int. 2023, 164, 112292. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Yadav, M.P.; Li, J. Effect of various hydrocolloids on the physical and fermentation properties of dough. Food Chem. 2019, 271, 165–173. [Google Scholar] [CrossRef]
- Balic, R.; Miljkovic, T.; Ozsisli, B.; Simsek, S. Utilization of modified wheat and tapioca starches as fat replacements in bread formulation. J. Food Process. Preserv. 2017, 41, 12888. [Google Scholar] [CrossRef]
- Niu, M.; Hou, G.G.; Kindelspire, J.; Krishnan, P.; Zhao, S. Microstructural, textural, and sensory properties of whole-wheat noodle modified by enzymes and emulsifiers. Food Chem. 2017, 223, 16–24. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Yang, T.; Ma, Y.; McClements, D.J.; Ren, F.; Tian, Y.; Jin, Z. A review of structural transformations and properties changes in starch during thermal processing of foods. Food Hydrocoll. 2021, 113, 106543. [Google Scholar] [CrossRef]
- Chen, H.; Fu, M.; Zhang, Y.; Ma, C.; Kan, J. Effect of temperature during acetylation and heat moisture treatment on the structural and physicochemical properties and application of wheat starch. Food Hydrocoll. 2023, 144, 109036. [Google Scholar] [CrossRef]
- Obadi, M.; Xu, B. Review on the physicochemical properties, modifications, and applications of starches and its common modified forms used in noodle products. Food Hydrocoll. 2021, 112, 106286. [Google Scholar] [CrossRef]
- Kaur, B.; Ariffin, F.; Bhat, R.; Karim, A. Progress in starch modification in the last decade. Food Hydrocoll. 2012, 26, 398–404. [Google Scholar] [CrossRef]
- Klaochanpong, N.; Puncha-arnon, S.; Uttapap, D.; Puttanlek, C.; Rungsardthong, V. Octenyl succinylation of granular and debranched waxy starches and their application in low-fat salad dressing. Food Hydrocoll. 2017, 66, 296–306. [Google Scholar] [CrossRef]
- Xu, L.; Bai, Z.; Feng, J.; He, L.; Ren, J.; Chai, S.; Chen, X. Effects of the degree of substitution of octenyl succinic anhydride on the physicochemical characteristics of adlay starch. Int. J. Biol. Macromol. 2023, 241, 124535. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, H.; Liu, S.; Wang, Z.; Kan, J. Insight into esterified and granular esterified-pregelatinized starch formation during esterification modification: Key role of temperature. Food Chem. 2024, 460, 140809. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, S.; Chi, G.; Li, H.; Chen, K.; Wang, Z.; Kan, J. Preparation and functional characteristics of starch-lipid complexes with different oleic acid-rich glycerolipids. Food Chem. 2025, 476, 143450. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhao, X.; Chen, Z.; Zhou, Z.; Ji, S.; Xu, Y.; Zhang, C.; Shen, J.; Chen, Q.; Li, K.; et al. Insights into the improved cold-water solubility and digestibility of alkaline-alcohol modified cassava starch: A discussion from the perspective of fine structure. Int. J. Biol. Macromol. 2025, 305, 140952. [Google Scholar] [CrossRef]
- Wen, Y.; Yao, T.; Xu, Y.; Corke, H.; Sui, Z. Pasting, thermal and rheological properties of octenylsuccinylate modified starches from diverse small granule starches differing in amylose content. J. Cereal Sci. 2020, 95, 103030. [Google Scholar] [CrossRef]
- Chen, H.J.; Huang, J.; Su, Y.; Fu, M.; Kan, J. Effects of oil and heating on the physicochemical and microstructural properties of gluten-starch dough. Food Chem. 2024, 436, 137571. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, M.; Li, W.; Liang, C.; Huang, X.; Hu, H.; Huang, Z.; Gan, T.; Zhang, Y. Effects of the addition of cassava starch and the size of water clusters on physicochemical and cooking properties of rice noodles. Food Chem. 2025, 470, 142665. [Google Scholar] [CrossRef]
- Bajaj, R.; Singh, N.; Kaur, A. Properties of octenyl succinic anhydride (OSA) modified starches and their application in low fat mayonnaise. Int. J. Biol. Macromol. 2019, 131, 147–157. [Google Scholar] [CrossRef]
- Huang, Q.; Fu, X.; He, X.W.; Luo, F.X.; Yu, S.J.; Li, L. The effect of enzymatic pretreatments on subsequent octenyl succinic anhydride modifications of cornstarch. Food Hydrocoll. 2010, 24, 60–65. [Google Scholar] [CrossRef]
- Hajihashemi, Z.; Nasirpour, A.; Scher, J.; Desobry, S. Interactions among lactose, β-lactoglobulin and starch in co-lyophilized mixtures as determined by Fourier Transform Infrared Spectroscopy. Int. J. Food Sci. Technol. 2012, 51, 3376–3382. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Li, Y.; Qu, J.; Zhang, X.; Seytahmetovna, S.A.; Blennow, A.; Guo, D. Structural features of five types of maize starch granule subgroups sorted by flow cytometry. Food Chem. 2021, 356, 129657. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Z.; Wang, Z.; Chen, R.; Zhang, S. Molecular interactions between apigenin and starch with different amylose/amylopectin ratios revealed by X-ray diffraction, FT-IR and solid-state NMR. Carbohydr. Polym. 2023, 310, 120737. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Fu, J.; Huang, J.; Wang, L. Fish collagen mediated alteration of wheat starch thermal properties during multi-species co-fermentation. Int. J. Biol. Macromol. 2025, 295, 139987. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Wu, Y.; Kan, J. Functionality differences between esterified and pregelatinized esterified starches simultaneously prepared by octenyl succinic anhydride modification and its application in dough. Int. J. Biol. Macromol. 2024, 260, 129594. [Google Scholar] [CrossRef]
- Meng, F.; Tian, S.; Chen, Y.; Liu, Z. Preparation and physicochemical properties of OSA modified Cyperus esculentus starch nanoparticles. Int. J. Biol. Macromol. 2025, 292, 140045. [Google Scholar] [CrossRef]
- Sharma, A.; Jindal, N.; Singh, S. Influence of 2-octenyl-1-succinic anhydride (OSA) concentration on the functional, rheological, structural and flow properties of starch from Indian Teff (Eragrostis tef). Int. J. Biol. Macromol. 2025, 284, 138055. [Google Scholar] [CrossRef]
- Fazeli, M.; Islam, S.; Baniasadi, H.; Abidnejad, R.; Schlapp-Hackl, I.; Hummel, M.; Lipponen, J. Exploring the potential of regenerated Ioncell fiber composites: A sustainable alternative for high-strength applications. Green Chem. 2024, 26, 6822–6835. [Google Scholar] [CrossRef]
- Zhang, J.; Ran, C.; Jiang, X.; Dou, J. Impact of octenyl succinic anhydride (OSA) esterification on microstructure and physicochemical properties of sorghum starch. LWT-Food Sci. Technol. 2021, 152, 112320. [Google Scholar] [CrossRef]
- No, J.; Shin, M. Preparation and characteristics of octenyl succinic anhydride-modified partial waxy rice starches and encapsulated paprika pigment powder. Food Chem. 2019, 295, 466–474. [Google Scholar] [CrossRef]
- Fang, C.; Huang, J.; Pu, H.; Yang, Q.; Chen, Z.; Zhu, Z. Cold-water solubility, oil-adsorption and enzymolysis properties of amorphous granular starches. Food Hydrocoll. 2021, 117, 106669. [Google Scholar] [CrossRef]
- Zaidul, I.; Norulaini, N.; Omar, A.M.; Yamauchi, H.; Noda, T. RVA analysis of mixtures of wheat flour and potato, sweet potato, yam, and cassava starches. Carbohydr. Polym. 2007, 69, 784–791. [Google Scholar] [CrossRef]
- Liu, X.; Huang, S.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. Changes of starch during thermal processing of foods: Current status and future directions. Trends Food Sci. Technol. 2022, 119, 320–337. [Google Scholar] [CrossRef]
- Punia, S.; Siroha, A.K.; Sandhu, K.S.; Kaur, M. Rheological and pasting behavior of OSA modified mungbean starches and its utilization in cake formulation as fat replacer. Int. J. Biol. Macromol. 2019, 128, 230–236. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, P.; Li, P.; Zhao, J.; Olnood, C.; Zhao, S.; Yang, X.; Wang, Q.; Chen, X. Effects of Salecan on the gelatinization and retrogradation behaviors of wheat starch. LWT-Food Sci. Technol. 2023, 186, 115238. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F.; Xiao, Q.; Cai, M.; Yang, Q.; Weng, H.; Xiao, A. Structure and physicochemical properties of amphiphilic agar modified with octenyl succinic anhydride. Carbohydr. Polym. 2021, 251, 117031. [Google Scholar] [CrossRef]
- Peng, P.; Wang, X.; Zou, X.; Zhang, X.; Hu, X. Dynamic behaviors of protein and starch and interactions associated with glutenin composition in wheat dough matrices during sequential thermo-mechanical treatments. Food Res. Int. 2022, 154, 110986. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Wang, C.; Li, Y.; Bai, Z.; Luo, D.; Hu, Y.; Chen, S. Effects of konjac glucomannan and freezing on thermal properties, rheology, digestibility and microstructure of starch isolated from wheat dough. LWT-Food Sci. Technol. 2023, 177, 114588. [Google Scholar] [CrossRef]
- Dapčević-Hadnađev, T.; Hadnađev, M.; Dokić, L.; Krstonošić, V. Small deformation rheological behaviour of wheat gluten-octenyl succinyl modified corn starches mixtures. J. Cereal Sci. 2021, 97, 103150. [Google Scholar] [CrossRef]
- Xu, X.; Meng, L.; Gao, C.; Cheng, W.; Yang, Y.; Shen, X.; Tang, X. Construction of starch-sodium alginate interpenetrating polymer network and its effects on structure, cooking quality and in vitro starch digestibility of extruded whole buckwheat noodles. Food Hydrocoll. 2023, 143, 108876. [Google Scholar] [CrossRef]
- Song, C.; Baik, M.; Kim, B. Rheological properties of native maize, waxy maize, and acetylated maize starches, and applications in the development of food products. J. Korean Soc. Appl. Biol. Chem. 2023, 56, 63–68. [Google Scholar] [CrossRef]

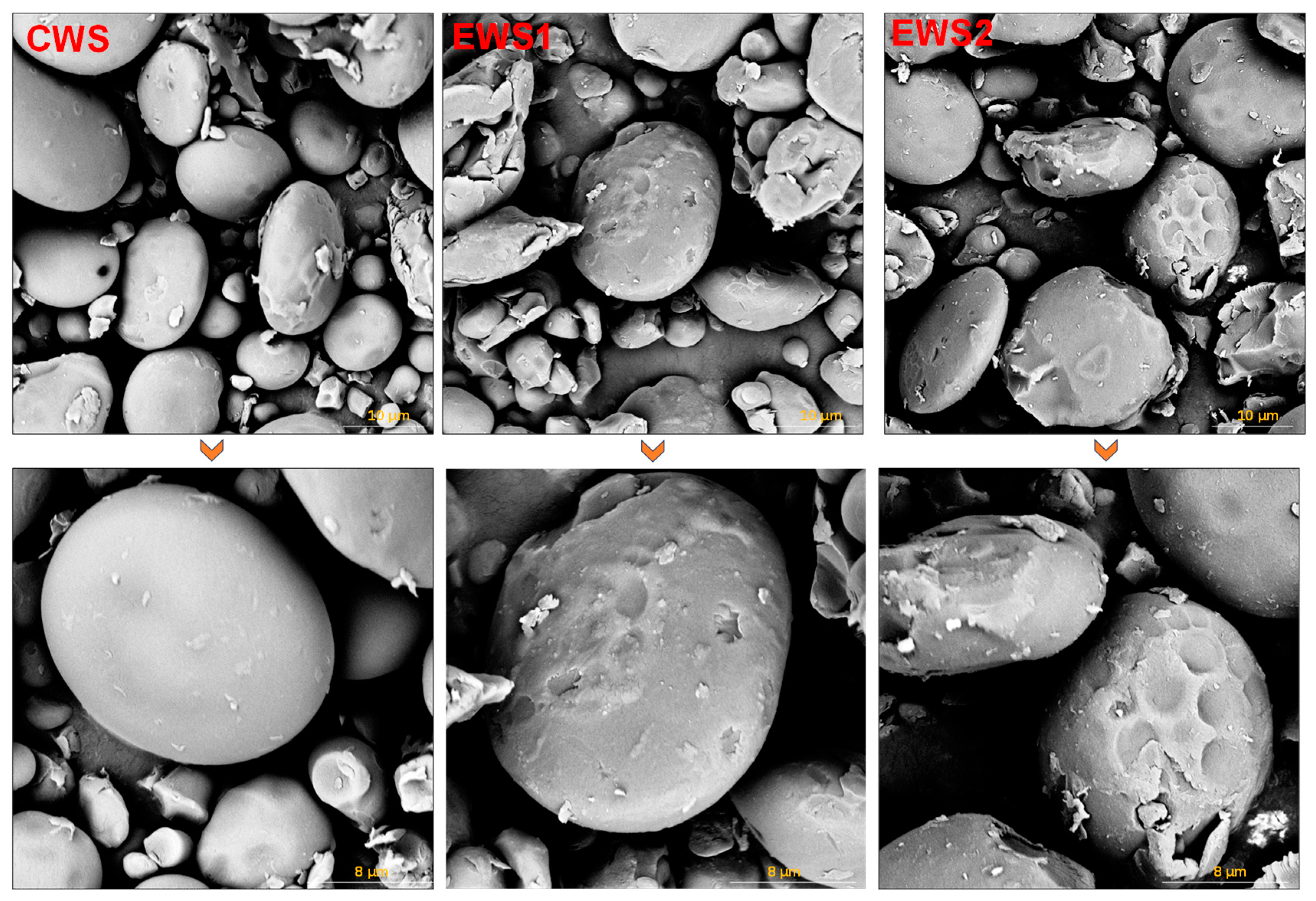

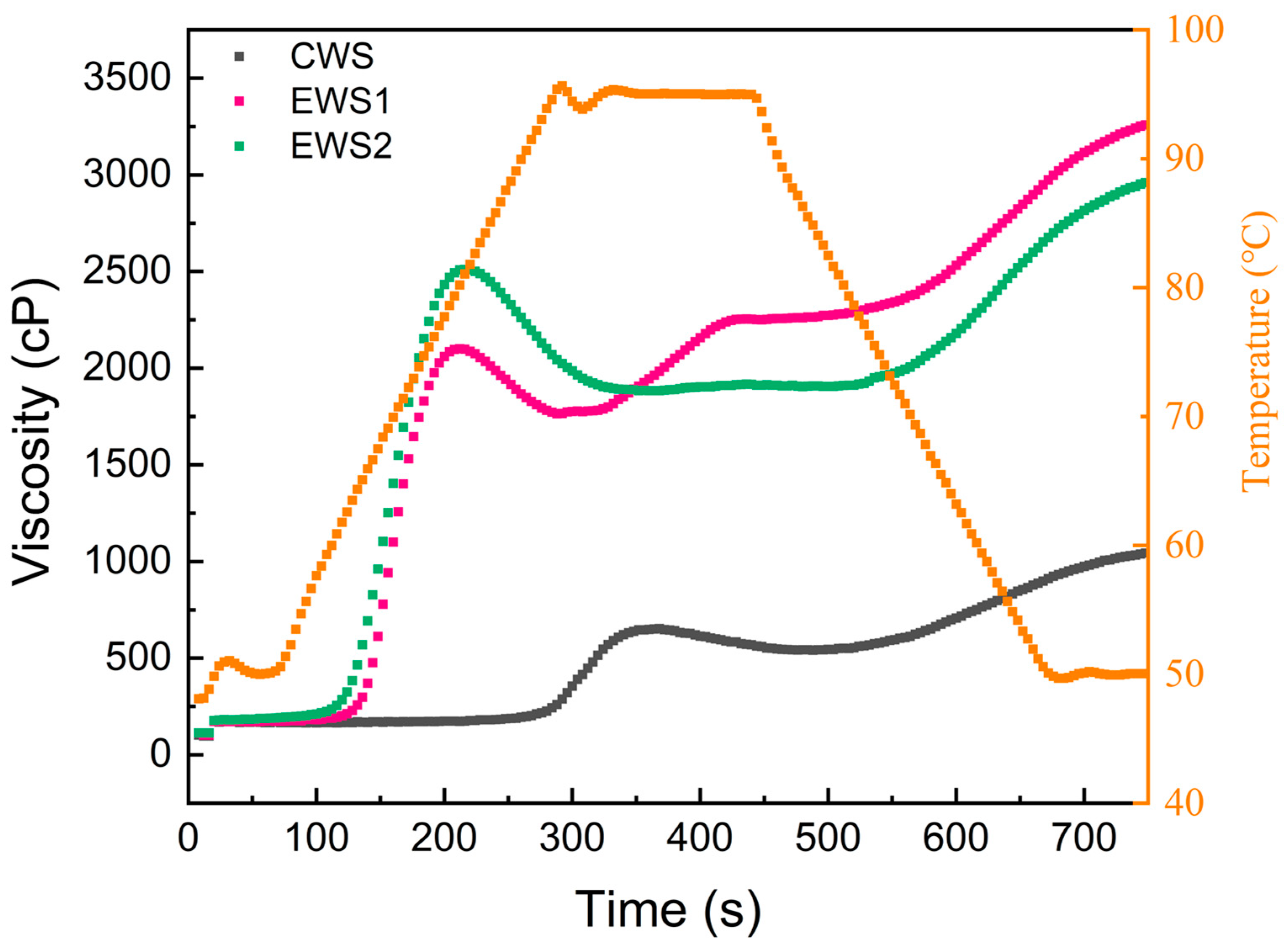
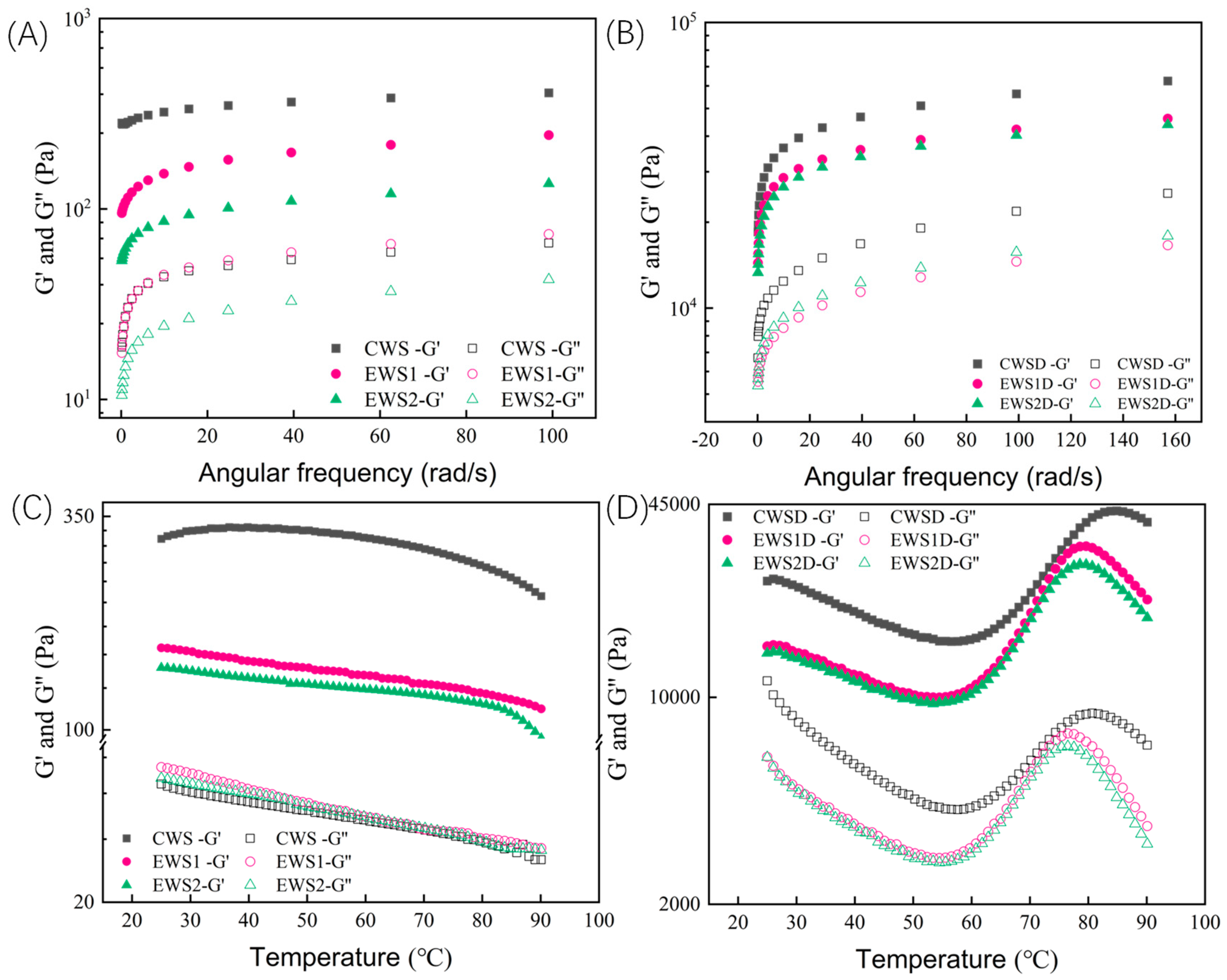
| Samples | Peak Viscosity (cP) | Breakdown Viscosity (cP) | Final Viscosity (cP) | Setback Viscosity (cP) | Pasting Temperature (°C) |
|---|---|---|---|---|---|
| CWS | 653 ± 14 c | 112 ± 13 c | 1044 ± 10 c | 503 ± 13 c | 93.90 ± 0.23 a |
| EWS1 | 2239 ± 32 b | 275 ± 19 b | 3259 ± 23 a | 1295 ± 20 a | 62.60 ± 0.53 b |
| EWS2 | 2511 ± 8 a | 627 ± 24 a | 2960 ± 18 b | 1076 ± 9 b | 60.00 ± 0.12 c |
| Samples | C1/ (N·m) | C2/ (N·m) | C3/ (N·m) | C4/ (N·m) | C5/ (N·m) | C5 − C4/ (N·m) |
|---|---|---|---|---|---|---|
| CWSD | 1.087 ± 0.010 b | 0.644 ± 0.003 c | 3.286 ± 0.014 a | 2.061 ± 0.024 b | 2.999 ± 0.015 a | 0.938 ± 0.387 a |
| EWS1D | 1.082 ± 0.021 b | 0.687 ± 0.024 b | 3.309 ± 0.012 a | 2.181 ± 0.009 a | 2.994 ± 0.009 a | 0.813 ± 0.004 b |
| EWS2D | 1.136 ± 0.013 a | 0.746 ± 0.019 a | 2.222 ± 0.009 b | 2.152 ± 0.010 a | 2.942 ± 0.005 b | 0.790 ± 0.008 b |
| Samples | Cooking Time (min) | Water Absorption Rate (%) | Cooking Loss Rate (%) |
|---|---|---|---|
| CWSD | 8.6 ± 0.31 a | 220.3 ± 5.7 c | 11.9 ± 1.1 a |
| EWS1D | 7.2 ± 0.12 b | 245.7 ± 4.2 b | 9.1 ± 0.8 b |
| EWS2D | 6.1 ± 0.24 c | 257.8 ± 6.1 a | 8.9 ± 0.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Yang, L.; Zhang, D.; Chen, H.; Kan, J. Functionalities of Octenyl Succinic Anhydride Wheat Starch and Its Effect on the Quality of Model Dough and Noodles. Foods 2025, 14, 1688. https://doi.org/10.3390/foods14101688
Ma H, Yang L, Zhang D, Chen H, Kan J. Functionalities of Octenyl Succinic Anhydride Wheat Starch and Its Effect on the Quality of Model Dough and Noodles. Foods. 2025; 14(10):1688. https://doi.org/10.3390/foods14101688
Chicago/Turabian StyleMa, Hongxue, Liai Yang, Dunhe Zhang, Huijing Chen, and Jianquan Kan. 2025. "Functionalities of Octenyl Succinic Anhydride Wheat Starch and Its Effect on the Quality of Model Dough and Noodles" Foods 14, no. 10: 1688. https://doi.org/10.3390/foods14101688
APA StyleMa, H., Yang, L., Zhang, D., Chen, H., & Kan, J. (2025). Functionalities of Octenyl Succinic Anhydride Wheat Starch and Its Effect on the Quality of Model Dough and Noodles. Foods, 14(10), 1688. https://doi.org/10.3390/foods14101688







