Purification and Characterization of Endogenous α-Amylase from Glutinous Rice Flour
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification of Endogenous α-Amylase
2.2.1. Extraction of Crude Enzyme Solution
2.2.2. Graded Precipitation of Ammonium Sulfate
2.2.3. Ion Exchange Chromatography
2.2.4. I Gel Filtration Chromatography
2.3. Determination of Protein Content
2.4. Determination of Endogenous α-Amylase Activity
2.5. SDS-PAGE and Enzyme Activity Staining Analysis
2.6. Optimal pH for Endogenous α-Amylase
2.7. pH Stability of Endogenous α-Amylase
2.8. Optimal Temperature for Endogenous α-Amylase
2.9. Temperature Stability of Endogenous α-Amylase
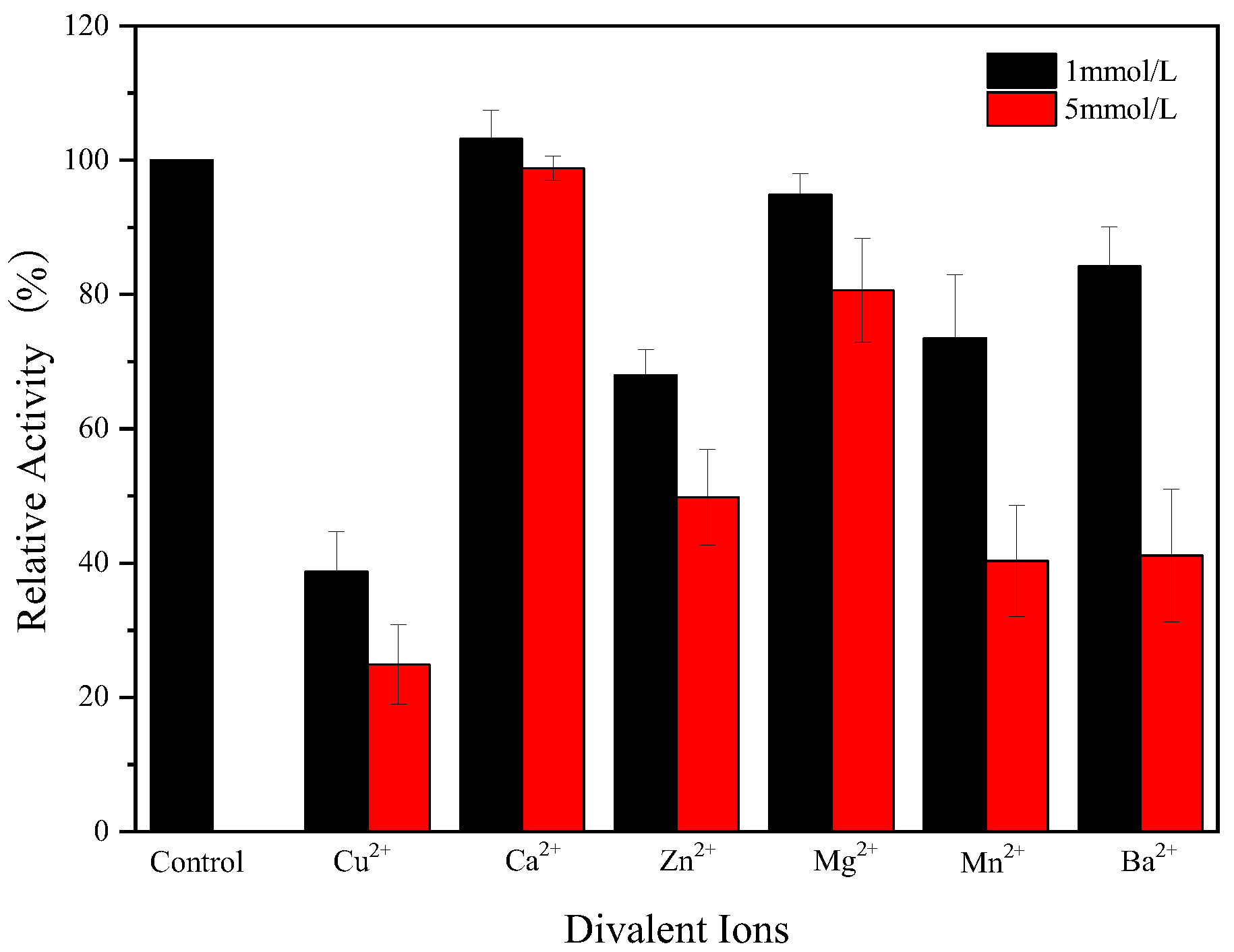
2.10. The Effect of Metal Ions on the Activity of Endogenous α-Amylase
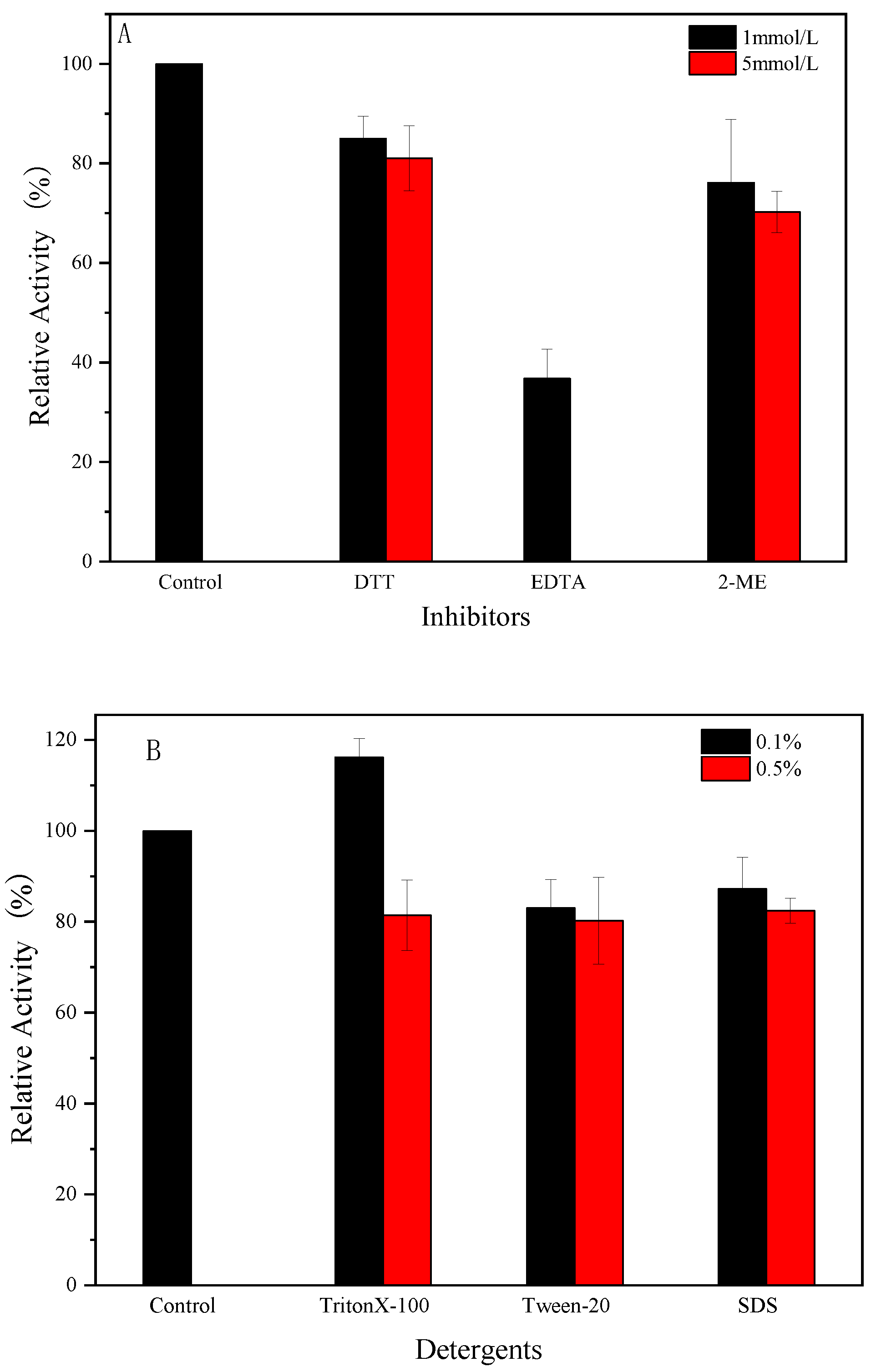
2.11. The Impact of Various Compounds on the Activity of Endogenous α-Amylase
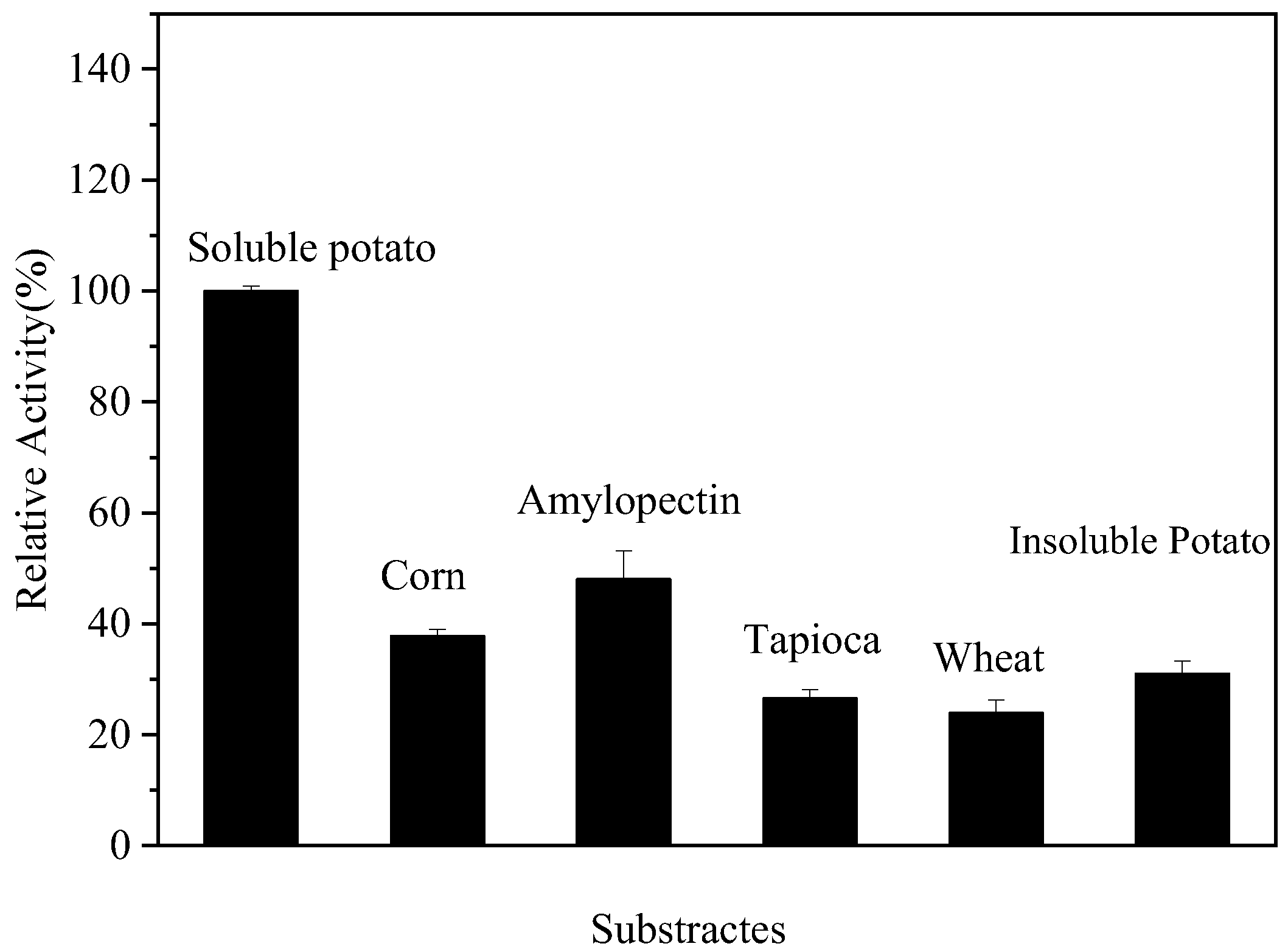
2.12. Substrate Specificity of Endogenous α-Amylase
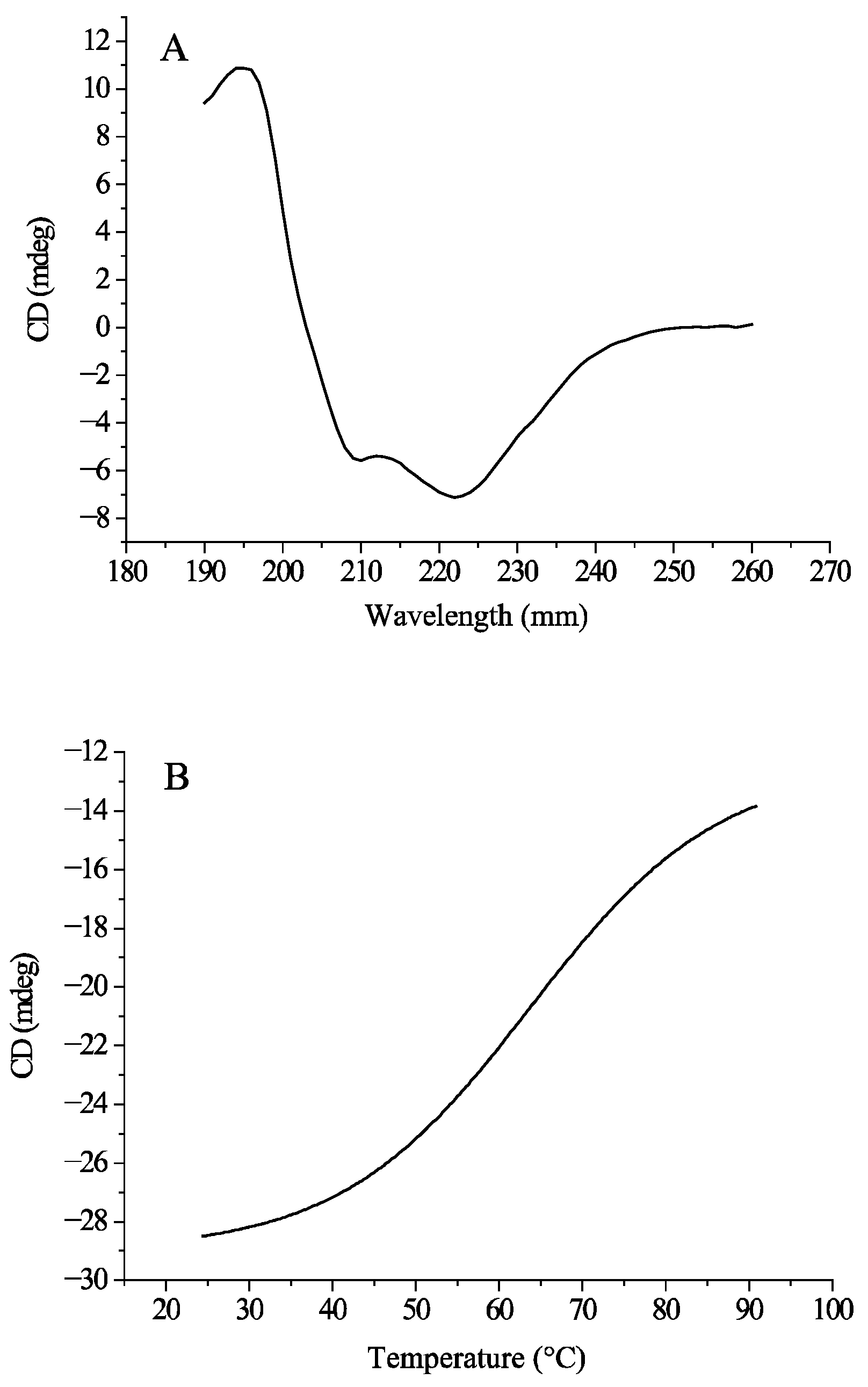
2.13. Circular Dichroism Spectroscopy Determination
3. Results and Discussion
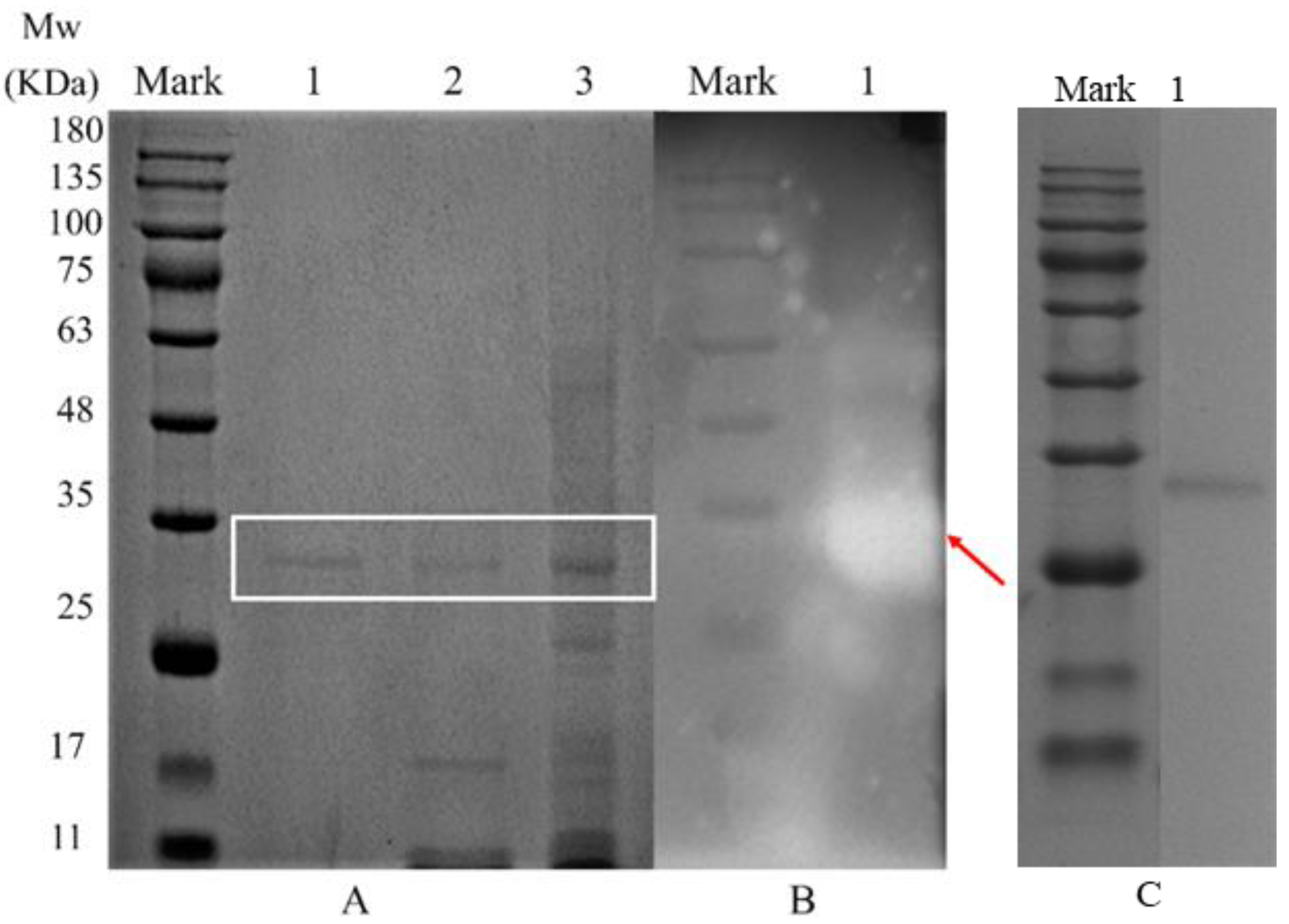
3.1. Isolation and Purification of Endogenous α-Amylases
3.2. Molecular Weight Determination
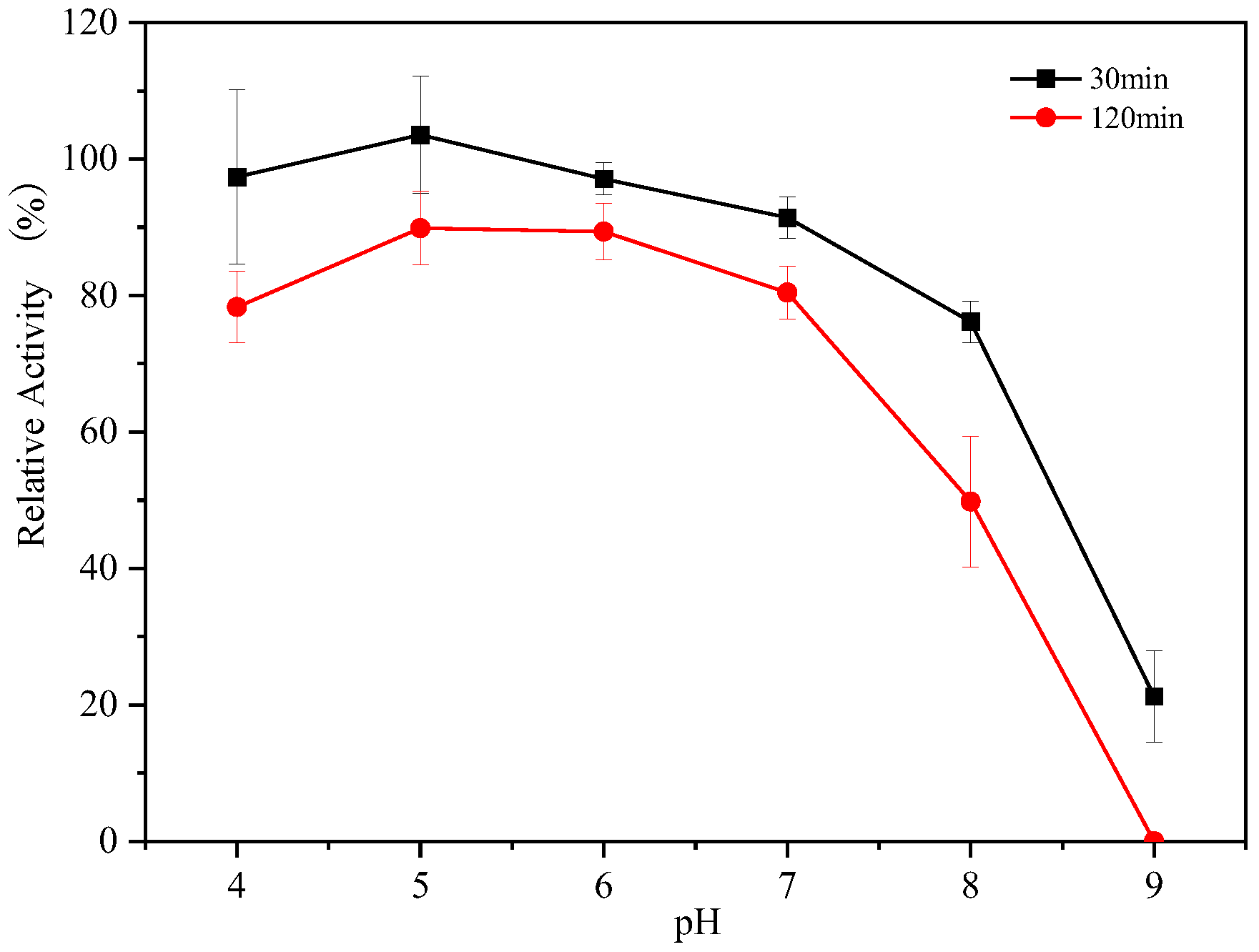
3.3. Optimum pH of Endogenous α-Amylase
3.4. The pH Stability of Endogenous α-Amylases
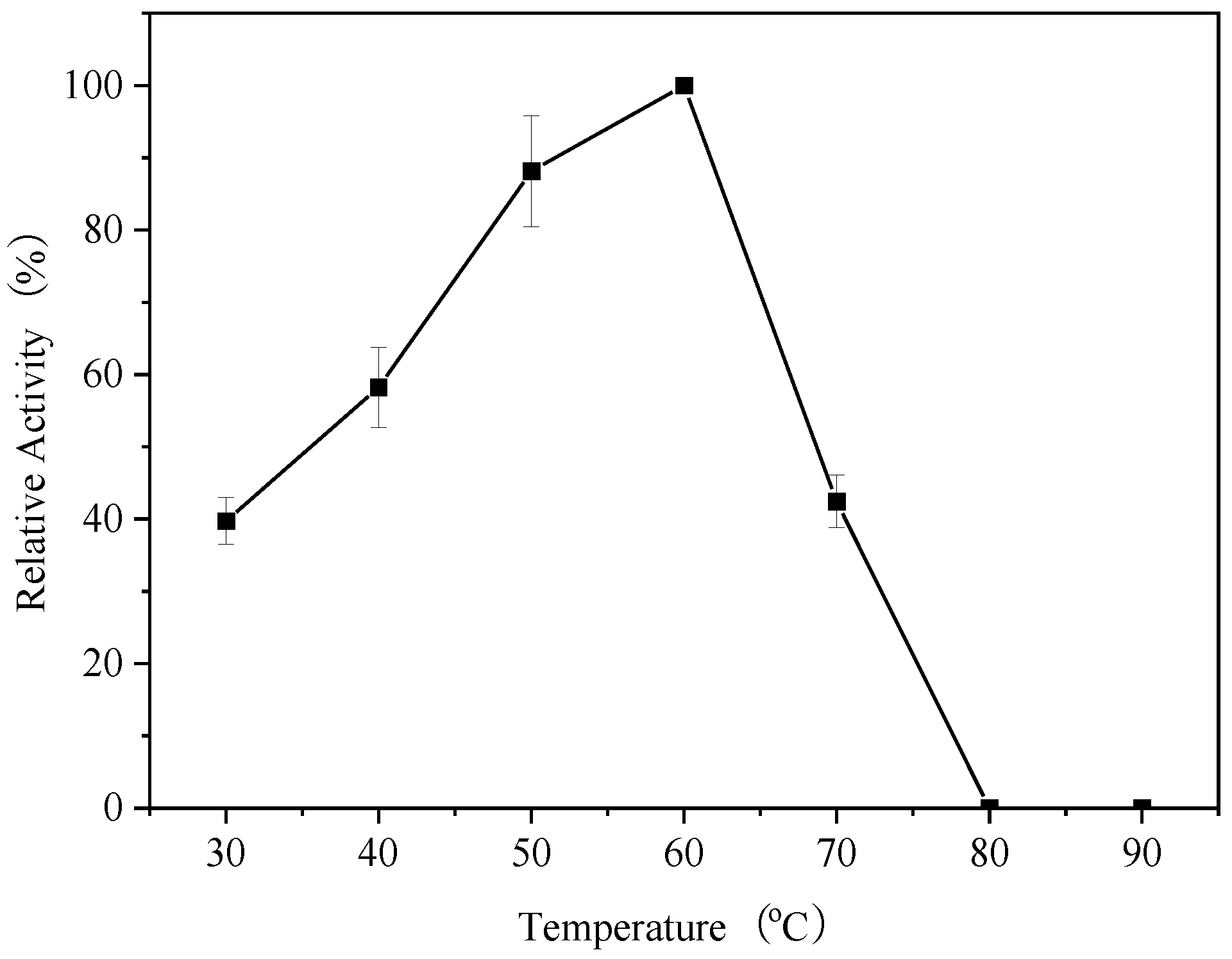
3.5. Optimum Temperature of Endogenous α-Amylase
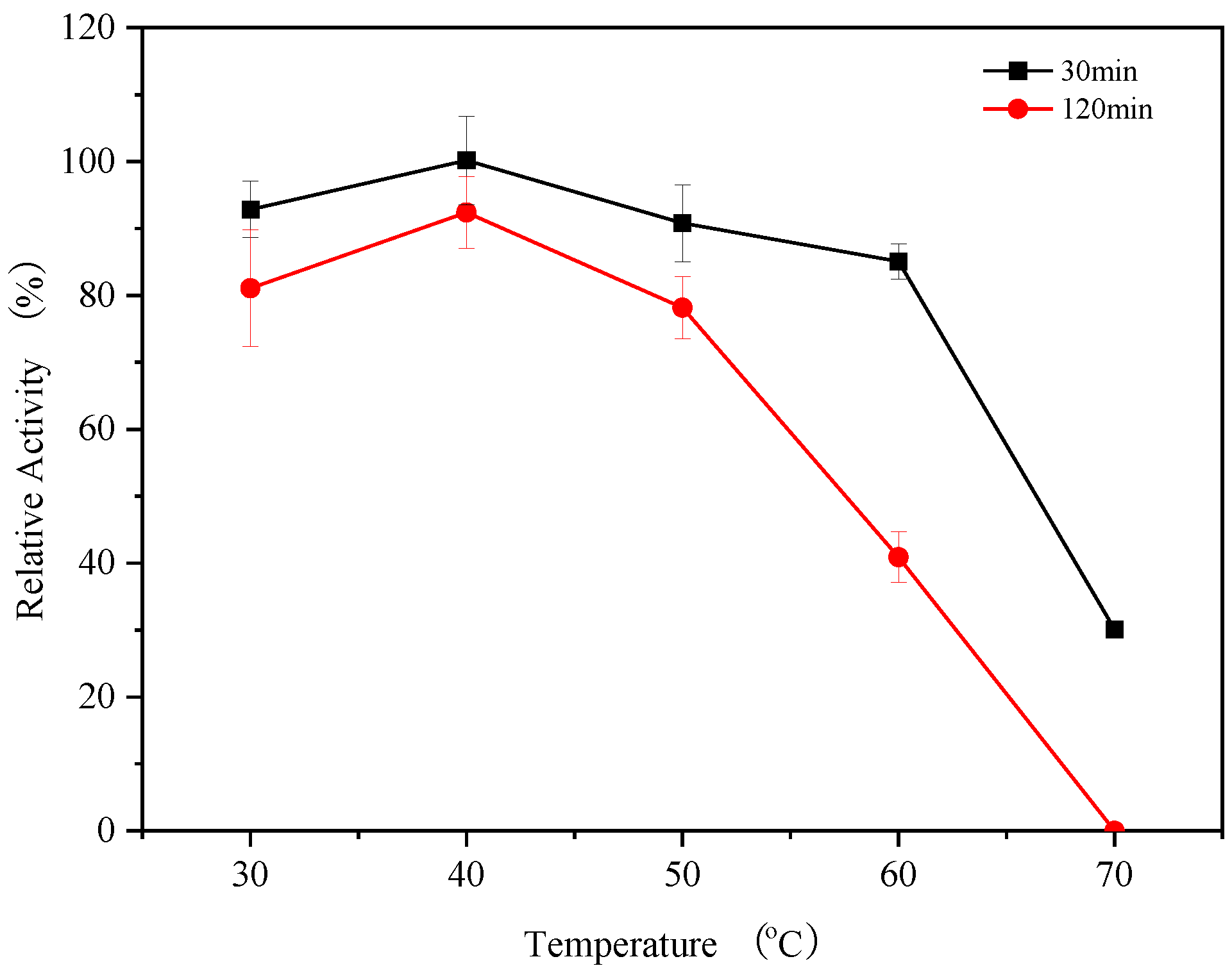
3.6. Thermal Stability of Endogenous α-Amylases
3.7. Effect of Metal Ions on Endogenous α-Amylase Activity
3.8. Effect of Compounds on Enzyme Activity
3.9. Substrate Specificity of Endogenous α-Amylase
3.10. Circular Dichroism (CD) Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Nakamur, S.; Ohtsub, K. Morphological, molecular structural and physicochemical characterization of starch granules formed in endosperm of rice with ectopic overexpression of α-amylase. J. Appl. Glycosci. 2024, 71, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, D.; Feng, N.; Khan, A. Regulation of Exogenous strigolactone on storage substance metabolism and endogenous hormone levels in the early germination stage of rice seeds under salt stress. Antioxidants 2025, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Song, S.; Yin, Q.; Zhao, T.; Liu, H.; He, A.; Wang, W. Enhancement in seed priming-induced starch degradation of rice seed under chilling stress via GA-mediated α-Amylase expression. Rice 2022, 15, 19–26. [Google Scholar] [CrossRef]
- Damaris, R.; Lin, Z.; Yang, P.; He, D. The rice alpha-amylase, conserved regulator of seed maturation and germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef]
- Nakata, M.; Fukamatsu, Y.; Miyashita, T.; Hakata, M.; Kimura, R.; Nakata, Y. High temperature-induced expression of rice α-amylases in developing endosperm produces chalky grains. Front. Plant. Sci. 2017, 8, 2089–2099. [Google Scholar] [CrossRef]
- Mangan, D.; Draga, A.; Ivory, R.; Cornaggia, C.; Blundell, M.; Howitt, C.; McCleary, B. A novel enzymatic method discriminating wheat pre-harvest sprouting from Late Maturity alpha-amylase. J. Cereal Sci. 2022, 105, 103480. [Google Scholar] [CrossRef]
- Zhang, Q.; Pritchard, J.; Mieog, J. Over-expression of a wheat late maturity alpha-amylase type 1 impact on starch properties during grain development and germination. Front. Plant. Sci. 2022, 13, 811728. [Google Scholar] [CrossRef]
- Aljabi, H.; Pawelzik, E. Impact of cultivar and growing conditions on alpha-amylase properties in wheat. Starch 2021, 73, 3–4. [Google Scholar] [CrossRef]
- Olaerts, H.; Courtin, C. Impact of preharvest sprouting on endogenous hydrolases and technological quality of wheat and bread: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 698–713. [Google Scholar] [CrossRef]
- Kaur, N.; Gasparre, N.; Rosell, C. Expanding the application of germinated wheat by examining the impact of varying alpha-amylase levels from grain to bread. J. Cereal Sci. 2024, 120, 104059. [Google Scholar] [CrossRef]
- Arunachallam, P.; Kumaravel, V.; Gopal, S. Purification and biochemical characterization of α-amylase from Aspergillus tamarii MTCC5152. Prep. Biochem. Biotechnol. 2024, 54, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Azad, R.; Thakur, D. Purification and characterization of α-amylase from Acanthoscelides obtectus (Say) (Coleoptera: Chrysomelidae). Int. J. Biol. Macromol. 2024, 278, 135009. [Google Scholar]
- Singh, K.; Ahmad, F.; Singh, V.; Kayastha, K.; Kayastha, A. Purification, biochemical characterization and Insilico modeling of α-amylase from Vicia faba. J. Mol. Liq. 2017, 234, 133–141. [Google Scholar] [CrossRef]
- Carrasco, M.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Purification and characterization of a novel cold adapted fungal glucoamylase. Microb. Cell Fact. 2017, 16, 75. [Google Scholar] [CrossRef]
- Allala, F.; Bouacem, K.; Boucherba, N.; Azzouz, Z.; Mechri, S.; Sahnoun, M.; Benallaoua, S.; Hacene, H.; Jaouadi, B.; Darenfed, A. Purification, biochemical, and molecular characterization of a novel extracellular thermostable and alkaline α-amylase from Tepidimonas fonticaldi strain HB23. Int. J. Biol. Macromol. 2019, 132, 558–574. [Google Scholar]
- Yuan, S.; Li, R.; Lin, B.; Yan, R.; Ye, X. Engineering of Bacillus amyloliquefaciens α-Amylase for Improved Catalytic Efficiency by Error-Prone PCR. Starch/Staerke 2023, 75, 202200082. [Google Scholar] [CrossRef]
- Wang, C.; Lu, L.; Huang, C.; He, B.; Huang, R. Simultaneously Improved Thermostability and Hydrolytic Pattern of Alpha-Amylase by Engineering Central Beta Strands of TIM Barrel. Appl. Biochem. Biotechnol. 2020, 192, 57–70. [Google Scholar]
- Zhang, H.; Wu, F.; Xu, D.; Xu, X. Endogenous alpha-amylase explains the different pasting and rheological properties of wet and dry milled glutinous rice flour. Food Hydrocoll. 2021, 113, 106425. [Google Scholar] [CrossRef]
- Olajuyigbe, F.; Fatokun, C. Biochemical characterization of an extremely stable pH-versatile laccase from Sporothrix carnis CPF-05. Int. J. Biol. Macromol. 2017, 94, 535–543. [Google Scholar] [CrossRef]
- Fincan, S.; Özdemir, S.; Karakaya, A.; Enez, B. Purification and characterization of thermostable α-amylase produced from Bacillus licheniformis So-B3 and its potential in hydrolyzing raw starch. Life Sci. 2021, 264, 118639. [Google Scholar]
- Sudan, S.; Kumar, N.; Kaur, I.; Sahni, G. Production, purification and characterization of raw starch hydrolyzing thermostable acidic α-amylase from hot springs, India. Int. J. Biol. Macromol. 2018, 117, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.J.; Sigh, S.P. Purification and Characterization of an Amylase from a Newly Isolated Geobacillus thermoleovorans TTIO4 from the Hot Spring of Tuwa-Timba. Starch 2023, 75, 9–10. [Google Scholar]
- Santorelli, M.; Maurelli, L.; Pocsfalvi, G.; Fiume, I.; Squillaci, G.; Cara, F.L.; Monaco, G.D.; Morana, A. Isolation and characterisation of a novel alpha-amylase from the extreme haloarchaeon Haloterrigena turkmenica. Int. J. Biol. Macromol. 2016, 92, 174–184. [Google Scholar] [CrossRef]
- Posoongnoen, S.; Thummavongsa, T. Purification and characterization of thermostable α-amylase from germinating Sword bean (Canavalia gladiata (Jacq.) DC.) seeds. Plant Biotechnol. 2020, 37, 31–38. [Google Scholar]
- Uno-Okamura, K.; Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Purification and properties of apoplastic amylase from oat (Avena sativa) seedlings. Physiol. Plant 2004, 121, 117–123. [Google Scholar] [PubMed]
- Muralikrishna, G.; Nirmala, M. Cereal α-amylases—An overview. Carbohydr. Polym. 2005, 60, 163–173. [Google Scholar]
- Bertoft, E.; Andtfolk, C.; Kulp, S. Effect of pH, temperature, and calcium ions on barley malt α-amylase isoenzymes. J. Inst. Brew. 1984, 90, 298–302. [Google Scholar]
- Yadav, J.; Prakash, V. Stabilization of α-amylase, the key enzyme in carbohydrates properties alterations, at low pH. Int. J. Food Prop. 2011, 14, 1182–1196. [Google Scholar]
- Asoodeh, A.; Chamani, J.; Lagzian, M. A novel thermostable, acidophilic α-amylase from a new thermophilic “Bacillus sp. Ferdowsicous” isolated from Ferdows hot mineral spring in Iran: Purification and biochemical characterization. Int. J. Biol. Macromol. 2010, 46, 289–297. [Google Scholar]
- Zhang, F.; Yang, X.; Geng, L.; Zhang, Z.; Yin, Y.; Li, W. Purification and characterization of a novel and versatile α-amylase from thermophilic Anoxybacillus sp. YIM 342. Starch/Staerke 2016, 68, 446–453. [Google Scholar]
- Bekler, F.; Güven, K.; Güven, R. Purification and characterization of novel α-amylase from Anoxybacillus ayderensis FMB1. Biocatal. Biotransform. 2021, 39, 322–332. [Google Scholar] [CrossRef]
- Guillaume, J.; Da Lage, J.; Mezdou, S.; Marion-Poll, F.; Terrol, C.; Brouzes, M.; Schmidely, P. Amylase activity across black soldier fly larvae development and feeding substrates: Insights on starch digestibility and external digestion. Animal 2024, 18, 101337. [Google Scholar] [CrossRef]
- Murakami, S.; Nishimoto, H.; Toyama, Y.; Shimamoto, E. Purification and biochemical characterization of a novel alpha-amylase from Bacillus licheniformis NH1—Cloning, nucleotide sequence and expression of amyN gene in Escherichia coli. Process Biochem. 2008, 43, 499–510. [Google Scholar]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Porfiri, M.; Melnichuk, N.; Braia, M.; Brinatti, C.; Loh, W.; Romanini, D. Analysis of the structure-function relationship of alpha amylase complexed with polyacrylic acid. Colloids Surf. B 2020, 188, 110787. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Liang, G.; Zhu, J.; Lu, B.; Peng, L.; Wang, Q.; Wei, Y. Influence of Calcium Ions on the Thermal Characteristics of α-amylase from Thermophilic Anoxybacillus sp. GXS-BL. Curr. Med. Chem. 2019, 26, 148–157. [Google Scholar] [CrossRef]
- Sokočević, A.; Han, S.; Engels, W.J. Biophysical characterization of α-amylase inhibitor Parvulustat (Z-2685) and comparison with Tendamistat (HOE-467). Biochim. Biophys. Acta 2011, 18, 1383–1393. [Google Scholar] [CrossRef]
- Li, M.; Li, T.; Lu, X.; Sun, L.; Chen, Y.; Liu, H.; Cao, M. Site-directed mutagenesis of myofibril-bound serine proteinase from Crucian carp: Possible role of Pro95, A127 and I130 on thermal stability. Biochem. Eng. J. 2017, 125, 196–205. [Google Scholar] [CrossRef]

| Procedure | Total Activity/U | Total Protein/mg | Specific Activity U/mg | Recovery /% | Purification Fold |
| Crude Enzyme | 67.4 | 662 | 0.102 | 100 | 1.00 |
| Ammonium Sulfate Precipitation | 45.0 | 162 | 0.277 | 66.8 | 2.72 |
| DEAE Sepharose Fast Flow | 16.8 | 27.0 | 0.623 | 24.9 | 6.11 |
| Superdex-200 | 5.92 | 1.75 | 3.37 | 8.78 | 33.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, F.; Wu, F.; Guo, L.; Xu, X. Purification and Characterization of Endogenous α-Amylase from Glutinous Rice Flour. Foods 2025, 14, 1679. https://doi.org/10.3390/foods14101679
Zhang H, Zhang F, Wu F, Guo L, Xu X. Purification and Characterization of Endogenous α-Amylase from Glutinous Rice Flour. Foods. 2025; 14(10):1679. https://doi.org/10.3390/foods14101679
Chicago/Turabian StyleZhang, Huang, Fengjiao Zhang, Fengfeng Wu, Lichun Guo, and Xueming Xu. 2025. "Purification and Characterization of Endogenous α-Amylase from Glutinous Rice Flour" Foods 14, no. 10: 1679. https://doi.org/10.3390/foods14101679
APA StyleZhang, H., Zhang, F., Wu, F., Guo, L., & Xu, X. (2025). Purification and Characterization of Endogenous α-Amylase from Glutinous Rice Flour. Foods, 14(10), 1679. https://doi.org/10.3390/foods14101679





