Sustainable Extraction of Actinostemma lobatum Kernel Oil by 2-Methyltetrahydrofuran: A Comparative Study on Physicochemical Properties and Bioactive Compounds Against Petro-Sourced Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oil Content
2.3. Oil Extraction
2.4. Physicochemical Properties Analyses
2.4.1. Oil Yield
2.4.2. Oil Color
2.4.3. Study of the Oxidative Status of Oil
2.4.4. Oxidative Stability Index (OSI)
2.4.5. Fatty Acid Composition
2.4.6. Triacylglycerol Composition
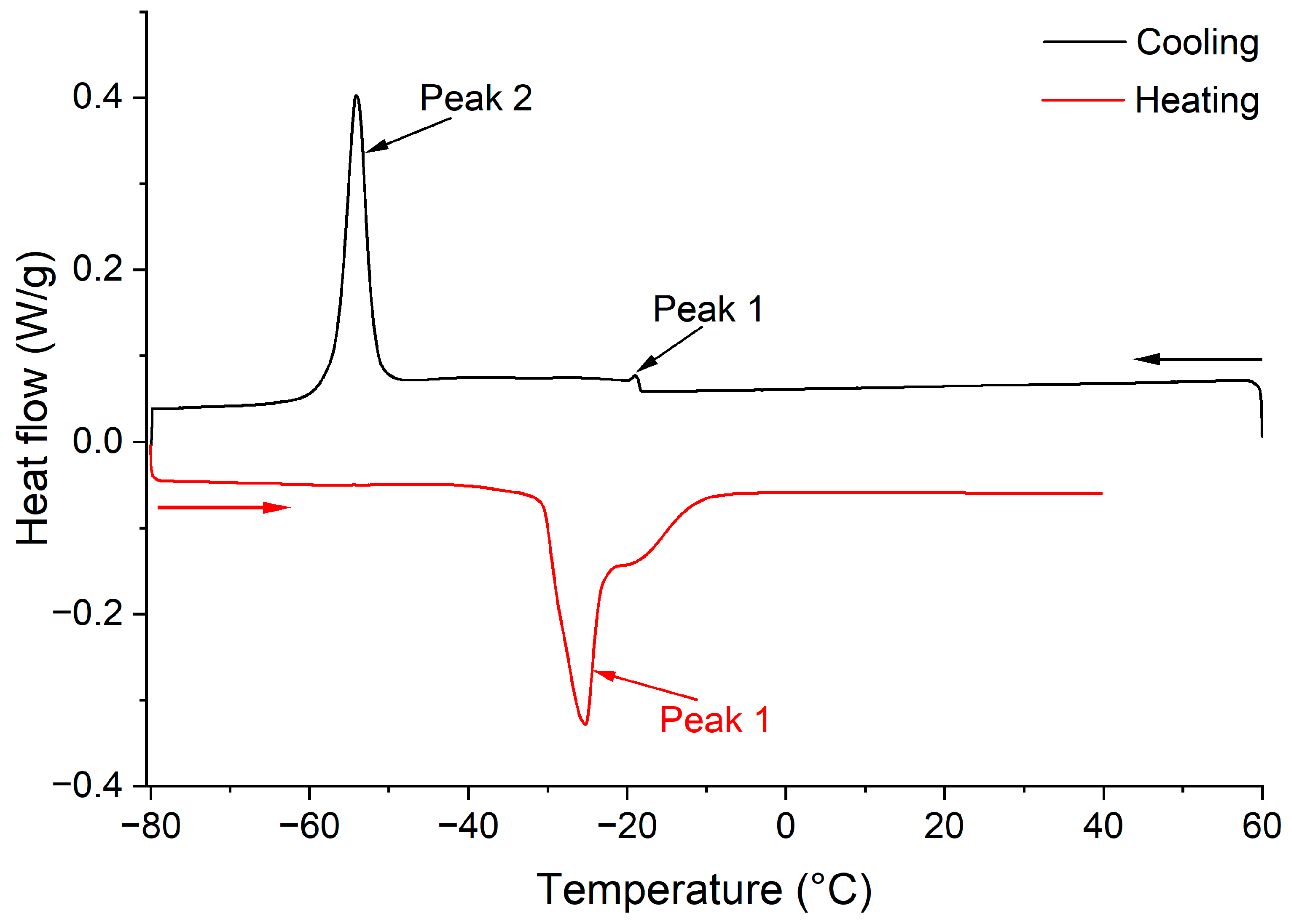
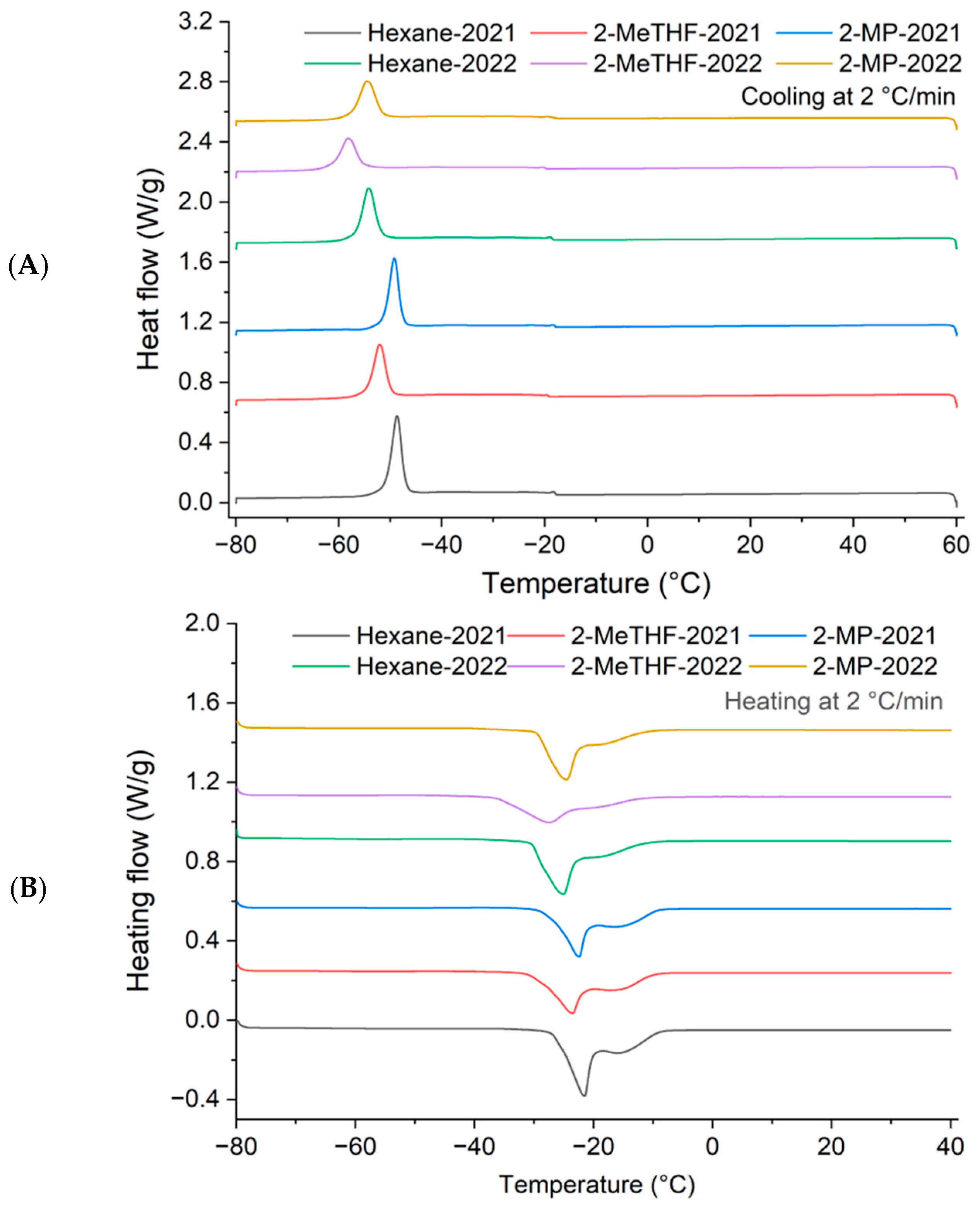
2.4.7. Thermal Analysis by Differential Scanning Calorimetry (DSC)
2.4.8. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5. Bioactive Compounds Analyses
2.5.1. Tocopherol and Tocotrienol
2.5.2. Phytosterols
2.5.3. β-Carotene
2.6. Statistical Analyses
3. Results and Discussions
3.1. Physicochemical Properties
3.1.1. Oil Yield
3.1.2. Oil Color
3.1.3. Oxidative Status
K232 and K268
AV and p-AnV
3.1.4. OSI
3.1.5. Fatty Acid
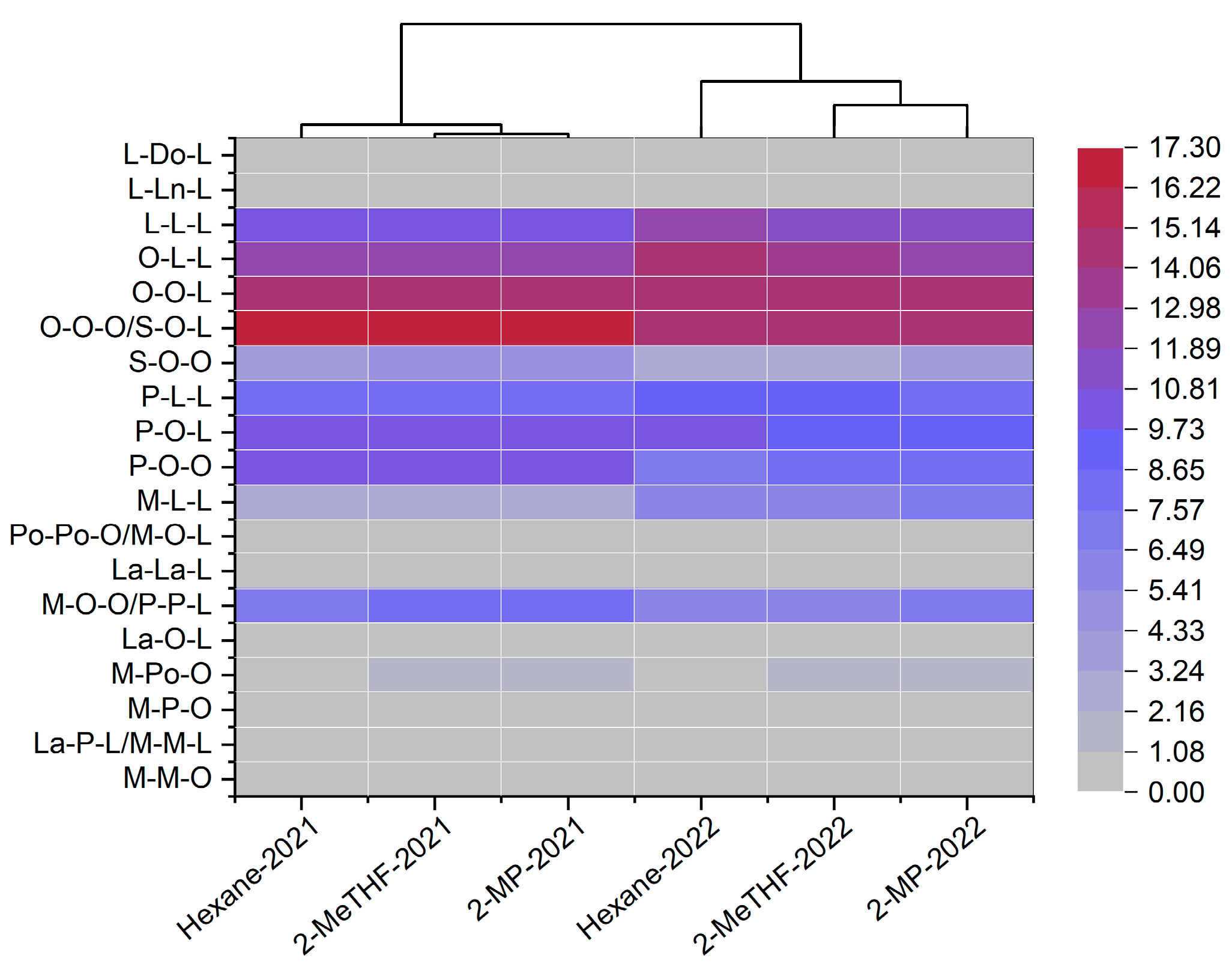
3.1.6. Triacylglycerol Analysis
3.1.7. DSC Melting and Crystallization Profiles
3.1.8. FT-IR Analysis
3.2. Bioactive Compounds Analyses
3.2.1. Tocopherol and Tocotrienol
3.2.2. Phytosterols
3.2.3. β-Carotene
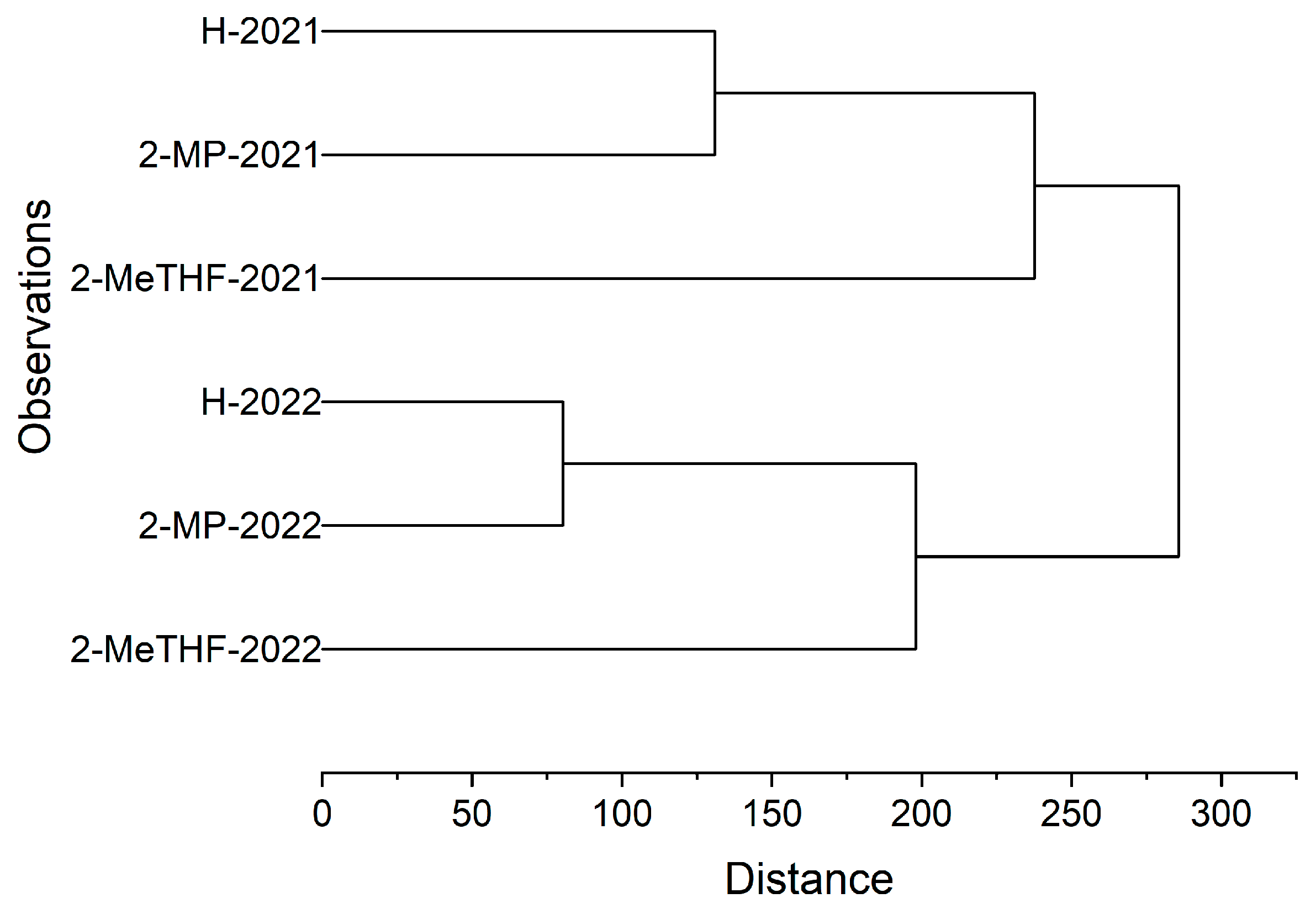
3.3. Hierarchical Cluster Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, L.; Zhang, T.; Xie, L.; Karrar, E.; Shi, L.; Jin, J.; Wang, X.; Jin, Q. Physicochemical characteristics of Actinostemma lobatum Maxim. kernel oil by supercritical fluid extraction and conventional methods. Ind. Crops Prod. 2020, 152, 112516. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Jolliet, O.; Meijaard, E.; Slavin, J.; Rasetti, M.; Aleta, A.; Moreno, Y.; Agostoni, C. Sustainable nutrition and the case of vegetable oils to match present and future dietary needs. Front. Public Health 2023, 11, 1106083. [Google Scholar] [CrossRef]
- Zheng, L.; Zhou, X.; Ye, Z.; Zhang, T. AlphaFold 3.0 prediction reveals stronger interaction between oleic acid and colipase than palmitic acid. J. Agric. Food Chem. 2024, 72, 27521–27527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xie, L.; Guo, Y.; Wang, Z.; Guo, X.; Liu, R.; Jin, Q.; Chang, M.; Wang, X. 4, 4-Dimethylsterols reduces fat accumulation via inhibiting fatty acid amide hydrolase in vitro and in vivo. Research 2024, 7, 0377. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, G.; Bucheli, M.E.; Aguilera, M.P.; Belaj, A.; Jimenez, A. Squalene in virgin olive oil: Screening of variability in olive cultivars. Eur. J. Lipid Sci. Technol. 2016, 118, 1250–1253. [Google Scholar] [CrossRef]
- Shahidi, F. Bailey’s Industrial oil and Fat Products, 7th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Claux, O.; Rapinel, V.; Goupy, P.; Patouillard, N.; Vian, M.A.; Jacques, L.; Chemat, F. Dry and aqueous 2-methyloxolane as green solvents for simultaneous production of soybean oil and defatted meal. ACS Sustain. Chem. Eng. 2021, 9, 7211–7223. [Google Scholar] [CrossRef]
- Trad, S.; Chaabani, E.; Aidi Wannes, W.; Dakhlaoui, S.; Nait Mohamed, S.; Khammessi, S.; Hammami, M.; Bourgou, S.; Saidani Tounsi, M.; Fabiano-Tixier, A.-S.; et al. Quality of edible sesame oil as obtained by green solvents: In silico versus experimental screening approaches. Foods 2023, 12, 3263. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, Z.; Wang, Y.; Li, Y. The effect of alternative solvents to n-hexane on the green extraction of Litsea cubeba kernel oils as new oil sources. Ind. Crops Prod. 2018, 126, 340–346. [Google Scholar] [CrossRef]
- Cravotto, C.; Claux, O.; Bartier, M.; Fabiano-Tixier, A.-S.; Tabasso, S. Leading edge technologies and perspectives in industrial oilseed extraction. Molecules 2023, 28, 5973. [Google Scholar] [CrossRef]
- Morón-Ortiz, Á.; Mapelli-Brahm, P.; León-Vaz, A.; Benitez-González, A.M.; Martín-Gómez, A.N.; León, R.; Meléndez-Martínez, A.J. Assessment of milling and the green biosolvents ethyl lactate and 2-methyltetrahydrofuran (2-methyloxolane) for the ultrasound-assisted extraction of carotenoids in common and phytoene-rich Dunaliella bardawil microalgae. LWT-Food Sci. Technol. 2024, 213, 117007. [Google Scholar] [CrossRef]
- Smets, R.; Goos, P.; Claes, J.; Van Der Borght, M. Optimisation of the lipid extraction of fresh black soldier fly larvae (Hermetia illucens) with 2-methyltetrahydrofuran by response surface methodology. Sep. Purif. Technol. 2021, 258, 118040. [Google Scholar] [CrossRef]
- Sicaire, A.-G.; Vian, M.A.; Fine, F.; Carré, P.; Tostain, S.; Chemat, F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason. Sonochem. 2016, 31, 319–329. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; de Barros, S.T.D.; Gimenes, M.L. The extraction of passion fruit oil with green solvents. J. Food Eng. 2013, 117, 458–463. [Google Scholar] [CrossRef]
- Sicaire, A.-G.; Vian, M.; Fine, F.; Joffre, F.; Carré, P.; Tostain, S.; Chemat, F. Alternative bio-based solvents for extraction of fat and oils: Solubility prediction, global yield, extraction kinetics, chemical composition and cost of manufacturing. Int. J. Mol. Sci. 2015, 16, 8430–8453. [Google Scholar] [CrossRef]
- Rapinel, V.; Claux, O.; Abert-Vian, M.; McAlinden, C.; Bartier, M.; Patouillard, N.; Jacques, L.; Chemat, F. 2-Methyloxolane (2-MeOx) as sustainable lipophilic solvent to substitute hexane for green extraction of natural products. Properties, applications, and perspectives. Molecules 2020, 25, 3417. [Google Scholar] [CrossRef] [PubMed]
- Jennifer, B.; Galvin, G.B. 2-METHYLPENTANE (ISOHEXANE). J. Toxicol. Environ. Health Part A 1999, 58, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zheng, L.; Mwinyi Pembe, W.; Zhang, J.; Xie, D.; Wang, X.; Huang, J.; Jin, Q.; Wang, X. Production of sn-1,3-distearoyl-2-oleoyl-glycerol-rich fats from mango kernel fat by selective fractionation using 2-methylpentane based isohexane. Food Chem. 2017, 234, 46–54. [Google Scholar] [CrossRef]
- Cui, J.; Yang, Z.; Xu, Y.; Tan, C.-P.; Zhang, W. Lipidomics insight on differences in lipid profiles and phytosterol compositions of coconut oils extracted by classical and green solvents. Food Res. Int. 2023, 174, 113653. [Google Scholar] [CrossRef]
- Morón-Ortiz, Á.; Mapelli-Brahm, P.; León-Vaz, A.; Benitez-González, A.M.; León, R.; Meléndez-Martínez, A.J. Ultrasound-assisted extraction of carotenoids from phytoene-accumulating Chlorella sorokiniana microalgae: Effect of milling and performance of the green biosolvents 2-methyltetrahydrofuran and ethyl lactate. Food Chem. 2024, 434, 137437. [Google Scholar] [CrossRef]
- Rapinel, V.; Chemat, A.; Santerre, C.; Belay, J.; Hanaei, F.; Vallet, N.; Jacques, L.; Fabiano-Tixier, A.S. 2-Methyloxolane as a bio-based solvent for green extraction of aromas from hops (Humulus lupulus L.). Molecules 2020, 25, 1727. [Google Scholar] [CrossRef]
- Breil, C.; Meullemiestre, A.; Vian, M.; Chemat, F. Bio-based solvents for green extraction of lipids from oleaginous yeast biomass for sustainable aviation biofuel. Molecules 2016, 21, 196. [Google Scholar] [CrossRef]
- Ravi, H.K.; Vian, M.A.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative solvents for lipid extraction and their effect on protein quality in black soldier fly (Hermetia illucens) larvae. J. Clean. Prod. 2019, 238, 117861. [Google Scholar] [CrossRef]
- Rekha, B.; Lokesh, B.R.; Gopala Krishna, A.G. Chemistry of color fixation in crude, physically refined and chemically refined rice bran oils upon heating. J. Am. Oil Chem. Soc. 2014, 91, 1665–1676. [Google Scholar] [CrossRef]
- Zheng, L.; Jin, J.; Karrar, E.; Xie, L.; Huang, J.; Chang, M.; Wang, X.; Zhang, H.; Jin, Q. Antioxidant activity evaluation of tocored through chemical assays, evaluation in stripped corn oil, and CAA assay. Eur. J. Lipid Sci. Technol. 2020, 122, 1900354. [Google Scholar] [CrossRef]
- Zheng, L.; Ji, C.; Jin, J.; Xie, D.; Liu, R.; Wang, X.; Jin, Q.; Huang, J. Effect of moisture and heat treatment of corn germ on oil quality. J. Am. Oil Chem. Soc. 2018, 95, 383–390. [Google Scholar] [CrossRef]
- Rajagukguk, Y.V.; Islam, M.; Grygier, A.; Tomaszewska-Gras, J. Thermal and spectroscopic profiles variation of cold-pressed raspberry seed oil studied by DSC, UV/VIS, and FTIR techniques. J. Food Compos. Anal. 2023, 124, 105723. [Google Scholar] [CrossRef]
- Mohammad Taghi Gharibzahedi, S.; Altintas, Z. Lesser mealworm (Alphitobius diaperinus L.) larvae oils extracted by pure and binary mixed organic solvents: Physicochemical and antioxidant properties, fatty acid composition, and lipid quality indices. Food Chem. 2023, 408, 135209. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, C.; Zhang, Y.; Wei, W.; Jin, Q.; Xu, Z.; Tao, G.; Wang, X. Identification and quantification of triacylglycerols in human milk fat using ultra-performance convergence chromatography and quadrupole time-of-flight mass spectrometery with supercritical carbon dioxide as a mobile phase. Food Chem. 2019, 275, 712–720. [Google Scholar] [CrossRef]
- Embaby, H.E.; Miyakawa, T.; Hachimura, S.; Muramatsu, T.; Nara, M.; Tanokura, M. Crystallization and melting properties studied by DSC and FTIR spectroscopy of goldenberry (Physalis peruviana) oil. Food Chem. 2022, 366, 130645. [Google Scholar] [CrossRef]
- ISO 9936:2016; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. International Organization for Standardization: Geneva, Switzerland, 2016.
- Zhang, F.; Zhu, F.; Chen, B.; Su, E.; Chen, Y.; Cao, F. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef]
- Wan Mahmood, W.M.A.; Theodoropoulos, C.; Gonzalez-Miquel, M. Enhanced microalgal lipid extraction using bio-based solvents for sustainable biofuel production. Green Chem. 2017, 19, 5723–5733. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Bourgou, S.; Detry, P.; Wannes, W.A.; Kenny, T.; Ksouri, R.; Sellami, I.H.; Fauconnier, M.-L. Green Extraction of Fennel and Anise Edible Oils Using Bio-Based Solvent and Supercritical Fluid: Assessment of Chemical Composition, Antioxidant Property, and Oxidative Stability. Food Bioprocess Technol. 2019, 12, 1798–1807. [Google Scholar] [CrossRef]
- Mohammadi, N.; Ostovar, N.; Granato, D. Pyrus glabra seed oil as a new source of mono and polyunsaturated fatty acids: Composition, thermal, and FTIR spectroscopic characterization. LWT-Food Sci. Technol. 2023, 181, 114790. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Kouřimská, L. Assessment of the nutritional quality of plant lipids using atherogenicity and thrombogenicity indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.E.; Rikal, L.; Schneider-Teixeira, A.; Deladino, L.; Ixtaina, V. Extraction method impact on the physicochemical characteristics of lipids from chia nutlets applicable to long-term storage studies. Food Chem. 2023, 427, 136706. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Li, D.; Zhang, L.-X.; Liu, Y.-l.; Wang, X.-d. Heating effect on the DSC melting curve of flaxseed oil. J. Therm. Anal. Calorim. 2014, 115, 2129–2135. [Google Scholar] [CrossRef]
- Chiavaro, E.; Rodriguez-Estrada, M.T.; Barnaba, C.; Vittadini, E.; Cerretani, L.; Bendini, A. Differential scanning calorimetry: A potential tool for discrimination of olive oil commercial categories. Anal. Chim. Acta 2008, 625, 215–226. [Google Scholar] [CrossRef]
- Gloria, H.; Aguilera, J.M. Assessment of the Quality of Heated Oils by Differential Scanning Calorimetry. J. Agric. Food Chem. 1998, 46, 1363–1368. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañán, G.; Juan, T.; Socias i Company, R. Tocopherol concentration in almond oil from Moroccan seedlings: Geographical origin and post-harvest implications. J. Food Compos. Anal. 2014, 33, 161–165. [Google Scholar] [CrossRef]
- Shahidi, F.; Camargo, D.; Costa, A. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Chen, X.; Memory Kunda, L.S.; Li, X.; Wang, N.; Huang, Y.; Hao, Y.; He, Q.; Liao, W.; Chen, J. A Comprehensive Review of Beneficial Effects of Phytosterols on Glycolipid Metabolism and Related Mechanisms. J. Agric. Food Chem. 2025, 73, 3826–3841. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is it possible to substitute hexane with green solvents for extraction of carotenoids? A theoretical versus experimental solubility study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef]
| Variable | Oil Samples | |||||
|---|---|---|---|---|---|---|
| Hexane-2021 | 2-MeTHF-2021 | 2-MP-2021 | Hexane-2022 | 2-MeTHF-2022 | 2-MP-2022 | |
| Oil Yield (%) | 24.71 ± 0.14 c | 27.60 ± 0.76 b | 22.74 ± 0.63 d | 28.12 ± 0.10 b | 29.77 ± 0.48 a | 25.71 ± 0.09 c |
| Color (R and Y) Lovibond units 1” cell | R, 1.65; Y, 15 | R, 18.05; Y, 39 | R, 2.15; Y, 15 | R, 1.20; Y, 18 | R, 16.40; Y, 38 | R, 1.40; Y, 19 |
| 5R + Y | 23.25 ± 0.35 e | 129.25 ± 1.77 a | 25.75 ± 0.35 c | 24.00 ± 0.00 d | 120.00 ± 0.71 b | 26.00 ± 0.00 c |
| K232 | 2.39 ± 0.25 c | 11.58 ± 0.54 a | 2.47 ± 0.02 c | 1.90 ± 0.07 c | 10.14 ± 0.75 b | 2.04 ± 0.13 c |
| K268 | 0.18 ± 0.01 c | 2.65 ± 0.21 a | 0.22 ± 0.04 c | 0.12 ± 0.03 c | 2.46 ± 0.13 b | 0.14 ± 0.02 c |
| AV (mg KOH/g) | 1.21 ± 0.13 c | 3.75 ± 0.77 a | 0.75 ± 0.07 e | 0.81 ± 0.04 de | 2.57 ± 0.30 b | 0.85 ± 0.17 d |
| p-AnV | 2.61 ± 0.34 f | 183.97 ± 1.45 b | 4.48 ± 0.51 e | 11.76 ± 3.20 c | 201.00 ± 4.30 a | 8.59 ± 1.20 d |
| OSI (h) | 11.12 ± 0.39 d | 17.13 ± 1.32 c | 41.50 ± 2.58 a | 28.47 ± 3.87 b | 1.35 ± 0.51 e | 25.91 ± 2.10 b |
| Lauric acid (C12:0) | 0.61 ± 0.00 b | 0.61 ± 0.00 b | 0.61 ± 0.00 b | 0.68 ± 0.01 a | 0.67 ± 0.00 a | 0.68 ± 0.01 a |
| Myristic acid (C14:0) | 4.13 ± 0.04 b | 4.10 ± 0.04 b | 4.12 ± 0.05 b | 4.35 ± 0.02 a | 4.33 ± 0.03 a | 4.34 ± 0.03 a |
| Palmitic acid (C16:0) | 7.15 ± 0.07 a | 7.26 ± 0.08 a | 7.14 ± 0.08 a | 6.54 ± 0.05 b | 6.60 ± 0.08 b | 6.54 ± 0.03 b |
| Palmitoleic acid (C16:1) | 0.14 ± 0.01 a | 0.14 ± 0.01 a | 0.14 ± 0.01 a | 0.09 ± 0.00 b | 0.09 ± 0.00 b | 0.09 ± 0.01 b |
| Margaric acid (C17:0) | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.01 a | 0.05 ± 0.00 a | 0.05 ± 0.01 a | 0.05 ± 0.00 a |
| Stearic acid (C18:0) | 2.68 ± 0.01 b | 2.71 ± 0.01 b | 2.68 ± 0.00 b | 2.98 ± 0.01 a | 3.01 ± 0.02 a | 2.97 ± 0.00 a |
| Oleic acid (C18:1n9c) | 46.86 ± 0.20 a | 46.78 ± 0.31 a | 46.91 ± 0.13 a | 36.68 ± 0.02 b | 36.56 ± 0.09 b | 36.75 ± 0.05 b |
| Linoleic acid (C18:2n6c) | 36.8 ± 0.04 b | 36.76 ± 0.15 b | 36.75 ± 0.02 b | 46.51 ± 0.13 a | 46.55 ± 0.04 a | 46.47 ± 0.10 a |
| Arachidic acid (C20:0) | 0.27 ± 0.04 a | 0.27 ± 0.04 a | 0.27 ± 0.04 a | 0.28 ± 0.04 a | 0.28 ± 0.04 a | 0.28 ± 0.04 a |
| Eicosenoic acid (C20:1) | 0.24 ± 0.00 a | 0.24 ± 0.01 a | 0.24 ± 0.00 a | 0.23 ± 0.00 a | 0.23 ± 0.01 a | 0.22 ± 0.00 a |
| Linolenic acid (C18:3n3) | 0.62 ± 0.01 b | 0.63 ± 0.00 b | 0.63 ± 0.00 b | 1.10 ± 0.01 a | 1.11 ± 0.02 a | 1.10 ± 0.02 a |
| Behenic acid (C22:0) | 0.33 ± 0.01 b | 0.34 ± 0.00 b | 0.34 ± 0.00 b | 0.44 ± 0.01 b | 0.43 ± 0.00 b | 0.43 ± 0.00 b |
| Lignoceric acid (C24:0) | 0.12 ± 0.00 a | 0.12 ± 0.00 a | 0.12 ± 0.00 a | 0.09 ± 0.00 b | 0.09 ± 0.00 b | 0.09 ± 0.00 b |
| SFAs | 15.30 ± 0.18 a | 15.17 ± 0.49 a | 15.05 ± 0.49 a | 15.08 ± 0.50 a | 15.16 ± 0.52 a | 15.06 ± 0.45 a |
| MUFAs | 47.24 ± 0.20 a | 47.05 ± 0.14 a | 47.18 ± 0.04 a | 36.88 ± 0.17 b | 36.76 ± 0.07 b | 36.95 ± 0.20 b |
| PUFAs | 37.42 ± 0.03 a | 37.39 ± 0.15 a | 37.38 ± 0.03 a | 47.61 ± 0.14 b | 47.66 ± 0.06 b | 47.57 ± 0.12 b |
| UFAs/SFAs | 5.54 ± 0.08 a | 5.57 ± 0.18 a | 5.62 ± 0.18 a | 5.61 ± 0.18 a | 5.57 ± 0.19 a | 5.62 ± 0.16 a |
| n-3/n-6 | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| AI | 0.29 ± 0.00 a | 0.29 ± 0.00 a | 0.29 ± 0.00 a | 0.29 ± 0.00 a | 0.29 ± 0.00 a | 0.29 ± 0.00 a |
| TI | 0.27 ± 0.01 a | 0.27 ± 0.00 a | 0.27 ± 0.00 a | 0.26 ± 0.00 a | 0.26 ± 0.01 a | 0.26 ± 0.00 a |
| HH | 7.03 ± 0.09 a | 6.98 ± 0.08 a | 7.05 ± 0.09 a | 7.20 ± 0.05 a | 7.16 ± 0.08 a | 7.20 ± 0.03 a |
| Parameters | Peaks | Hexane-2021 | 2-MeTHF-2021 | 2-MP-2021 | Hexane-2022 | 2-MeTHF-2022 | 2-MP-2022 |
|---|---|---|---|---|---|---|---|
| Onset temperature (°C) | Peak 1 | −18.03 ± 2.69 a | −19.64 ± 5.44 b | −17.89 ± 0.35 a | −18.48 ± 0.85 a | −19.62 ± 2.40 b | −18.53 ± 4.10 a |
| Peak temperature (°C) | −18.36 ± 2.40 a | −19.98 ± 5.16 cd | −18.33 ± 0.21 a | −18.99 ± 0.85 ab | −20.19 ± 2.62 d | −19.39 ± 1.48 bc | |
| Offset temperature (°C) | −19.19 ± 1.98 ab | −20.63 ± 5.94 de | −19.12 ± 0.07 a | −19.82 ± 0.35 bc | −21.03 ± 2.19 e | −20.21 ± 0.85 cd | |
| Peak height (W/g) | 0.13 ± 0.00 a | 0.07 ± 0.00 c | 0.08 ± 0.01 bc | 0.11 ± 0.01 ab | 0.07 ± 0.00 c | 0.07 ± 0.03 c | |
| Enthalpy (ΔH, J/g) | 0.26 ± 0.35 a | 0.12 ± 0.60 a | 0.17 ± 0.10 a | 0.27 ± 0.44 a | 0.12 ± 0.17 a | 0.17 ± 0.12 a | |
| Onset temperature (°C) | Peak 2 | −47.11 ± 0.13 a | −50.22 ± 0.46 b | −47.43 ± 0.23 a | −51.74 ± 0.08 c | −55.03 ± 0.32 d | −51.81 ± 0.48 c |
| Peak temperature (°C) | −48.63 ± 0.13 a | −52.13 ± 0.16 b | −49.02 ± 0.11 a | −53.90 ± 0.37 c | −57.89 ± 0.52 d | −54.39 ± 0.20 c | |
| Offset temperature (°C) | −51.41 ± 0.30 a | −54.96 ± 0.20 b | −51.67 ± 0.24 a | −57.63 ± 0.14 c | −62.39 ± 0.31 c | −57.63 ± 0.87 b | |
| Peak height (W/g) | 0.54 ± 0.03 a | 0.38 ± 0.05 ab | 0.43 ± 0.04 ab | 0.40 ± 0.07 ab | 0.22 ± 0.02 c | 0.32 ± 0.10 bc | |
| Enthalpy (ΔH, J/g) | 41.57 ± 1.24 a | 33.58 ± 0.69 b | 33.43 ± 1.90 b | 36.82 ± 3.28 ab | 26.71 ± 1.48 c | 33.30 ± 4.38 b |
| Parameters | Onset Temperature (°C) | Peak Temperature (°C) | Offset Temperature (°C) | Peak Height (W/g) | Enthalpy (ΔH, J/g) |
|---|---|---|---|---|---|
| Hexane-2021 | −25.49 ± 0.04 a | −21.76 ± 0.18 a | −20.54 ± 0.28 a | −0.23 ± 0.02 c | 14.78 ± 0.30 abc |
| 2-MeTHF-2021 | −28.85 ± 1.29 b | −24.12 ± 0.71 b | −22.41 ± 0.69 bc | −0.12 ± 0.03 a | 10.42 ± 1.45 c |
| 2-MP-2021 | −25.84 ± 0.56 a | −22.22 ± 0.40 a | −20.92 ± 1.17 ab | −0.18 ± 0.00 b | 12.66 ± 4.65 bc |
| Hexane-2022 | −30.62 ± 0.93 b | −25.42 ± 0.32 b | −23.53 ± 0.29 cd | −0.19 ± 0.02 bc | 20.94 ± 0.19 a |
| 2-MeTHF-2022 | −34.03 ± 0.46 c | −27.58 ± 0.15 c | −24.87 ± 0.30 d | −0.08 ± 0.00 a | 9.29 ± 0.27 c |
| 2-MP-2022 | −30.47 ± 2.31 c | −25.40 ± 1.05 b | −23.35 ± 0.81 cd | −0.18 ± 0.02 b | 19.35 ± 4.42 ab |
| Variable | Oil Samples | |||||
|---|---|---|---|---|---|---|
| Hexane-2021 | 2-MeTHF-2021 | 2-MP-2021 | Hexane-2022 | 2-MeTHF-2022 | 2-MP-2022 | |
| Tocopherols | ||||||
| α | 172.48 ± 0.90 a | 157.35 ± 0.31 b | 172.38 ± 7.97 a | 138.93 ± 6.90 c | 136.11 ± 1.90 cd | 126.61 ± 0.75 d |
| β | 353.13 ± 2.79 b | 321.60 ± 0.09 c | 358.87 ± 17.39 b | 390.85 ± 18.04 a | 386.07 ± 0.25 a | 356.77 ± 0.48 b |
| γ | 42.21 ± 1.58 a | 38.07 ± 1.27 ab | 39.12 ± 1.04 ab | 42.04 ± 3.78 a | 42.71 ± 2.06 a | 35.35 ± 0.59 b |
| δ | 396.32 ± 1.77 c | 359.68 ± 0.27 d | 396.58 ± 19.74 c | 512.76 ± 25.80 a | 505.79 ± 0.02 a | 463.85 ± 0.19 b |
| Tocotrienols | ||||||
| α | 6.53 ± 1.39 a | 10.58 ± 5.13 a | 8.10 ± 1.15 a | 13.64 ± 5.27 a | 25.76 ± 18.39 a | 15.33 ± 1.85 a |
| γ | 4.33 ± 0.19 ab | 5.56 ± 2.66 ab | 4.45 ± 0.69 ab | 7.05 ± 2.72 ab | 14.63 ± 9.62 a | 0.31 ± 0.04 b |
| δ | 1.49 ± 0.08 b | 2.32 ± 0.83 b | 2.02 ± 0.33 b | 2.68 ± 0.98 b | 7.77 ± 4.72 a | 0.26 ± 0.16 b |
| Total tocols | 976.49 ± 8.55 bc | 895.15 ± 9.95 c | 981.52 ± 43.98 bc | 1107.95 ± 63.50 a | 1118.83 ± 36.41 a | 998.48 ± 0.82 b |
| Phytosterols | ||||||
| Brassicasterol | 65.56 ± 0.80 c | 66.65 ± 0.49 bc | 69.73 ± 0.38 a | 65.48 ± 0.67 c | 68.39 ± 0.87 ab | 67.11 ± 1.25 bc |
| Campesterol | 326.11 ± 8.64 a | 295.00 ± 7.07 a | 320.89 ± 8.34 a | 348.97 ± 12.69 a | 338.20 ± 2.55 a | 314.23 ± 69.62 a |
| Campestanol | 23.00 ± 0.00 a | 24.74 ± 1.78 a | 22.87 ± 0.19 ab | 21.56 ± 2.03 ab | 22.17 ± 2.59 ab | 18.42 ± 2.01 b |
| Stigmasterol | 21.06 ± 1.33 b | 24.39 ± 0.86 a | 20.10 ± 0.15 b | 13.66 ± 0.48 cd | 15.09 ± 1.29 c | 12.56 ± 0.79 d |
| Clerosterol | 19.38 ± 1.95 c | 26.18 ± 1.66 b | 18.00 ± 0.00 c | 24.89 ± 0.16 b | 32.09 ± 2.69 a | 18.86 ± 0.93 c |
| β-Sitosterol | 1358.34 ± 2.34 a | 1269.84 ± 21.44 c | 1323.08 ± 11.42 ab | 1285.37 ± 10.43 bc | 1205.01 ± 7.09 d | 1320.44 ± 27.67 ab |
| Δ5-Avenasterol | 112.24 ± 1.07 a | 76.60 ± 2.26 c | 105.62 ± 3.36 b | 57.49 ± 0.73 d | 48.57 ± 3.63 e | 55.03 ± 1.36 d |
| Stigmastadienol | 116.40 ± 6.50 a | 103.88 ± 0.17 bc | 114.91 ± 5.78 ab | 90.50 ± 4.94 de | 80.82 ± 1.15 e | 94.43 ± 5.05 cd |
| Stigmastenol | 208.40 ± 4.80 a | 167.80 ± 3.11 b | 201.35 ± 1.91 a | 146.25 ± 6.71 c | 139.62 ± 0.54 c | 141.67 ± 2.36 c |
| Δ7-Avenasterol | 16.83 ± 1.66 bc | 21.74 ± 2.47 a | 17.81 ± 1.15 bc | 15.09 ± 0.12 bc | 14.86 ± 1.21 c | 18.62 ± 0.54 ab |
| Total sterols | 2267.33 ± 3.30 a | 2076.83 ± 14.38 c | 2214.37 ± 13.25 b | 2069.26 ± 8.85 c | 1964.80 ± 2.54 d | 2061.36 ± 39.83 c |
| β-carotene | 13.16 ± 0.02 d | 24.15 ± 0.44 a | 13.45 ± 0.18 c | 14.55 ± 0.01 c | 23.55 ± 0.03 a | 15.02 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Guo, H.; Song, H.; Yu, M.; Xie, M.; Korma, S.A.; Zhang, T. Sustainable Extraction of Actinostemma lobatum Kernel Oil by 2-Methyltetrahydrofuran: A Comparative Study on Physicochemical Properties and Bioactive Compounds Against Petro-Sourced Solvents. Foods 2025, 14, 1682. https://doi.org/10.3390/foods14101682
Zheng L, Guo H, Song H, Yu M, Xie M, Korma SA, Zhang T. Sustainable Extraction of Actinostemma lobatum Kernel Oil by 2-Methyltetrahydrofuran: A Comparative Study on Physicochemical Properties and Bioactive Compounds Against Petro-Sourced Solvents. Foods. 2025; 14(10):1682. https://doi.org/10.3390/foods14101682
Chicago/Turabian StyleZheng, Liyou, Hongyan Guo, Haozhi Song, Miao Yu, Mengxi Xie, Sameh A. Korma, and Tao Zhang. 2025. "Sustainable Extraction of Actinostemma lobatum Kernel Oil by 2-Methyltetrahydrofuran: A Comparative Study on Physicochemical Properties and Bioactive Compounds Against Petro-Sourced Solvents" Foods 14, no. 10: 1682. https://doi.org/10.3390/foods14101682
APA StyleZheng, L., Guo, H., Song, H., Yu, M., Xie, M., Korma, S. A., & Zhang, T. (2025). Sustainable Extraction of Actinostemma lobatum Kernel Oil by 2-Methyltetrahydrofuran: A Comparative Study on Physicochemical Properties and Bioactive Compounds Against Petro-Sourced Solvents. Foods, 14(10), 1682. https://doi.org/10.3390/foods14101682








