The Effects of Sourdough Fermentation on the Biochemical Properties, Aroma Profile and Leavening Capacity of Carob Flour
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Media, and Culture Conditions
2.2. Sourdough Preparation
2.3. Sourdough Homogenization, pH, and TTA Determination
2.4. LAB and Yeast Counting
2.5. Sequencing
2.6. Bacterial and Fungal Analysis
2.7. Metabolite Target and Sugar Analyses
2.8. Volatile Compound Analysis
2.9. Total Phenols Assay
2.10. Antioxidant Activity
2.11. Leavening Ability
2.12. Statistical Analysis
3. Results and Discussion
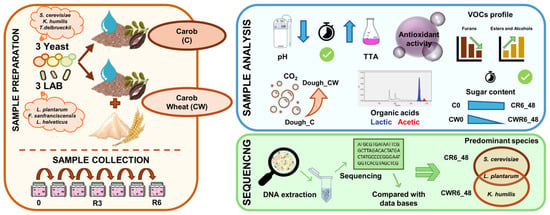
3.1. Sourdough Formulation, Flours and Starter Selection
3.2. Sourdough Fermentation, pH and TTA
3.3. By-Product Profile
3.4. Soluble Sugars
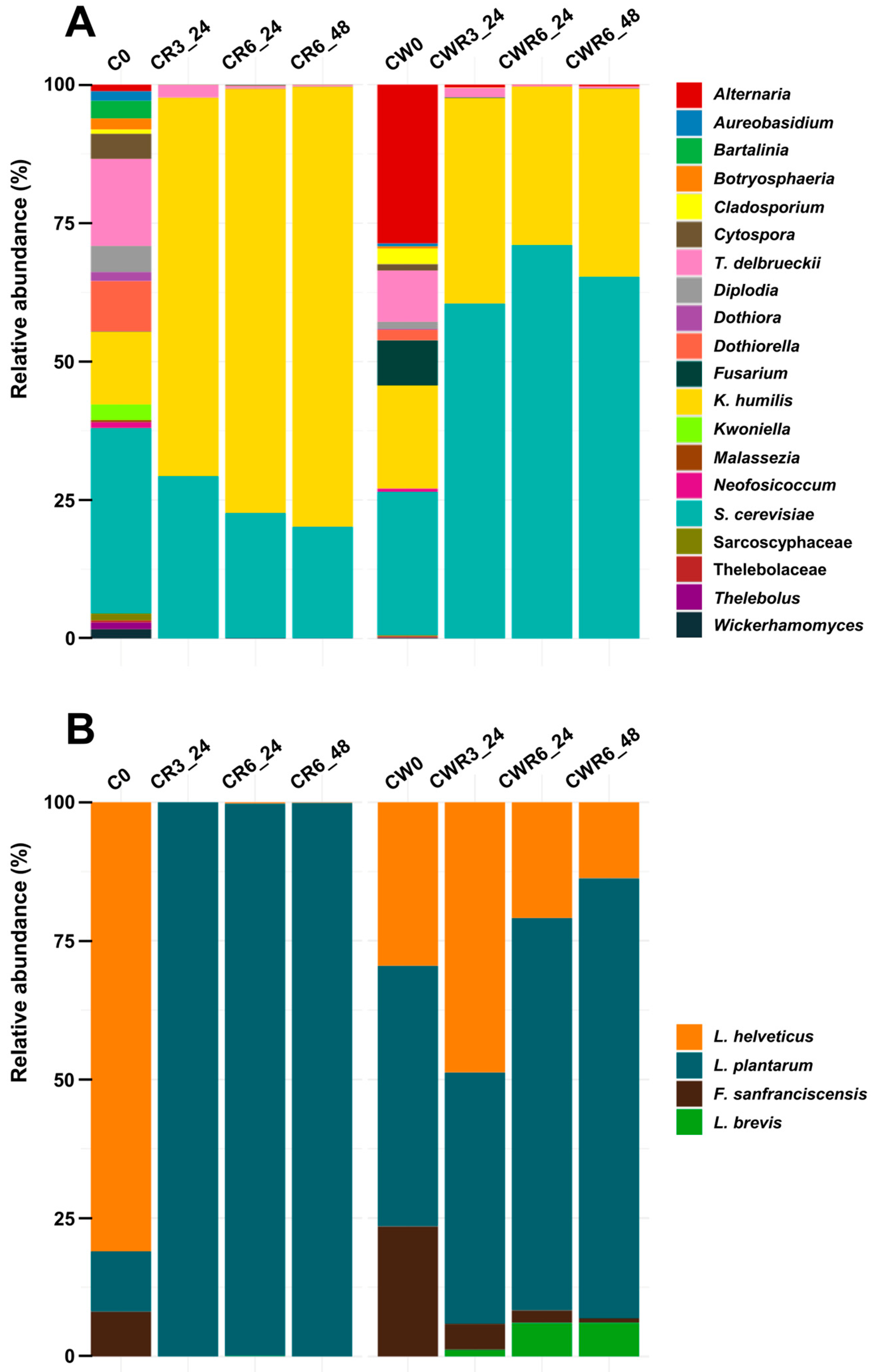
3.5. Counting and Sequencing
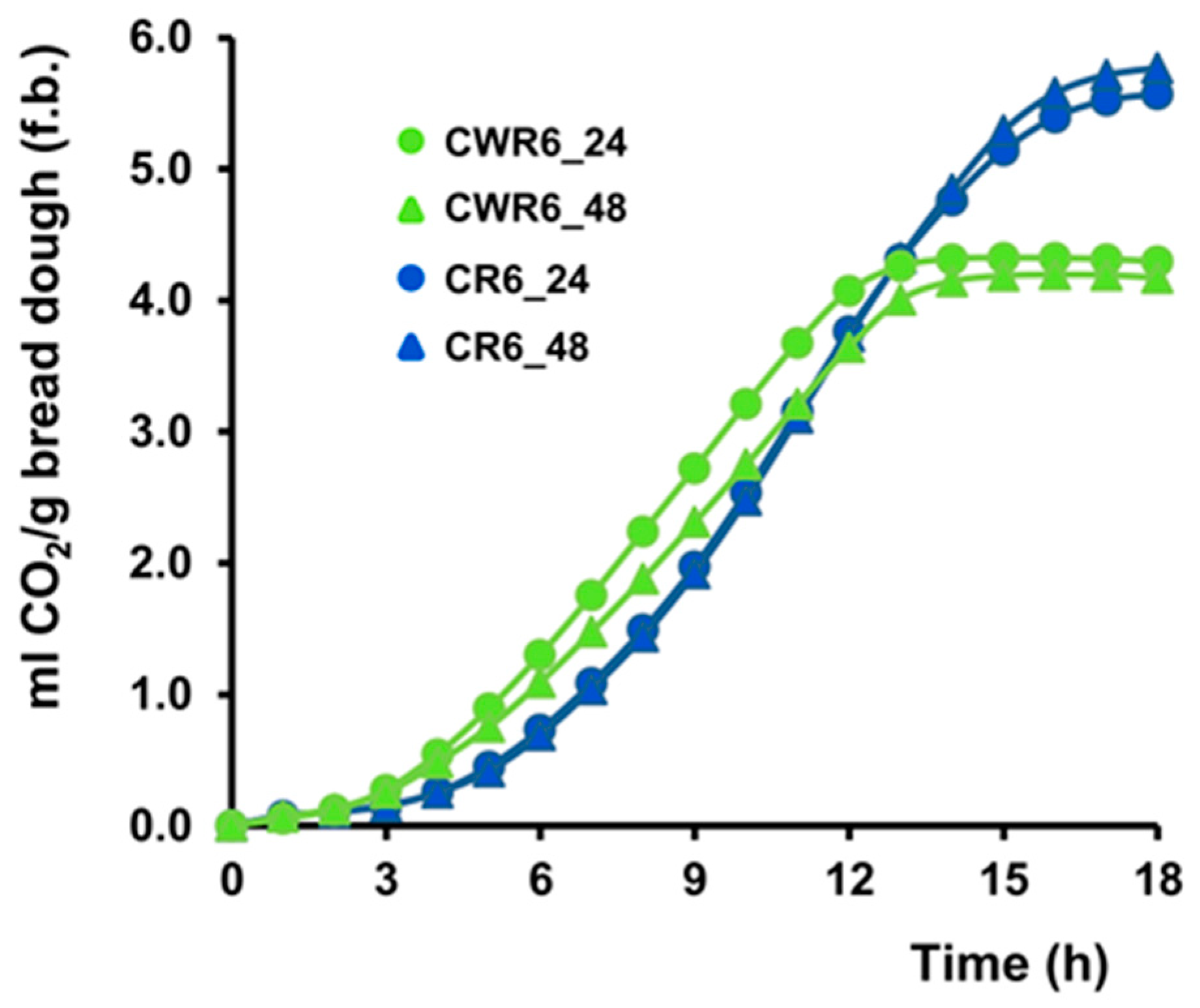
3.6. Leavening Ability of Sourdough
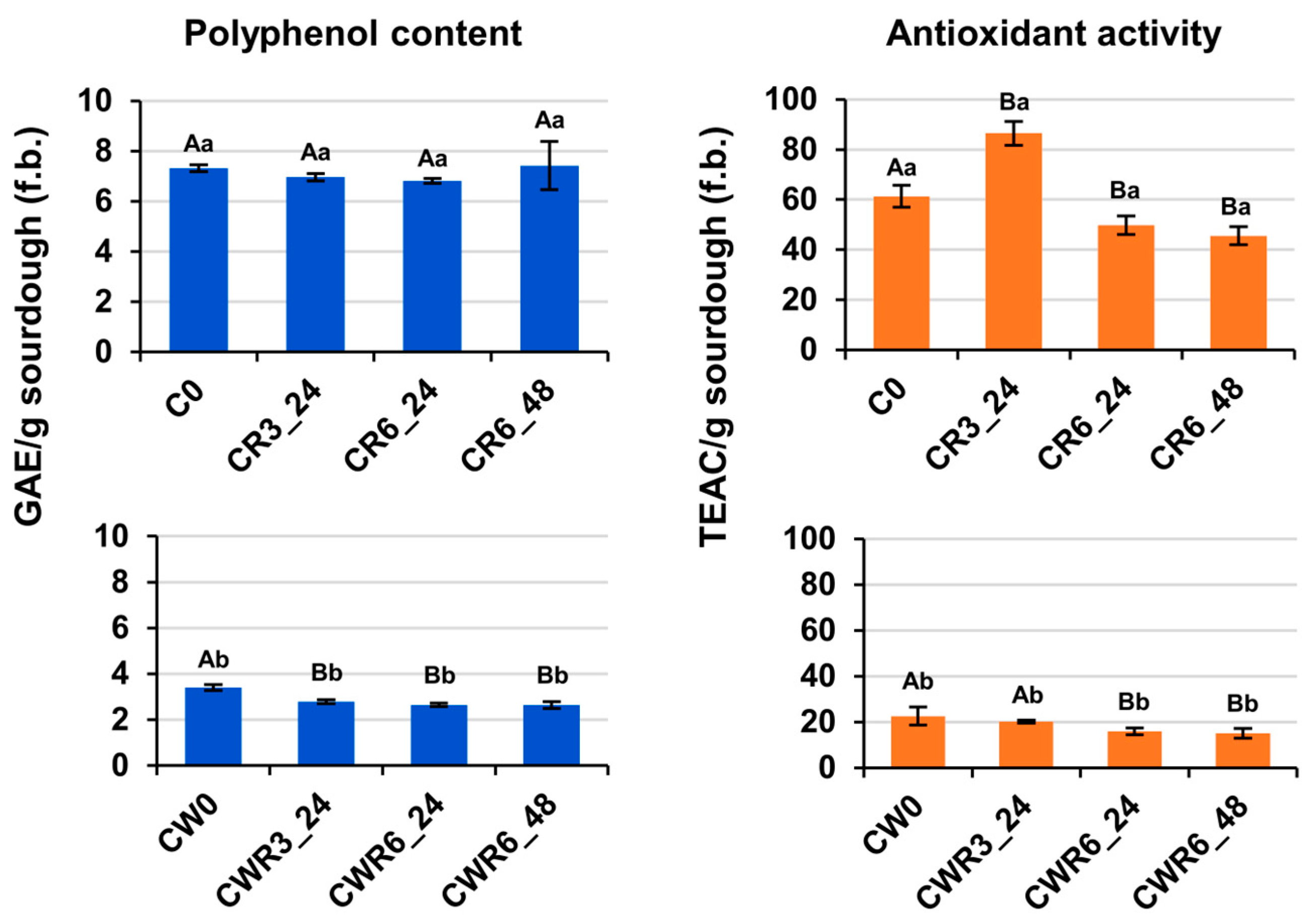
3.7. Antioxidant Activity and Polyphenol Content
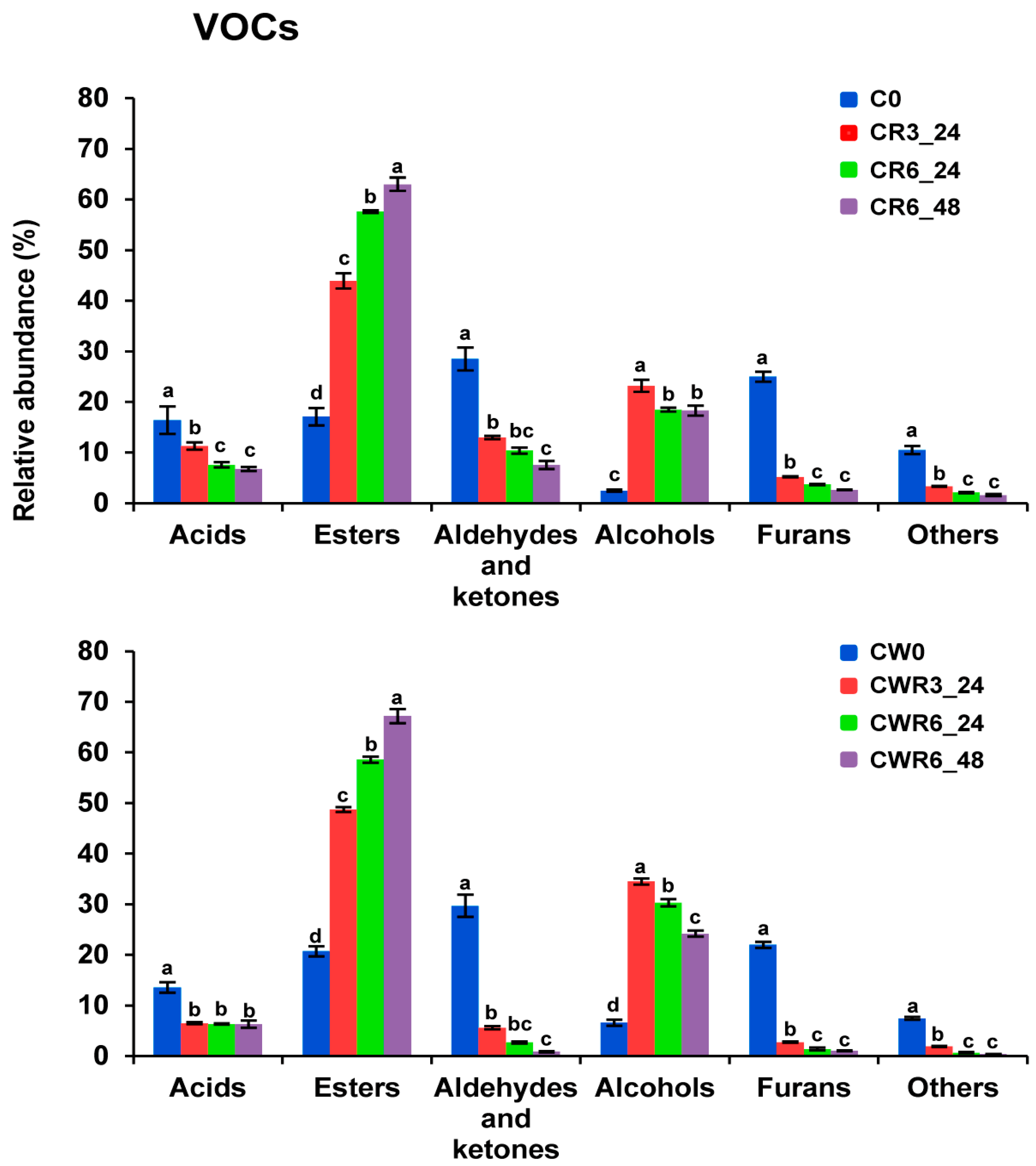
3.8. Volatile Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MAPA. Anuario de Estadística Agraria; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2023; Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/2023/default.aspx?parte=3&capitulo=07&grupo=13&seccion=2 (accessed on 23 March 2025).
- Ikram, A.; Khalid, W.; Zafar, K.U.; Ali, A.; Afzal, M.F.; Aziz, A.; Rasool, I.F.U.; Al-Farga, A.; Aqlan, F.; Koraqi, H. Nutritional, biochemical, and clinical applications of carob: A review. Food Sci. Nutr. 2023, 11, 3641–3654. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W. Carob (Ceratonia siliqua L.), pharmacological and phytochemical activities of neglected legume of the Mediterranean Basin, as functional food. Rev. Recent Clin. Trials 2024, 19, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Turfani, V.; Narducci, V.; Azzini, E.; Maiani, G.; Carcea, M. Nutritional characterisation and bioactive components of commercial carob flours. Food Chem. 2014, 153, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.J.; Pestana, M. Sugars and phenols in carob tree fruits from different producing countries: A short review. Heliyon 2024, 10, e30922. [Google Scholar] [CrossRef]
- Turhan, I. Relationship between sugar profile and D-pinitol content of pods of wild and cultivated types of carob bean (Ceratonia siliqua L.). Int. J. Food Prop. 2014, 17, 363–370. [Google Scholar] [CrossRef]
- Azab, A. D-pinitol-active natural product from carob with notable insulin regulation. Nutrients 2022, 14, 1453. [Google Scholar] [CrossRef]
- García-Díez, E.; López-Oliva, M.E.; Caro-Vadillo, A.; Pérez-Vizcaíno, F.; Pérez-Jiménez, J.; Ramos, S.; Martín, M.Á. Supplementation with a cocoa-carob blend, alone or in combination with metformin, attenuates diabetic cardiomyopathy, cardiac oxidative stress and inflammation in Zucker diabetic rats. Antioxidants 2022, 11, 432. [Google Scholar] [CrossRef]
- Dahmani, W.; Elaouni, N.; Abousalim, A.; Akissi, Z.L.E.; Legssyer, A.; Ziyyat, A.; Sahpaz, S. Exploring carob (Ceratonia siliqua L.): A comprehensive assessment of its characteristics, ethnomedicinal uses, phytochemical aspects, and pharmacological activities. Plants 2023, 12, 3303. [Google Scholar] [CrossRef]
- Petitjean, M.; Isasi, J.R. Locust bean gum, a vegetable hydrocolloid with industrial and biopharmaceutical applications. Molecules 2022, 27, 8265. [Google Scholar] [CrossRef]
- Igual, M.; Cámara, R.M.; Fortuna, F.; García-Herrera, P.; Pedrosa, M.M.; García-Segovia, P.; Martínez-Monzó, J.; Cámara, M. Enhancement of corn flour with carob bean for innovative gluten-free extruded products. Foods 2024, 13, 3352. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Esposito, L.; Caputo, P.; Martuscelli, M.; Gaglianò, M.; Clodoveo, M.L.; De Luca, G.; Rossi, C.O.; Savastano, M.; Scarcelli, E.; et al. Carob pulp flour as an innovative source of bioactive molecules for the preparation of high-value-added jellies. Heliyon 2024, 10, e38354. [Google Scholar] [CrossRef]
- Loullis, A.; Pinakoulaki, E. Carob as cocoa substitute: A review on composition, health benefits and food applications. Eur. Food Res. Technol. 2018, 244, 959–977. [Google Scholar] [CrossRef]
- Zahorec, J.; Šoronja-Simović, D.; Petrović, J.; Šereš, Z.; Pavlić, B.; Božović, D.; Perović, L.; Martić, N.; Bulut, S.; Kocić-Tanackov, S. Development of functional bread: Exploring the nutritional, bioactive and microbial potential of carob (Ceratonia siliqua L.) pulp powder. Processes 2024, 12, 2882. [Google Scholar] [CrossRef]
- Salinas, M.V.; Carbas, B.; Brites, C.; Puppo, M.C. Influence of different carob fruit flours (Ceratonia siliqua L.) on wheat dough performance and bread quality. Food Bioprocess Technol. 2015, 8, 1561–1570. [Google Scholar] [CrossRef]
- Šoronja-Simović, D.M.; Smole-Možina, S.; Raspor, P.; Maravić, N.R.; Zahorec, J.J.; Luskar, L.; Šereš, Z.I. Carob flour and sugar beet fiber as functional additives in bread. Acta Period. Technol. 2016, 47, 83–93. [Google Scholar] [CrossRef]
- Rico, D.; Alonso de Linaje, A.; Herrero, A.; Asensio-Vegas, C.; Miranda, J.; Martínez-Villaluenga, C.; de Luis, D.A.; Martin-Diana, A.B. Carob by-products and seaweeds for the development of functional bread. J. Food Process Preserv. 2018, 42, e13700. [Google Scholar] [CrossRef]
- Cauvain, S.P.; Clark, R.H. An introduction to the history of the manufacture of bakery products and relevant studies in human nutrition. In Baking Technology and Nutrition: Towards a Healthier World; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Brandt, M.J. Industrial production of sourdoughs for the baking branch—An overview. Int. J. Food Microbiol. 2019, 302, 3–7. [Google Scholar] [CrossRef]
- Sanmartín, G.; Sánchez-Adriá, I.E.; Salvador, A.; Prieto, J.A.; Estruch, F.; Randez-Gil, F. Quantitative assessment of volatile profile and sensory perception of artisan bread made in the city of Valencia. Foods 2024, 13, 3872. [Google Scholar] [CrossRef]
- De Vuyst, L.; Comasio, A.; Van Kerrebroeck, S. Sourdough production: Fermentation strategies, microbial ecology, and use of non-flour ingredients. Crit. Rev. Food Sci. Nutr. 2023, 63, 2447–2479. [Google Scholar] [CrossRef]
- Khlestkin, V.K.; Lockachuk, M.N.; Savkina, O.A.; Kuznetsova, L.I.; Pavlovskaya, E.N.; Parakhina, O.I. Taxonomic structure of bacterial communities in sourdoughs of spontaneous fermentation. Vavilovskii Zh. Genet. Sel. 2022, 26, 385–393. [Google Scholar] [CrossRef]
- Zannini, E.; Gobbetti, M. The 7th International Symposium on Sourdough—“Sourdough for health”. Int. J. Food Microbiol. 2019, 302, 1–2. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Portincasa, P.; Montemurro, M.; Di Palo, D.M.; Lorusso, M.P.; De Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough fermented breads are more digestible than those started with baker’s yeast alone: An in vivo challenge dissecting distinct gastrointestinal responses. Nutrients 2019, 11, 2954. [Google Scholar] [CrossRef]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Calasso, M.; Campanella, D.; De Angelis, M.; Gobbetti, M. Use of sourdough fermentation and mixture of wheat, chickpea, lentil and bean flours for enhancing the nutritional, texture and sensory characteristics of white bread. Int. J. Food Microbiol. 2014, 180, 78–87. [Google Scholar] [CrossRef]
- Curiel, J.A.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C.G. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sourdough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef]
- Chinma, C.E.; Ezeocha, V.C.; Adedeji, O.E.; Jolayemi, O.S.; Onwuka, Q.I.; Ilowefah, M.A.; Adebo, J.A.; Rosell, C.M.; Bamidele, O.P.; Adebo, O.A. Germinated/fermented legume flours as functional ingredients in wheat-based bread: A review. J. Food Sci. 2025, 90, e70022. [Google Scholar] [CrossRef]
- Novotni, D.; Mutak, N.; Nanjara, L.; Drakula, S.; Čukelj Mustač, N.; Voučko, B.; Ćurić, D. Sourdough fermentation of carob flour and its application in wheat bread. Food Technol. Biotechnol. 2020, 58, 465–474. [Google Scholar] [CrossRef]
- Voučko, B.; Čukelj Mustač, N.; Nanjara, L.; Drakula, S.; Grgić, T.; Ćurić, D.; Novotni, D. Fermentation Performance of Carob Flour, Proso Millet Flour and Bran for Gluten-Free Flat-Bread. Foods 2024, 13, 3458. [Google Scholar] [CrossRef]
- FEDIMA. Information Paper on Sourdough in Europe. 2014. Available online: https://www.fedima.org/resources/informed-customers-consumers/70-sourdough-in-eu (accessed on 12 February 2025).
- Sanmartín, G.; Sánchez-Adriá, I.E.; Prieto, J.A.; Estruch, F.; Randez-Gil, F. Bioprospecting of sourdough microbial species from artisan bakeries in the city of Valencia. Food Microbiol. 2024, 120, 104474. [Google Scholar] [CrossRef]
- Guthrie, C.; Fink, G.R. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991, 194, 21–37. [Google Scholar] [PubMed]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar] [CrossRef]
- Einarsson, S.V.; Rivers, A.R. ITSxpress version 2: Software to rapidly trim internal transcribed spacer sequences with quality scores for amplicon sequencing. Microbiol. Spectr. 2024, 12, e0060124. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; May, T.W.; Frøslev, T.G.; Pawlowska, J.; Lindahl, B.; Põldmaa, K.; Truong, C.; et al. The UNITE database for molecular identification and taxonomic communication of fungi and other eukaryotes: Sequences, taxa and classifications reconsidered. Nucleic Acids Res. 2024, 52, D791–D797. [Google Scholar] [CrossRef]
- Sánchez-Adriá, I.E.; Sanmartín, G.; Prieto, J.A.; Estruch, F.; Fortis, E.; Randez-Gil, F. Technological and acid stress performance of yeast isolates from industrial sourdough. LWT 2023, 184, 114957. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Datta, S.; Timson, D.J.; Annapure, U.S. Antioxidant properties and global metabolite screening of the probiotic yeast Saccharomyces cerevisiae var. boulardii. J. Sci. Food Agric. 2017, 97, 3039–3049. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 25 November 2024).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests (R Package Version 0.7.2). 2023. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 7 January 2025).
- Bahry, H.; Pons, A.; Abdallah, R.; Pierre, G.; Delattre, C.; Fayad, N.; Taha, S.; Vial, C. Valorization of carob waste: Definition of a second-generation bioethanol production process. Bioresour. Technol. 2017, 235, 25–34. [Google Scholar] [CrossRef]
- Bahry, H.; Abdalla, R.; Pons, A.; Taha, S.; Vial, C. Optimization of lactic acid production using immobilized Lactobacillus rhamnosus and carob pod waste from the Lebanese food industry. J. Biotechnol. 2019, 306, 81–88. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Leroy, F. Microbial ecology and process technology of sourdough fermentation. Adv. Appl. Microbiol. 2017, 100, 49–160. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Tsiasiotou, S.; Drosinos, E.H. Comparative study of spontaneously fermented sourdoughs originating from two regions of Greece: Peloponnesus and Thessaly. Eur. Food Res. Technol. 2010, 231, 883–890. [Google Scholar] [CrossRef]
- Carbonetto, B.; Nidelet, T.; Guezenec, S.; Perez, M.; Segond, D.; Sicard, D. Interactions between Kazachstania humilis yeast species and lactic acid bacteria in sourdough. Microorganisms 2020, 8, 240. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Sieuwerts, S.; Bron, P.A.; Smid, E.J. Mutually stimulating interactions between lactic acid bacteria and Saccharomyces cerevisiae in sourdough fermentation. LWT 2018, 90, 201–206. [Google Scholar] [CrossRef]
- Landis, E.A.; Oliverio, A.M.; McKenney, E.A.; Nichols, L.M.; Kfoury, N.; Biango-Daniels, M.; Shell, L.K.; Madden, A.A.; Shapiro, L.; Sakunala, S.; et al. The diversity and function of sourdough starter microbiomes. eLife 2021, 10, e61644. [Google Scholar] [CrossRef]
- Rodicio, R.; Heinisch, J.J. Sugar Metabolism by Saccharomyces and non-Saccharomyces Yeasts. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Martínez-Anaya, M.A.; Llin, M.L.; Macías, M.P.; Collar, C. Regulation of acetic acid production by homo- and heterofermentative lactobacilli in whole-wheat sourdoughs. Z. Lebensm. Unters. Forsch. 1994, 199, 186–190. [Google Scholar] [CrossRef]
- Nevoigt, E.; Stahl, U. Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 1997, 21, 231–241. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M.; Chandrashekar, N. Effects of d-pinitol on diabetes mellitus: An updated review. Bull. Natl. Res. Cent. 2022, 46, 130. [Google Scholar] [CrossRef]
- Basharat, Z.; Afzaal, M.; Saeed, F.; Islam, F.; Hussain, M.; Ikram, A.; Pervaiz, M.U.; Awuchi, C.G. Nutritional and functional profile of carob bean (Ceratonia siliqua): A comprehensive review. Int. J. Food Prop. 2023, 26, 389–413. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef]
- Rogalski, E.; Ehrmann, M.A.; Vogel, R.F. Intraspecies diversity and genome-phenotype-associations in Fructilactobacillus sanfranciscensis. Microbiol. Res. 2021, 243, 126625. [Google Scholar] [CrossRef]
- Wittwer, A.E.; Sicard, D.; Howell, K.S. Kazachstania humilis. Trends Microbiol. 2022, 30, 1012–1013. [Google Scholar] [CrossRef]
- Vu, T.H.; Johnson, S.K.; Fenton, H.; Nguyen, D.D.; Bhattarai, R.R. Submerged fermentation of empty carob pods to enhance D-pinitol, total phenolic and dietary fibre contents and associated bioactivity. Int. J. Food Sci. Technol. 2024, 59, 6388–6397. [Google Scholar] [CrossRef]
- Minutillo, S.A.; Ruano-Rosa, D.; Abdelfattah, A.; Schena, L.; Malacrinò, A. The fungal microbiome of wheat flour includes potential mycotoxin producers. Foods 2022, 11, 676. [Google Scholar] [CrossRef]
- Oshiro, M.; Tanaka, M.; Momoda, R.; Zendo, T.; Nakayama, J. Mechanistic insight into yeast bloom in a lactic acid bacteria relaying-community in the start of sourdough microbiota evolution. Microbiol. Spectr. 2021, 9, e0066221. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, K.; Li, H.; Ma, R.; Cui, M. Effect of mixed Saccharomyces cerevisiae Y10 and Torulaspora delbrueckii Y22 on dough fermentation for steamed bread making. Int. J. Food Microbiol. 2019, 303, 58–64. [Google Scholar] [CrossRef]
- Taillandier, P.; Lai, Q.P.; Julien-Ortiz, A.; Brandam, C. Interactions between Torulaspora delbrueckii and Saccharomyces cerevisiae in wine fermentation: Influence of inoculation and nitrogen content. World J. Microbiol. Biotechnol. 2014, 30, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Chaalal, M.; Ydjedd, S.; Chemache, L.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Kati, D.E. Evaluation of the antimicrobial potential of digested and undigested carob phenolic extracts: Impact on selected gut microbiota. Acta Aliment. 2023, 52, 612–622. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Tang, K.; Hu, Y.; Xu, X.; Gänzle, M.G. Effect of mixed cultures of yeast and lactobacilli on the quality of wheat sourdough bread. Front. Microbiol. 2019, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, E.; Prata, C.; Malaguti, M.; Marotti, I.; Segura-Carretero, A.; Catizone, P.; Dinelli, G.; Hrelia, S. Phytochemical profile and nutraceutical value of old and modern common wheat cultivars. PLoS ONE 2012, 7, e45997. [Google Scholar] [CrossRef]
- Li, Y.; Ma, D.; Sun, D.; Wang, C.; Zhang, J.; Xie, Y.; Guo, T. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015, 3, 328–334. [Google Scholar] [CrossRef]
- Owen, R.W.; Haubner, R.; Hull, W.E.; Erben, G.; Spiegelhalder, B.; Bartsch, H.; Haber, B. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem. Toxicol. 2003, 41, 1727–1738. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Guerrini, S.; Blandino, M.; Luti, S.; Pazzagli, L.; Granchi, L. Antioxidant properties of sourdoughs made with whole grain flours of hull-less barley or conventional and pigmented wheat and by selected lactobacilli strains. Foods 2020, 9, 640. [Google Scholar] [CrossRef]
- Srour, N.; Daroub, H.; Toufeili, I.; Olabi, A. Developing a carob-based milk beverage using different varieties of carob pods and two roasting treatments and assessing their effect on quality characteristics. J. Sci. Food Agric. 2016, 96, 3047–3057. [Google Scholar] [CrossRef]
- Brassesco, M.E.; Vilas-Boas, A.M.; Brandão, T.R.S.; Silva, C.L.M.; Azevedo, M.; Pintado, M. Impact of roasting temperature and seed presence on carob flour (Ceratonia siliqua L.): Physical, chemical, and functional properties. ACS Food Sci. Technol. 2023, 3, 1890–1902. [Google Scholar] [CrossRef]
- Antoniou, C.; Kyriacou, M.C.; Kyratzis, A.C.; Rouphael, Y. Linking colorimetric variation with non-volatile and volatile components of carob flour. Foods 2023, 12, 2556. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Kyratzis, A.C.; Soteriou, G.A.; Rouphael, Y.; Kyriacou, M.C. Configuration of the volatile aromatic profile of carob powder milled from pods of genetic variants harvested at progressive stages of ripening from high and low altitudes. Front. Nutr. 2021, 8, 789169. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.N.; Petersen, M.A.; Arneborg, N.; Hansen, Å.S. Influence of commercial baker’s yeasts on bread aroma profiles. Food Res. Int. 2013, 52, 160–166. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Hansen, Å.S. Aroma of wheat bread crumb. Cereal Chem. 2014, 91, 105–114. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Cirlini, M.; Morini, E.; Rinaldi, M.; Ganino, T.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and volatiles in bread making. LWT 2013, 53, 233–239. [Google Scholar] [CrossRef]
- Kaseleht, K.; Paalme, T.; Mihhalevski, A.; Sarand, I. Analysis of volatile compounds produced by different species of lactobacilli in rye sourdough using multiple headspace extraction. Int. J. Food Sci. Technol. 2011, 46, 1940–1946. [Google Scholar] [CrossRef]
| Organic Acids (mg/g Sourdough) 3 | log10 cfu/g Sourdough 3 | |||||
|---|---|---|---|---|---|---|
| Sourdough 1 | pH | TTA 2 | Lactic Acid | Acetic Acid | LAB 4 | Yeast |
| C0 | 4.79 ± 0.05 Aa | 6.4 ± 0.6 Aa | n.d. Aa | n.d. | 5.0 ± 0.2 Aa | 5.19 ± 0.02 Aa |
| CR3_24 | 4.69 ± 0.04 Aa | 6.4 ± 0.6 Aa | n.d. Aa | n.d. | 6.99 ± 0.18 Ba | 7.33 ± 0.08 Ba |
| CR6_24 | 4.71 ± 0.12 Aa | 5.8 ± 0.7 Aa | n.d. Aa | n.d. | 7.7 ± 0.2 Ba | 7.41 ± 0.02 Ba |
| CR6_48 | 4.65 ± 0.13 Aa | 6.4 ± 0.7 Aa | n.d. Aa | n.d. | 7.9 ± 0.2 Ba | 7.410 ± 0.008 Ba |
| CW0 | 4.95 ± 0.06 Ab | 3.94 ± 0.09 Ab | n.d. Aa | n.d. | 5.2 ± 0.2 Aa | 5.27 ± 0.12 Aa |
| CWR3_24 | 4.40 ± 0.08 Bb | 5.4 ± 0.3 Bb | 6.3 ± 1.05 Bb | n.d. | 8.58 ± 0.04 Bb | 7.60 ± 0.07 Ba |
| CWR6_24 | 4.30 ± 0.07 Bb | 6.1 ± 0.4 Ba | 7.4 ± 0.7 Bb | n.d. | 8.69 ± 0.17 Bb | 7.61 ± 0.02 Ba |
| CWR6_48 | 4.04 ± 0.12 Bb | 8.1 ± 0.5 Bb | 8.6 ± 0.9 Bb | n.d. | 8.76 ± 0.15 Bb | 7.54 ± 0.08 Ba |
| Compounds (mg/g Sourdough) 2 | |||||||
|---|---|---|---|---|---|---|---|
| Sourdough 1 | Pinitol | Glucose | Fructose | Sucrose | Maltose | Glycerol | Ethanol |
| C0 | 25.5 ± 1.9 Aa | 10.8 ± 1.3 Aa | 14.9 ± 2.3 Aa | 100.8 ± 7.7 Aa | n.d. Aa | n.d. Aa | n.d. Aa |
| CR3_24 | 29.2 ± 2.3 Aa | 2.1 ± 0.6 Ba | 8.08 ± 1.12 Aa | 94.77 ± 5.03 Aa | n.d. Aa | 6.6 ± 0.7 Ba | 13.6 ± 0.8 Ba |
| CR6_24 | 29.1 ± 2.9 Aa | 5.0 ± 1.3 Ba | 9.8 ± 2.3 Aa | 84.34 ± 1.05 Aa | n.d. Aa | 7.2 ± 1.3 Ba | 15.2 ± 0.7 Ba |
| CR6_48 | 29.9 ± 0.9 Aa | 5.7 ± 1.2 Ba | 8.06 ± 0.6 Ba | 41.3 ± 7.3 Ba | n.d. Aa | 9.7 ± 0.9 Ba | 26.3 ± 3.4 Ba |
| CW0 | 12.6 ± 0.9 Ab | 5.0 ± 0.9 Ab | 7.3 ± 0.8 Ab | 45.3 ± 3.6 Ab | 1.0 ± 0.4 Aa | n.d. Aa | n.d. Aa |
| CWR3_24 | 13.8 ± 0.8 Ab | 2.0 ± 0.3 Ba | 4.6 ± 0.4 Bb | 16.0 ± 1.3 Bb | 0.16 ± 0.06 Bb | 5.1 ± 0.6 Bb | 24.0 ± 2.2 Bb |
| CWR6_24 | 14.3 ± 0.5 Ab | 1.86 ± 0.07 Bb | 5.0 ± 0.9 Bb | 10.1 ± 2.5 Bb | 0.12 ± 0.03 Bb | 5.2 ± 0.6 Ba | 24.9 ± 1.3 Bb |
| CWR6_48 | 14.6 ± 0.4 Ab | 0.55 ± 0.09 Bb | 0.5 ± 0.2 Bb | 0.63 ± 0.16 Bb | n.d. Ba | 5.6 ± 0.2 Bb | 31.8 ± 1.4 Ba |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmartín, G.; Prieto, J.A.; Morard, M.; Estruch, F.; Blasco-García, J.; Randez-Gil, F. The Effects of Sourdough Fermentation on the Biochemical Properties, Aroma Profile and Leavening Capacity of Carob Flour. Foods 2025, 14, 1677. https://doi.org/10.3390/foods14101677
Sanmartín G, Prieto JA, Morard M, Estruch F, Blasco-García J, Randez-Gil F. The Effects of Sourdough Fermentation on the Biochemical Properties, Aroma Profile and Leavening Capacity of Carob Flour. Foods. 2025; 14(10):1677. https://doi.org/10.3390/foods14101677
Chicago/Turabian StyleSanmartín, Gemma, Jose A. Prieto, Miguel Morard, Francisco Estruch, Josep Blasco-García, and Francisca Randez-Gil. 2025. "The Effects of Sourdough Fermentation on the Biochemical Properties, Aroma Profile and Leavening Capacity of Carob Flour" Foods 14, no. 10: 1677. https://doi.org/10.3390/foods14101677
APA StyleSanmartín, G., Prieto, J. A., Morard, M., Estruch, F., Blasco-García, J., & Randez-Gil, F. (2025). The Effects of Sourdough Fermentation on the Biochemical Properties, Aroma Profile and Leavening Capacity of Carob Flour. Foods, 14(10), 1677. https://doi.org/10.3390/foods14101677