Myrica rubra Preharvest Treatment with Melatonin Improves Antioxidant and Phenylpropanoid Pathways During Postharvest Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
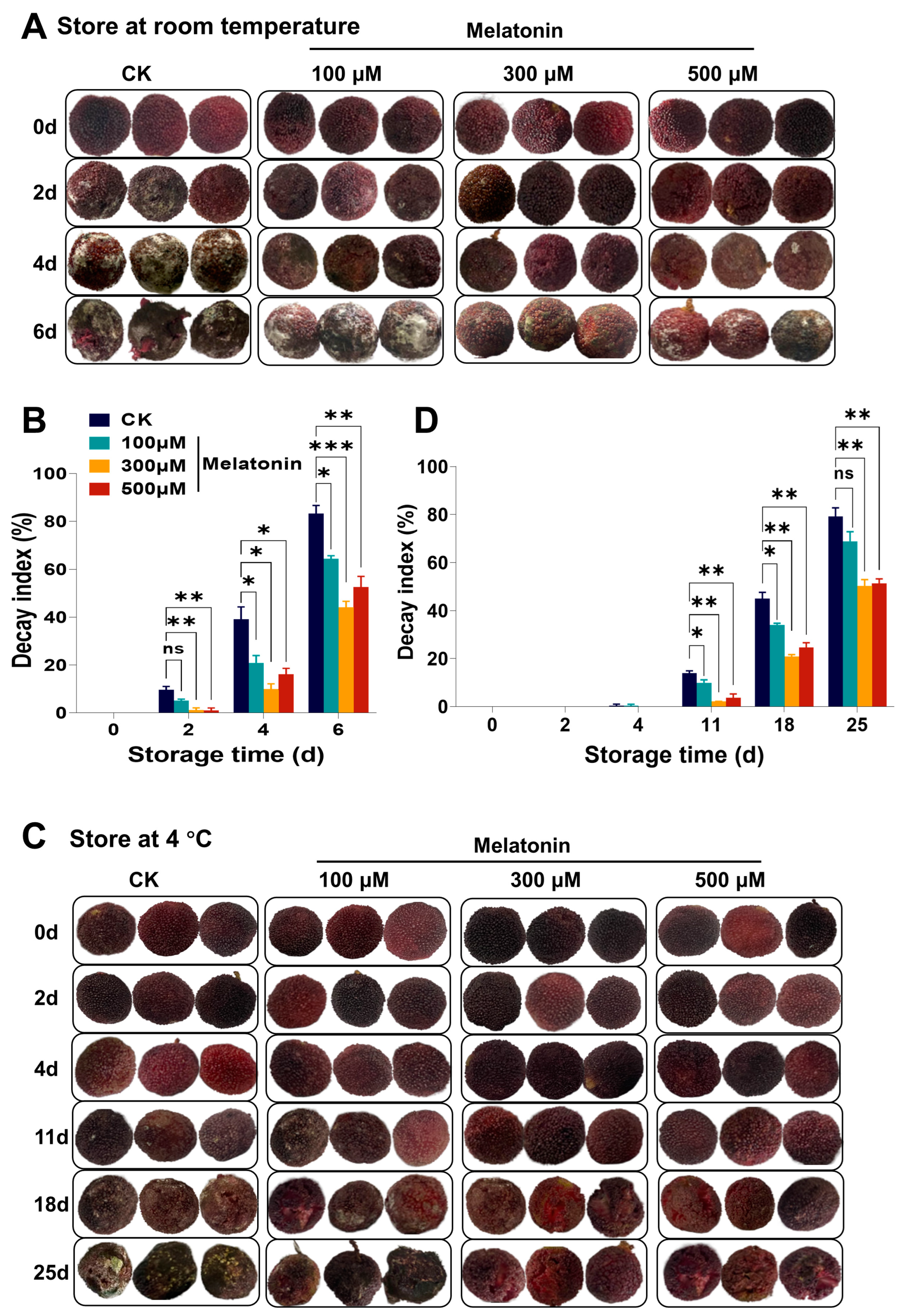
2.2. Determination of Decay Index
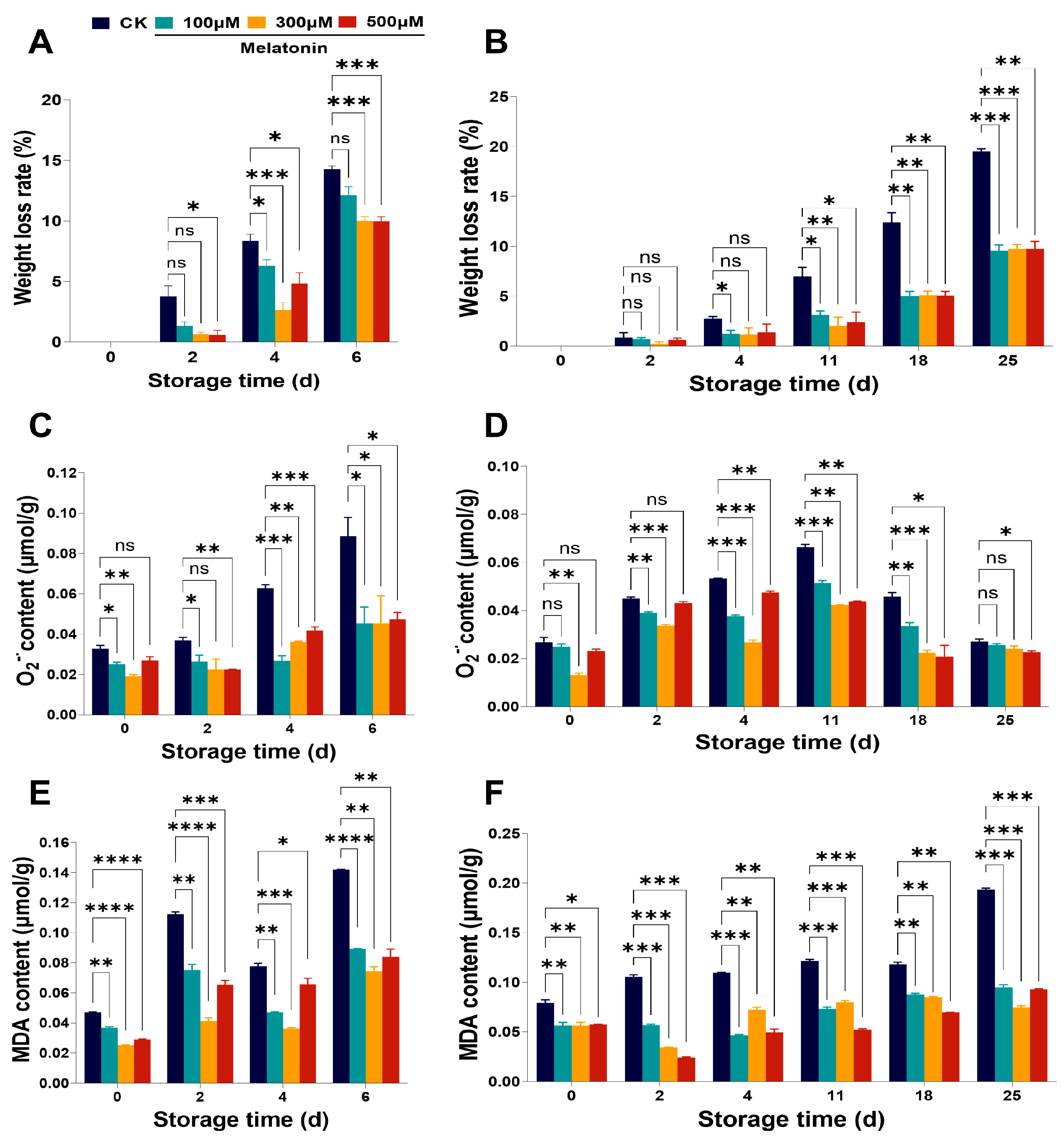
2.3. Determination of Weight Loss, O2−• and MDA Contents
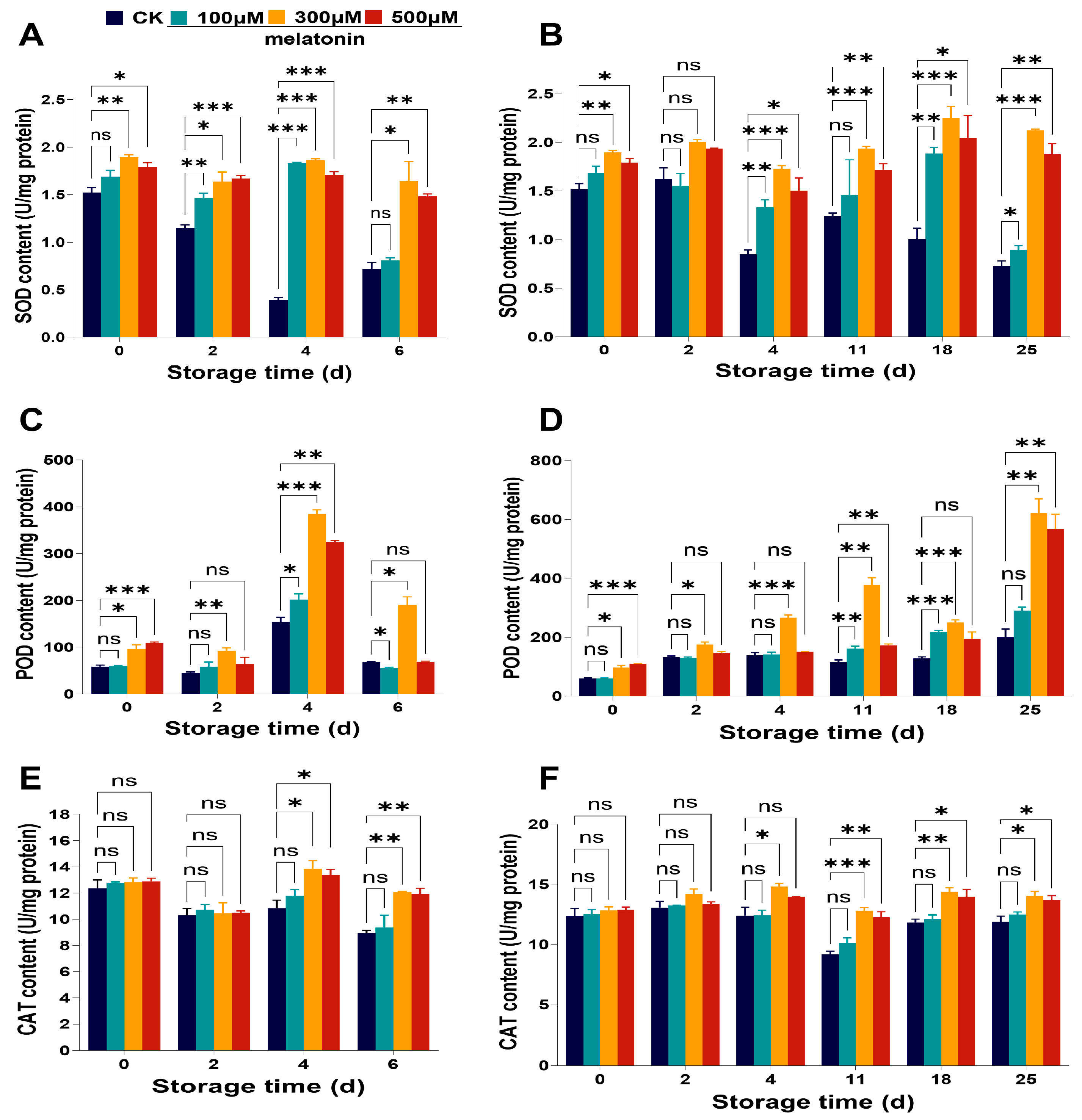
2.4. Detecting the Contents of SOD, POD, and CAT
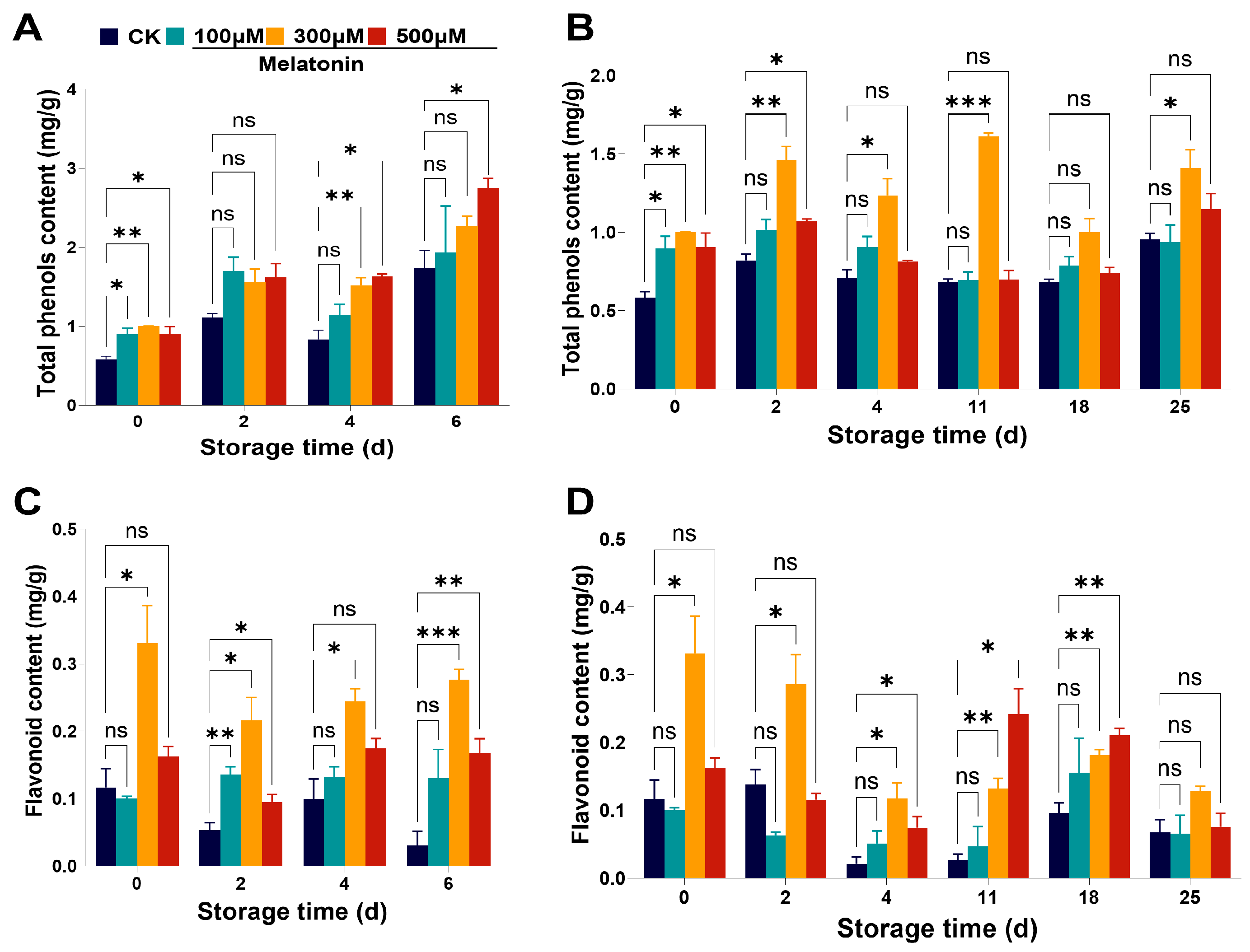
2.5. Total Phenolic and Flavonoid Content Determined
2.6. Total RNA Extraction, RNA Transcriptome Sequencing (RNA-Seq) and Data Analysis
2.7. Genes Expression Analysis Using Real-Time Quantitative PCR (qPCR)
2.8. Statistical Analysis
3. Results
3.1. Pre-Harvest Treatment with Melatonin Reduced Decay Index and Weight Loss of Myrica rubra Fruits
3.2. ROS Metabolism of Myrica rubra Fruits During Storage Could Be Affected by Pre-Harvest Treatment with Melatonin
3.3. Pre-Harvest Treatment with Melatonin Increased the Antioxidant Enzymes Activities in Myrica rubra Fruits
3.4. Pre-Harvest Melatonin Treatment Increased the Contents of Antioxidant Compounds in Myrica rubra Fruits
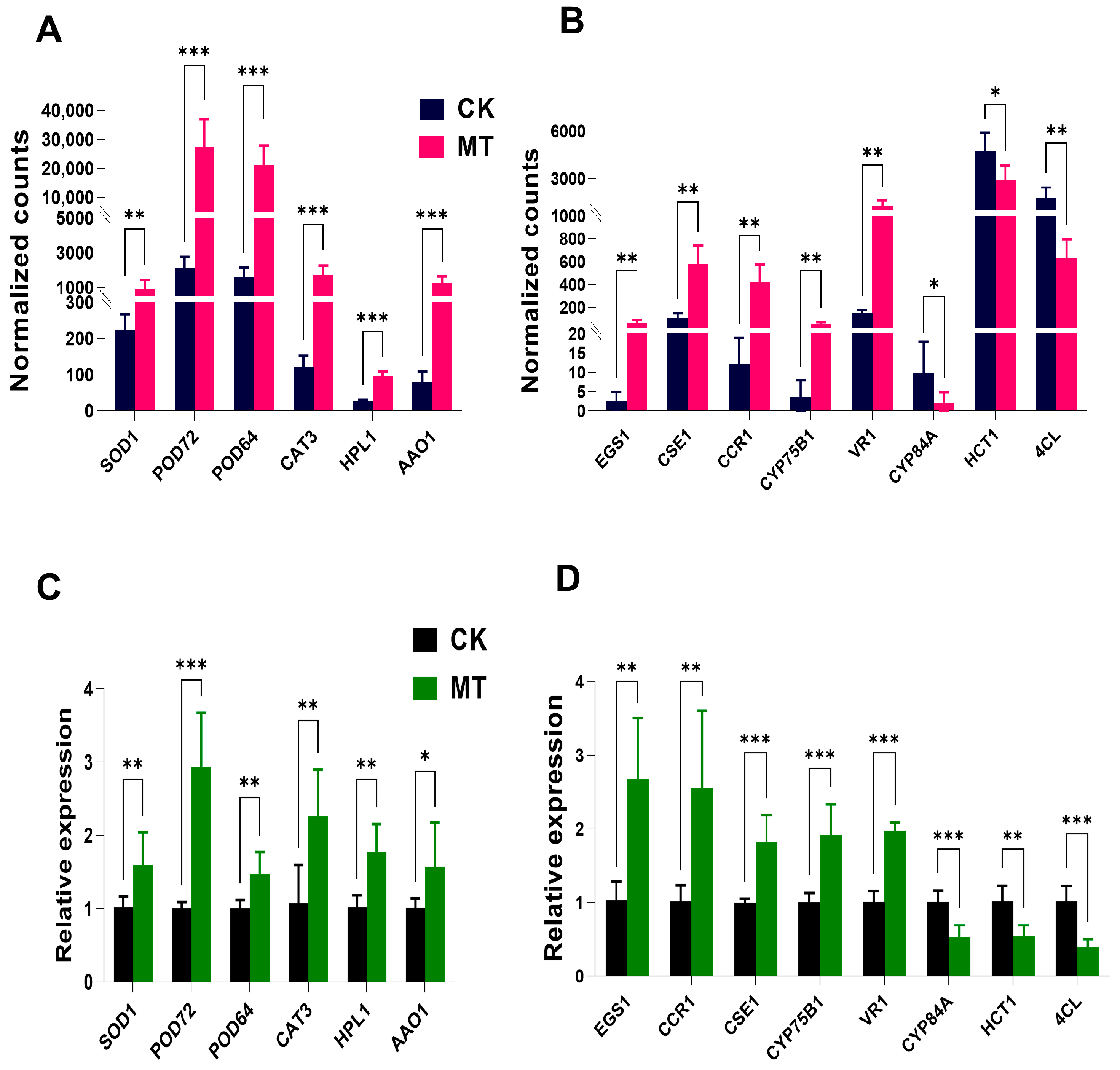
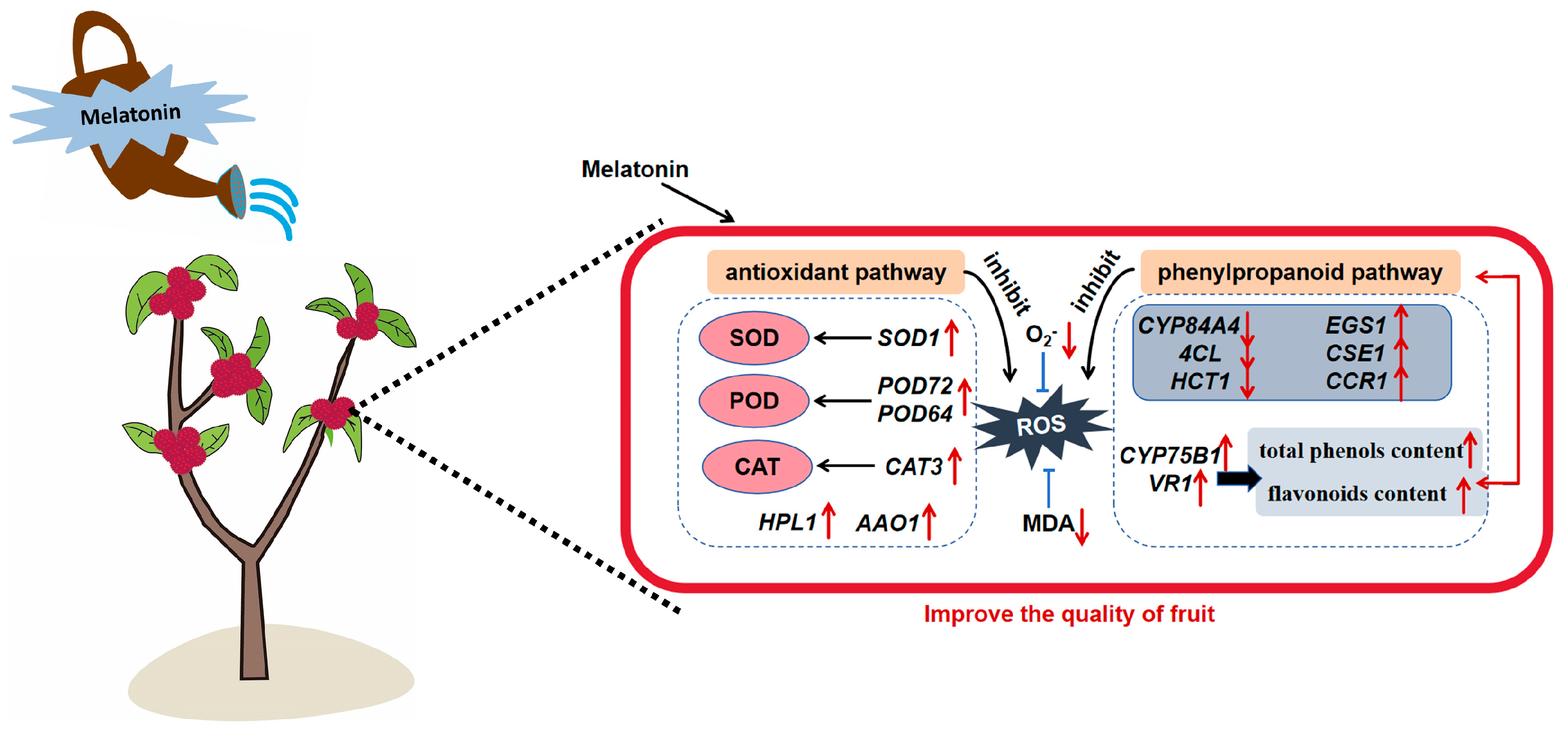
3.5. Pre-Harvest Melatonin Treatment Maintains Myrica rubra Quality via Antioxidant Pathway and Phenylpropanoid Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AsA | Ascorbate |
| APX | Ascorbate peroxidase |
| AAO1 | L-ascorbate oxidase |
| At1g02270 | Uncharacterized calcium-binding protein At1g02270 |
| CAT3 | Catalase isozyme 3 |
| C4H | Cinnamic acid 4-hydroxylase |
| CSE1 | Caffeoyl shikimase |
| CCR1 | Cinnamoyl coenzyme a reductase |
| CYP84A | Ferulate-5-hydroxylase |
| CYP75B1 | Flavonoid 3’-monooxygenase |
| ChiC | Class V chitinase |
| EGS1 | Eugenol synthase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSTF11 | Glutathione S-transferase F11 |
| HPL1 | Hydroperoxide lyase |
| HCT1 | O- hydroxycinnamoyltransferase |
| LYK3 | LysM domain receptor-like kinase 3 |
| Melatonin | MT |
| MDA | Malondialdehyde |
| MAN 7 | Mannan endo-1,4-beta-mannosidase 7 |
| MPK17 | Mitogen-activated protein kinase kinase kinase 17 |
| MYB26 | Transcription factor MYB26 |
| POD72 | Peroxidase 72 |
| POD64 | Peroxidase 64 |
| PAL | Phenylalanine ammonia-lyase |
| PDI1 | Protein disulfide-isomerase |
| PBL16 | Probable serine/threonine-protein kinase PBL16 |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SOD1 | Superoxide dismutase [Mn], mitochondrial |
| SCPL18 | Serine carboxypeptidase-like 18 |
| VR1 | Vestitone reductase |
| XTH23 | Probable xyloglucan endotransglucosylase/hydrolase protein 23 |
| XTH22 | Xyloglucan endotransglucosylase/hydrolase protein 22 |
| 4CL | 4-coumarate-CoA ligase |
References
- Wang, K.; Jin, P.; Cao, S.; Shang, H.; Yang, Z.; Zheng, Y. Methyl Jasmonate Reduces Decay and Enhances Antioxidant Capacity in Chinese Bayberries. J. Agric. Food Chem. 2009, 57, 5809–5815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, Z.; Sun, L.; Ren, H.; Zheng, X.; Liang, S.; Qi, X. An overview of the nutritional value, health properties, and future challenges of Chinese bayberry. PeerJ 2022, 10, 7717–7739. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yan, S.; Wang, Y.; Xu, G.; Zhao, D. Evaluation of the Effect of Preharvest Melatonin Spraying on Fruit Quality of ‘Yuluxiang’ Pear Based on Principal Component Analysis. Foods 2023, 12, 3507. [Google Scholar] [CrossRef]
- Xin, W.; Yuanyuan, C.; Chaowei, S.; Bin, W.; Yirong, Y.; Lin, L.; Yahong, Y.; Tianli, Y. Fungi with potential probiotic properties isolated from Fuzhuan brick tea. Food Sci. Hum. Wellness 2022, 11, 686–696. [Google Scholar]
- Ge, Y.; Li, X.; Li, C.; Tang, Q.; Duan, B.; Cheng, Y.; Hou, J.; Li, J. Effect of sodium nitroprusside on antioxidative enzymes and the phenylpropanoid pathway in blueberry fruit. Food Chem. 2019, 295, 607–612. [Google Scholar] [CrossRef]
- Chen, Y.; Hung, Y.C.; Chen, M.; Lin, M.; Lin, H. Enhanced storability of blueberries by acidic electrolyzed oxidizing water application may be mediated by regulating ROS metabolism. Food Chem. 2019, 270, 229–235. [Google Scholar] [CrossRef]
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P.M.A.; Canada, V.A.A. Oxidative stress: Importance for postharvest quality. HortScience 2004, 39, 924–925. [Google Scholar] [CrossRef]
- Ge, Y.; Wei, M.; Li, C.; Chen, Y.; Lv, J.; Meng, K.; Wang, W.; Li, J. Reactive oxygen species metabolism and phenylpropanoid pathway involved in disease resistance against Penicillium expansum in apple fruit induced by ϵ-poly-l-lysine. J. Sci. Food Agric. 2018, 98, 5082–5088. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Cheng, Y.; Hou, J.; Zhang, J.; Ge, Y. Postharvest Application of Acibenzolar-S-methyl Delays the Senescence of Pear Fruit by Regulating Reactive Oxygen Species and Fatty Acid Metabolism. J. Agric. Food Chem. 2020, 68, 4991–4999. [Google Scholar] [CrossRef]
- Biala, W.; Jasinski, M. The Phenylpropanoid Case—It Is Transport That Matters. Front. Plant Sci. 2018, 9, 1610–1617. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, C.; Wang, G.; He, J.; Zhu, S. Transcriptomic analysis reveals a role of phenylpropanoid pathway in the enhancement of chilling tolerance by pre-storage cold acclimation in cucumber fruit. Sci. Hortic. 2021, 288, 110282. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, M.; Liu, J.; Sun, W.; Min, D.; Li, F.; Li, X. Methyl salicylate pretreatment maintains quality and antioxidant capacity of fresh-cut pitaya fruit by modulating phenylpropanoid metabolism and antioxidant system. Sci. Hortic. 2023, 309, 111705. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, X.; Li, F. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT-Food Sci. Technol. 2019, 115, 108447. [Google Scholar] [CrossRef]
- Zhou, F.; Xu, D.; Liu, C.; Chen, C.; Tian, M.; Jiang, A. Ascorbic acid treatment inhibits wound healing of fresh-cut potato strips by controlling phenylpropanoid metabolism. Postharvest Biol. Technol. 2021, 181, 111644. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J. Biol. Chem. 1960, 235, 1992–1997. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Poeggeler, B.; Hardeland, R.; Cardinali, D.P. Drug Insight: The use of melatonergic agonists for the treatment of insomnia-focus on ramelteon. Nat. Clin. Pr. Neurol. 2007, 3, 221–228. [Google Scholar] [CrossRef]
- Li, R.; Luo, X.; Li, L.; Peng, Q.; Yang, Y.; Zhao, L.; Ma, M.; Hou, Z. The Protective Effects of Melatonin Against Oxidative Stress and Inflammation Induced by Acute Cadmium Exposure in Mice Testis. Biol. Trace Elem. Res. 2016, 170, 152–164. [Google Scholar] [CrossRef]
- Feng, Y.M.; Jia, Y.F.; Su, L.Y.; Wang, D.; Lv, L.; Xu, L.; Yao, Y.G. Decreased mitochondrial DNA copy number in the hippocampus and peripheral blood during opiate addiction is mediated by autophagy and can be salvaged by melatonin. Autophagy 2013, 9, 1395–1406. [Google Scholar] [CrossRef]
- Chen, S.J.; Huang, S.H.; Chen, J.W.; Wang, K.C.; Yang, Y.R.; Liu, P.F.; Lin, G.J.; Sytwu, H.K. Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2016, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Su, L.Y.; Li, H.; Lv, L.; Feng, Y.M.; Li, G.D.; Luo, R.; Zhou, H.J.; Lei, X.G.; Ma, L.; Li, J.L.; et al. Melatonin attenuates MPTP-induced neurotoxicity via preventing CDK5-mediated autophagy and SNCA/alpha-synuclein aggregation. Autophagy 2015, 11, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, L.Y.; Sun, C.; Jiao, L.; Miao, Y.; Xu, M.; Luo, R.; Zuo, X.; Zhou, R.; Zheng, P.; et al. Melatonin alleviates morphine analgesic tolerance in mice by decreasing NLRP3 inflammasome activation. Redox Biol. 2020, 34, 101560. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.; Requintina, P.; Bachurin, S. Antioxidant and antiaging activity of N-acetylserotonin and melatonin in the in vivo models. Ann. N. Y. Acad. Sci. 2001, 939, 190–199. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Popovich, I.G.; Zabezhinski, M.A.; Anisimov, S.V.; Vesnushkin, G.M.; Vinogradova, I.A. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim. Biophys. Acta 2006, 1757, 573–589. [Google Scholar] [CrossRef]
- Yilmaz, C.; Kocadagli, T.; Gokmen, V. Formation of melatonin and its isomer during bread dough fermentation and effect of baking. J. Agric. Food Chem. 2014, 62, 2900–2905. [Google Scholar] [CrossRef]
- Muszynska, B.; Sulkowska-Ziaja, K. Analysis of indole compounds in edible Basidiomycota species after thermal processing. Food Chem. 2012, 132, 455–459. [Google Scholar] [CrossRef]
- Escriva, L.; Manyes, L.; Barbera, M.; Martinez-Torres, D.; Meca, G. Determination of melatonin in Acyrthosiphon pisum aphids by liquid chromatography-tandem mass spectrometry. J. Insect Physiol. 2016, 86, 48–53. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Barbero, G.F.; Palma, M.; Garcia Barroso, C. Determination of Melatonin in Rice (Oryza sativa) Grains by Pressurized Liquid Extraction. J. Agric. Food Chem. 2015, 63, 1107–1115. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367–430. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2020, 105, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Shang, F.; Niu, B.; Wu, W.; Han, Y.; Chen, H.; Gao, H. Melatonin treatment delays the softening of blueberry fruit by modulating cuticular wax metabolism and reducing cell wall degradation. Food Res. Int. 2023, 173, 113357. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Ding, Z.; Xie, J. Prolong the postharvest shelf life of spinach through the antioxidative ability of melatonin. Food Chem. X 2023, 19, 100769. [Google Scholar] [CrossRef]
- Tang, Q.; Li, C.; Ge, Y.; Li, X.; Cheng, Y.; Hou, J.; Li, J. Exogenous application of melatonin maintains storage quality of jujubes by enhancing anti-oxidative ability and suppressing the activity of cell wall-degrading enzymes. LWT-Food Sci. Technol. 2020, 127, 109431. [Google Scholar] [CrossRef]
- Lin, X.; Huang, S.; Huber, D.J.; Zhang, Q.; Wan, X.; Peng, J.; Luo, D.; Dong, X.; Zhu, S. Melatonin Treatment Affects Wax Composition and Maintains Storage Quality in ‘Kongxin’ Plum (Prunus salicina L. cv) during Postharvest. Foods 2022, 11, 3972. [Google Scholar] [CrossRef]
- Fan, Y.; Li, C.; Li, Y.; Huang, R.; Guo, M.; Liu, J.; Sun, T.; Ge, Y. Postharvest melatonin dipping maintains quality of apples by mediating sucrose metabolism. Plant Physiol. Biochem. 2022, 174, 43–50. [Google Scholar] [CrossRef]
- Song, L.; Zhang, W.; Li, Q.; Jiang, Z.; Wang, Y.; Xuan, S.; Zhao, J.; Luo, S.; Shen, S.; Chen, X. Melatonin alleviates chilling injury and maintains postharvest quality by enhancing antioxidant capacity and inhibiting cell wall degradation in cold-stored eggplant fruit. Postharvest Biol. Technol. 2022, 194, 112092. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M.; Zhang, W.; Gao, Y.; Ma, X.; Cheng, S.; Chen, G. Exogenous melatonin activates the antioxidant system and maintains postharvest organoleptic quality in Hami melon (Cucumis. melo var. inodorus Jacq.). Front. Plant Sci. 2023, 14, 1274939. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Zhang, W.; Li, L.; Yang, W.; Liu, Y.; Jiang, L.; Guo, M.; Chen, G. Melatonin treatment delayed fruit softening by regulating postharvest carbohydrate metabolism of hami melon. Plant Physiol. Biochem. 2024, 219, 109328. [Google Scholar] [CrossRef]
- Dong, B.; Da, F.; Chen, Y.; Ding, X. Melatonin Treatment Maintains the Quality of Fresh-Cut Gastrodia elata under Low-Temperature Conditions by Regulating Reactive Oxygen Species Metabolism and Phenylpropanoid Pathway. Int. J. Mol. Sci. 2023, 24, 14284. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Kuang, C.; Chen, Y.; Da, F.; Yao, Q.; Zhu, D.; Ding, X. Melatonin Maintains Postharvest Quality in Fresh Gastrodia elata Tuber by Regulating Antioxidant Ability and Phenylpropanoid and Energy Metabolism During Storage. Int. J. Mol. Sci. 2024, 25, 11752. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Kou, X.; Qiao, L.; Li, J.; Luo, D.; Wang, X.; Cao, S. Maintaining quality of postharvest green pepper fruit using melatonin by regulating membrane lipid metabolism and enhancing antioxidant capacity. Food Chem. 2024, 460, 140671. [Google Scholar] [CrossRef]
- Cortes-Montana, D.; Bernalte-Garcia, M.J.; Velardo-Micharet, B.; Serrano, M.; Serradilla, M.J. Impact of Pre-Storage Melatonin Application on the Standard, Sensory, and Bioactive Quality of Early Sweet Cherry. Foods 2023, 12, 1723. [Google Scholar] [CrossRef]
- Qian, C.; Sun, Y.; Zhang, B.; Shao, Y.; Liu, J.; Kan, J.; Zhang, M.; Xiao, L.; Jin, C.; Qi, X. Effects of melatonin on inhibiting quality deterioration of postharvest water bamboo shoots. Food Chem. 2024, 8, 100208. [Google Scholar] [CrossRef]
- Wang, W.; Ni, Z.J.; Song, C.B.; Ma, W.P.; Cao, S.Q.; Wei, Z.J. Hydrogen sulfide treatment improves quality attributes via regulating the antioxidant system in goji berry (Lycium barbarum L.). Food Chem. 2023, 405, 134858. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Xu, M.; Dong, J.; Zhang, M.; Xu, X.; Sun, L. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Echeverria, G.; Ubach, D.; Aguiló-Aguayo, I.; López, M.L.; Larrigaudière, C. Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol. Biochem. 2017, 111, 216–225. [Google Scholar] [CrossRef]
- Carrión-Antolí, A.; Martínez-Romero, D.; Guillén, F.; Zapata, P.J.; Serrano, M.; Valero, D. Melatonin Pre-harvest Treatments Leads to Maintenance of Sweet Cherry Quality During Storage by Increasing Antioxidant Systems. Front. Plant Sci. 2022, 13, 863467. [Google Scholar] [CrossRef]
- Hu, H.; Liu, D.; Li, P.; Shen, W. Hydrogen sulfide delays leaf yellowing of stored water spinach (Ipomoea aquatica) during dark-induced senescence by delaying chlorophyll breakdown, maintaining energy status and increasing antioxidative capacity. Postharvest Biol. Technol. 2015, 108, 8–20. [Google Scholar] [CrossRef]
- Niu, S.S.; Xu, C.J.; Zhang, W.S.; Zhang, B.; Li, X.; Lin-Wang, K.; Ferguson, I.B.; Allan, A.C.; Chen, K.S. Coordinated regulation of anthocyanin biosynthesis in Chinese bayberry (Myrica rubra) fruit by a R2R3 MYB transcription factor. Planta 2010, 231, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Liu, Y.X.; Sang, Y.Y.; Ma, Y.Y.; Guo, M.R.; Bai, G.R.; Cheng, S.B.; Chen, G.G. Influences of ice-temperature storage on cell wall metabolism and reactive oxygen metabolism in Xinjiang (Diaogan) apricot. Postharvest Biol. Technol. 2021, 180, 111614. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Shi, T.; Sun, J.; Wu, X.; Weng, J.; Wang, P.; Qie, H.; Huang, Y.; Wang, H.; Gao, Z. Transcriptome analysis of Chinese bayberry (Myrica rubra Sieb. et Zucc.) fruit treated with heat and 1-MCP. Plant Physiol. Biochem. 2018, 133, 40–49. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Chen, T.; Ji, Y.; Shi, K.; Wang, L.; Zheng, X.; Kong, J. Melatonin in Apples and Juice: Inhibition of Browning and Microorganism Growth in Apple Juice. Molecules 2018, 23, 521. [Google Scholar] [CrossRef]
- Wang, X.; Liang, D.; Xie, Y.; Lv, X.; Wang, J.; Xia, H. Melatonin Application Increases Accumulation of Phenol substances in Kiwifruit during Storage. Emir. J. Food Agric. 2019, 31, 361–367. [Google Scholar]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef]
- Sharafi, Y.; Jannatizadeh, A.; Fard, J.R.; Aghdam, M.S. Melatonin treatment delays senescence and improves antioxidant potential of sweet cherry fruits during cold storage. Sci. Hortic. 2021, 288, 110304. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, E.; Lin, Z.; Miao, Z.; Li, Y.; Hu, S.; Gao, Y.; Jiang, Y.; Pang, L.; Li, X. Melatonin Rinsing Treatment Associated with Storage in a Controlled Atmosphere Improves the Antioxidant Capacity and Overall Quality of Lemons. Foods 2024, 13, 3298. [Google Scholar] [CrossRef]
- Hu, T.; Zheng, S.; Liu, Q.; Li, M.; Chen, J.; Zhang, H.; Lin, M.; Lin, H.; Chen, Y. Melatonin treatment maintains the quality properties and storability of carambola fruit by modulating energy metabolism. Food Chem. 2025, 464, 141661. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Singh, Z.; Mazhar, M.S.; Shah, H.M.S.; Afrifa-Yamoah, E.; Woodward, A. Postharvest melatonin application attenuates browning, delays softening, and maintains the antioxidant potential of jackfruit bulbs. Food Chem. 2025, 465, 141957. [Google Scholar] [CrossRef] [PubMed]
- Carrion-Antoli, A.; Lorente-Mento, J.M.; Valverde, J.M.; Castillo, S.; Valero, D.; Serrano, M. Effects of Melatonin Treatment on Sweet Cherry Tree Yield and Fruit Quality. Agronomy 2022, 12, 3. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Y.; Kang, H. Melatonin Is a Potential Target for Improving Post-Harvest Preservation of Fruits and Vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef]
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef]
- Rahman, M.A.; Woo, J.H.; Lee, S.H.; Park, H.S.; Kabir, A.H.; Raza, A.; El Sabagh, A.; Lee, K.W. Regulation of Na+/H+ exchangers, Na+/K+ transporters, and lignin biosynthesis genes, along with lignin accumulation, sodium extrusion, and antioxidant defense, confers salt tolerance in alfalfa. Front. Plant Sci. 2022, 13, 1041764. [Google Scholar] [CrossRef]
- Qu, G.; Wu, W.; Ba, L.; Ma, C.; Ji, N.; Cao, S. Melatonin Enhances the Postharvest Disease Resistance of Blueberries Fruit by Modulating the Jasmonic Acid Signaling Pathway and Phenylpropanoid Metabolites. Front. Chem. 2022, 10, 957581. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Liu, G.; Hu, M.; Yun, Z.; Duan, X.; Cai, K.; Jiang, G. Inhibition of downy blight and enhancement of resistance in litchi fruit by postharvest application of melatonin. Food Chem. 2021, 347, 129009. [Google Scholar] [CrossRef]
- Sharafi, Y.; Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Razavi, F.; Fard, J.R.; Farmani, B. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Sci. Hortic. 2019, 254, 222–227. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Luo, Z.; Li, L.; Jannatizadeh, A.; Fard, J.R.; Pirzad, F. Melatonin treatment maintains nutraceutical properties of pomegranate fruits during cold storage. Food Chem. 2020, 303, 125385. [Google Scholar] [CrossRef]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant Hormonomics: Multiple Phytohormone Profiling by Targeted Metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Ni, Z.; Hu, R.; Lin, L.; Deng, H.; Wang, J.; Tang, Y.; Sun, G.; Wang, X.; Li, H.; et al. Melatonin Alleviates Drought Stress by a Non-Enzymatic and Enzymatic Antioxidative System in Kiwifruit Seedlings. Int. J. Mol. Sci. 2020, 21, 852–869. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 9312–9337. [Google Scholar] [CrossRef]
- Kozieł, E.; Otulak-Kozieł, K.; Bujarski, J.J. Plant Cell Wall as a Key Player During Resistant and Susceptible Plant-Virus Interactions. Front. Microbiol. 2021, 12, 656809. [Google Scholar] [CrossRef]
- Jan, A.; Yang, G.; Nakamura, H.; Ichikawa, H.; Kitano, H.; Matsuoka, M.; Matsumoto, H.; Komatsu, S. Characterization of a Xyloglucan Endotransglucosylase Gene That Is Up-Regulated by Gibberellin in Rice. Plant Physiol. 2004, 136, 3670–3681. [Google Scholar] [CrossRef]
- Xue, L.; Sun, M.; Wu, Z.; Yu, L.; Yu, Q.; Tang, Y.; Jiang, F. LncRNA regulates tomato fruit cracking by coordinating gene expression via a hormone-redox-cell wall network. BMC Plant Biol. 2020, 20, 162–177. [Google Scholar] [CrossRef]
- Lin, Y.; Fan, L.; Xia, X.; Wang, Z.; Yin, Y.; Cheng, Y.; Li, Z. Melatonin decreases resistance to postharvest green mold on citrus fruit by scavenging defense-related reactive oxygen species. Postharvest Biol. Technol. 2019, 153, 21–30. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Q.; Ma, Y.-S.; Zhou, H.; Yu, R.-X.; Xiong, M.; Yang, N.; Wang, J.-Q.; Tian, Y.; Su, L.-Y. Myrica rubra Preharvest Treatment with Melatonin Improves Antioxidant and Phenylpropanoid Pathways During Postharvest Storage. Foods 2025, 14, 64. https://doi.org/10.3390/foods14010064
Chen J-Q, Ma Y-S, Zhou H, Yu R-X, Xiong M, Yang N, Wang J-Q, Tian Y, Su L-Y. Myrica rubra Preharvest Treatment with Melatonin Improves Antioxidant and Phenylpropanoid Pathways During Postharvest Storage. Foods. 2025; 14(1):64. https://doi.org/10.3390/foods14010064
Chicago/Turabian StyleChen, Jun-Quan, Yun-Shuang Ma, Hejiang Zhou, Rui-Xue Yu, Miao Xiong, Na Yang, Ji-Qiu Wang, Yang Tian, and Ling-Yan Su. 2025. "Myrica rubra Preharvest Treatment with Melatonin Improves Antioxidant and Phenylpropanoid Pathways During Postharvest Storage" Foods 14, no. 1: 64. https://doi.org/10.3390/foods14010064
APA StyleChen, J.-Q., Ma, Y.-S., Zhou, H., Yu, R.-X., Xiong, M., Yang, N., Wang, J.-Q., Tian, Y., & Su, L.-Y. (2025). Myrica rubra Preharvest Treatment with Melatonin Improves Antioxidant and Phenylpropanoid Pathways During Postharvest Storage. Foods, 14(1), 64. https://doi.org/10.3390/foods14010064




