Supercritical Extraction and Compound Profiling of Diverse Edible Mushroom Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Sc-CO2 Extraction
2.3. Soxhlet Extraction
2.4. GC–MS Analysis of Sc-CO2 Mushroom Extracts
2.5. GC–FID Analysis
2.6. Chemometric Methods
2.7. Statistical Methods
3. Results
3.1. Sc-CO2 Extraction of Mushrooms
3.2. GC–MS and GC–FID Analysis of Sc-CO2 Mushroom Samples
3.3. Chemometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Sheikha, A.F.; Hu, D.-M. How to Trace the Geographic Origin of Mushrooms? Trends Food Sci. Technol. 2018, 78, 292–303. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom Nutraceuticals for Improved Nutrition and Better Human Health: A Review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef]
- Reis, G.C.L.; Dala-Paula, B.M.; Tavano, O.L.; Guidi, L.R.; Godoy, H.T.; Gloria, M.B.A. In Vitro Digestion of Spermidine and Amino Acids in Fresh and Processed Agaricus Bisporus Mushroom. Food Res. Int. 2020, 137, 109616. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive Components of Mushrooms: Processing Effects and Health Benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.; James, A.; Black, L. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef]
- Samsudin, N.I.P.; Abdullah, N. Edible mushrooms from Malaysia; a literature review on their nutritional and medicinal properties. Int. Food Res. J. 2019, 26, 11–31. [Google Scholar]
- Roncero-Ramos, I.; Mendiola-Lanao, M.; Pérez-Clavijo, M.; Delgado-Andrade, C. Effect of Different Cooking Methods on Nutritional Value and Antioxidant Activity of Cultivated Mushrooms. Int. J. Food Sci. Nutr. 2017, 68, 287–297. [Google Scholar] [CrossRef]
- Rathore, H.; Sehwag, S.; Prasad, S.; Sharma, S. Technological, Nutritional, Functional and Sensorial Attributes of the Cookies Fortified with Calocybe Indica Mushroom. Food Meas. 2019, 13, 976–987. [Google Scholar] [CrossRef]
- Badalyan, S.M.; Barkhudaryan, A.; Rapior, S. Recent Progress in Research on the Pharmacological Potential of Mushrooms and Prospects for Their Clinical Application. In Medicinal Mushrooms; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 1–70. ISBN 9789811363818. [Google Scholar]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic Potential of Mushrooms in Diabetes Mellitus: Role of Polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, M.; Fang, Z. Efficient Physical Extraction of Active Constituents from Edible Fungi and Their Potential Bioactivities: A Review. Trends Food Sci. Technol. 2020, 105, 468–482. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef]
- Vidović, S.; Mujić, I.; Zeković, Z.; Lepojević, Ž.; Milošević, S.; Jokić, S. Extraction of Fatty Acids from Boletus Edulis by Subcritical and Supercritical Carbon Dioxide. J. Am. Oil Chem. Soc. 2011, 88, 1189–1196. [Google Scholar] [CrossRef]
- Jagannath, A.; Biradar, R. Comparative evaluation of soxhlet and ultrasonics on the structural morphology and extraction of bioactive compounds of lemon (Citrus limon L.) peel. J. Food Chem. Nanotechnol. 2019, 5, 56–64. [Google Scholar] [CrossRef]
- Kunene, P.N.; Mahlambi, P.N. Optimization and application of ultrasonic extraction and Soxhlet extraction followed by solid phase extraction for the determination of triazine pesticides in soil and sediment. J. Environ. Chem. Eng. 2020, 8, 103665. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.; Cravotto, G.; Binello, A.; Lorenzo, J.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef]
- Krivošija, S.; Jerković, I.; Nastić, N.; Zloh, M.; Jokić, S.; Banožić, M.; Aladić, K.; Vidović, S. Green pathway for utilisation of orange peel dust and in silico evaluation of pharmacological potential. Microchem. J. 2023, 193, 109132. [Google Scholar] [CrossRef]
- Sökmen, M.; Demir, E.; Alomar, S.Y. Optimization of Sequential Supercritical Fluid Extraction (SFE) of Caffeine and Catechins from Green Tea. J. Supercrit. Fluids 2018, 133, 171–176. [Google Scholar] [CrossRef]
- Živković, J.; Vladić, J.; Naffati, A.; Nastić, N.; Šavikin, K.; Tomić, M.; Vidović, S. Comparative chemical profiling of underexploited Arctostaphylos uva-ursi L. herbal dust extracts obtained by conventional, ultrasound-assisted and subcritical water extractions. Waste Biomass Valorization 2022, 13, 4147–4155. [Google Scholar] [CrossRef]
- Attard, T.M.; Bainier, C.; Reinaud, M.; Lanot, A.; McQueen-Mason, S.J.; Hunt, A.J. Utilisation of Supercritical Fluids for the Effective Extraction of Waxes and Cannabidiol (CBD) from Hemp Wastes. Ind. Crops Prod. 2018, 112, 38–46. [Google Scholar] [CrossRef]
- Nastić, N.; Vasić, A.; Šoronja Simović, D.; Vladić, J.; Jokić, S.; Aladić, K.; Vidović, S. Underutilized Rosa canina Herbal Dust as an Innovative Natural Functional and Health Promoting Ingredient: A Proposal of Two-Novel Approaches. Waste Biomass Valorization 2023, 14, 1207–1217. [Google Scholar] [CrossRef]
- Kavishree, S.; Hemavathy, J.; Lokesh, B.R.; Shashirekha, M.N.; Rajarathnam, S. Fat and Fatty Acids of Indian Edible Mushrooms. Food Chem. 2008, 106, 597–602. [Google Scholar] [CrossRef]
- Gavarić, A.; Vidović, S.; Aladić, K.; Jokić, S.; Vladić, J. Supercritical CO2 Extraction of Marrubium vulgare: Intensification of Marrubiin. RSC Adv. 2021, 11, 9067–9075. [Google Scholar] [CrossRef]
- Vidović, S.; Vasić, A.; Vladić, J.; Jokić, S.; Aladić, K.; Gavarić, A.; Nastić, N. Carbon Dioxide Supercritical Fluid Extracts from Yarrow and Rose Hip Herbal Dust as Valuable Source of Aromatic and Lipophilic Compounds. Sustain. Chem. Pharm. 2021, 22, 100494. [Google Scholar] [CrossRef]
- Vladić, J.; Kovačević, S.; Aladić, K.; Rebocho, S.; Jokić, S.; Podunavac-Kuzmanović, S.; Duarte, A.R.C.; Jerković, I. Novel Insights Into the Recovery and Stabilization of Rosmarinus Officinalis Volatile Aroma Compounds Using Green Solvents. Food Bioprocess Technol. 2023, 17, 1215–1230. [Google Scholar] [CrossRef]
- Vladić, J.; Kovačević, S.; Aladić, K.; Jokić, S.; Radman, S.; Podunavac-Kuzmanović, S.; Duarte, A.R.C.; Jerković, I. Innovative Strategy for Aroma Stabilization Using Green Solvents: Supercritical CO2 Extracts of Satureja Montana Dispersed in Deep Eutectic Solvents. Biomolecules 2023, 13, 1126. [Google Scholar] [CrossRef]
- Mujić, I.; Zeković, Z.; Vidović, S.; Radojković, M.; Živković, J.; Gođevac, D. Fatty acid profiles of four wild mushrooms and their potential benefits for hypertension treatment. J. Med. Food 2011, 14, 1330–1337. [Google Scholar] [CrossRef]
- Statistica (Data Analysis Software System), Version 14.0.0.15; StatSoft, Inc.: Tulsa, OK, USA; Available online: www.statsoft.com (accessed on 26 October 2023).
- NCSS 2023 Statistical Software; NCSS, LLC: Kaysville, UT, USA, 2023; Available online: www.ncss.com/software/ncss (accessed on 26 October 2023).
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Prentice Hall/Pearson: Harlow, UK, 2010; ISBN 9780273730422. [Google Scholar]
- Daszykowski, M.; Walczak, B.; Massart, D.L. Looking for Natural Patterns in Data. Chemom. Intell. Lab. Syst. 2001, 56, 83–92. [Google Scholar] [CrossRef]
- Mishra, J.; Khan, W.; Ahmad, S.; Misra, K. Supercritical Carbon Dioxide Extracts of Cordyceps Sinensis: Chromatography-Based Metabolite Profiling and Protective Efficacy Against Hypobaric Hypoxia. Front. Pharmacol. 2021, 12, 628924. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.S.; De Souza, A.C.S.; Soares, D.C.L.; Lima, C.C.; De Moraes, A.C.R.; Gkionis, S.V.; Arenhart, T.; Rodrigues, L.G.G.; Ferreira, S.R.S.; Pedrosa, R.C.; et al. Chemical Profile, Antimicrobial Potential, and Antiaggregant Activity of Supercritical Fluid Extract from Agaricus Bisporus. Chem. Pap. 2022, 76, 6205–6214. [Google Scholar] [CrossRef]
- Rodríguez-Seoane, P.; Díaz-Reinoso, B.; González-Muñoz, M.J.; Fernández de Ana Portela, C.; Domínguez, H. Innovative Technologies for the Extraction of Saccharidic and Phenolic Fractions from Pleurotus eryngii. LWT 2019, 101, 774–782. [Google Scholar] [CrossRef]
- Koubaa, M.; Lepreux, L.; Barba, F.J.; Mhemdi, H.; Vorobiev, E. Gas Assisted Mechanical Expression (GAME) for the Selective Recovery of Lipophilic and Hydrophilic Compounds from Olive Kernel. J. Clean. Prod. 2017, 166, 387–394. [Google Scholar] [CrossRef]
- Mazzutti, S.; Ferreira, S.R.S.; Riehl, C.A.S.; Smania, A.; Smania, F.A.; Martínez, J. Supercritical Fluid Extraction of Agaricus Brasiliensis: Antioxidant and Antimicrobial Activities. J. Supercrit. Fluids 2012, 70, 48–56. [Google Scholar] [CrossRef]
- Huynh, N.; Beltrame, G.; Tarvainen, M.; Suomela, J.-P.; Yang, B. Supercritical CO2 Extraction of Triterpenoids from Chaga Sterile Conk of Inonotus Obliquus. Molecules 2022, 27, 1880. [Google Scholar] [CrossRef]
- Morales, D.; Gil-Ramirez, A.; Smiderle, F.R.; Piris, A.J.; Ruiz-Rodriguez, A.; Soler-Rivas, C. Vitamin D-Enriched Extracts Obtained from Shiitake Mushrooms (Lentinula Edodes) by Supercritical Fluid Extraction and UV-Irradiation. Innov. Food Sci. Emerg. Technol. 2017, 41, 330–336. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.-M. Plant Sterols: Biosynthesis, Biological Function and Their Importance to Human Nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Mattila, P.; Lampi, A.-M.; Ronkainen, R.; Toivo, J.; Piironen, V. Sterol and Vitamin D2 Contents in Some Wild and Cultivated Mushrooms. Food Chem. 2002, 76, 293–298. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Peralta, R.M.; Bracht, A.; Ferreira, I.C.F.R. The Emerging Use of Mycosterols in Food Industry along with the Current Trend of Extended Use of Bioactive Phytosterols. Trends Food Sci. Technol. 2017, 67, 19–35. [Google Scholar] [CrossRef]
- Li, X.; Wu, Q.; Xie, Y.; Ding, Y.; Du, W.W.; Sdiri, M.; Yang, B.B. Ergosterol Purified from Medicinal Mushroom Amauroderma Rude Inhibits Cancer Growth in Vitro and in Vivo by up-Regulating Multiple Tumor Suppressors. Oncotarget 2015, 6, 17832–17846. [Google Scholar] [CrossRef] [PubMed]
- Villares, A.; García-Lafuente, A.; Guillamón, E.; Ramos, Á. Identification and Quantification of Ergosterol and Phenolic Compounds Occurring in Tuber Spp. Truffles. J. Food Compos. Anal. 2012, 26, 177–182. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Pietrzak, W.; Gawlik-Dziki, U. A New Look at Edible and Medicinal Mushrooms as a Source of Ergosterol and Ergosterol Peroxide—UHPLC-MS/MS Analysis. Food Chem. 2022, 369, 130927. [Google Scholar] [CrossRef]
- Sande, D.; Oliveira, G.P.D.; Moura, M.A.F.E.; Martins, B.D.A.; Lima, M.T.N.S.; Takahashi, J.A. Edible Mushrooms as a Ubiquitous Source of Essential Fatty Acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Papazav, P.; Vassilev, D.; Valchev, N.; Denev, P. Chemical and Lipid Composition of Wild Edible Mushrooms (Morchella esculenta) in Bulgaria. Oxid. Commun. 2020, 43, 194–203. [Google Scholar]
- Erbiai, E.H.; Da Silva, L.P.; Saidi, R.; Lamrani, Z.; Esteves Da Silva, J.C.G.; Maouni, A. Chemical composition, bioactive compounds, and antioxidant activity of two wild edible mushrooms armillaria mellea and macrolepiota procera from two countries(Morocco and portugal). Biomolecules 2021, 11, 575. [Google Scholar] [CrossRef]
- Shao, S.; Hernandez, M.; Kramer, J.K.G.; Rinker, D.L.; Tsao, R. Ergosterol Profiles, Fatty Acid Composition, and Antioxidant Activities of Button Mushrooms as Affected by Tissue Part and Developmental Stage. J. Agric. Food Chem. 2010, 58, 11616–11625. [Google Scholar] [CrossRef]
- Magnan, C.; Levin, B.E.; Luquet, S. Brain Lipid Sensing and the Neural Control of Energy Balance. Mol. Cell. Endocrinol. 2015, 418, 3–8. [Google Scholar] [CrossRef]
- Rajasekaran, A. Nutraceuticals. In Comprehensive Medicinal Chemistry III; Elsevier: Amsterdam, The Netherlands, 2017; pp. 107–134. ISBN 9780128032015. [Google Scholar]
- Moraes, C.D.; Oliveira, C.A.D.; Amaral, M.E.C.D.; Landini, G.A.; Catisti, R. Liver Metabolic Changes Induced by Conjugated Linoleic Acid in Calorie-Restricted Rats. Arch. Endocrinol. Metab. 2017, 61, 45–53. [Google Scholar] [CrossRef]
- Olson, J.M.; Haas, A.W.; Lor, J.; McKee, H.S.; Cook, M.E. A Comparison of the Anti-Inflammatory Effects of Cis-9, Trans-11 Conjugated Linoleic Acid to Celecoxib in the Collagen-Induced Arthritis Model. Lipids 2017, 52, 151–159. [Google Scholar] [CrossRef]
- Balci Yuce, H.; Akbulut, N.; Ocakli, S.; Kayir, O.; Elmastas, M. The Effect of Commercial Conjugated Linoleic Acid Products on Experimental Periodontitis and Diabetes Mellitus in Wistar Rats. Acta Odontol. Scand. 2017, 75, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, Y.; Park, Y. Trans-10, Cis-12 CLA Promotes Osteoblastogenesis via SMAD Mediated Mechanism in Bone Marrow Mesenchymal Stem Cells. J. Funct. Foods 2014, 8, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Guerendiain, M.; Castellote, A.I.; Estruch, R.; Covas, M.I.; Fitó, M.; Salas-Salvadó, J.; Martínez-González, M.A.; Aros, F.; Lamuela-Raventós, R.M.; et al. Plasma Fatty Acid Composition, Estimated Desaturase Activities, and Their Relation with the Metabolic Syndrome in a Population at High Risk of Cardiovascular Disease. Clin. Nutr. 2014, 33, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Librán-Pérez, M.; Pereiro, P.; Figueras, A.; Novoa, B. Antiviral Activity of Palmitic Acid via Autophagic Flux Inhibition in Zebrafish (Danio Rerio). Fish Shellfish Immunol. 2019, 95, 595–605. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Pal, P.K.; Chattopadhyay, A.; Bandyopadhyay, D. Oleic Acid Protects against Cadmium Induced Cardiac and Hepatic Tissue Injury in Male Wistar Rats: A Mechanistic Study. Life Sci. 2020, 244, 117324. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de La Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
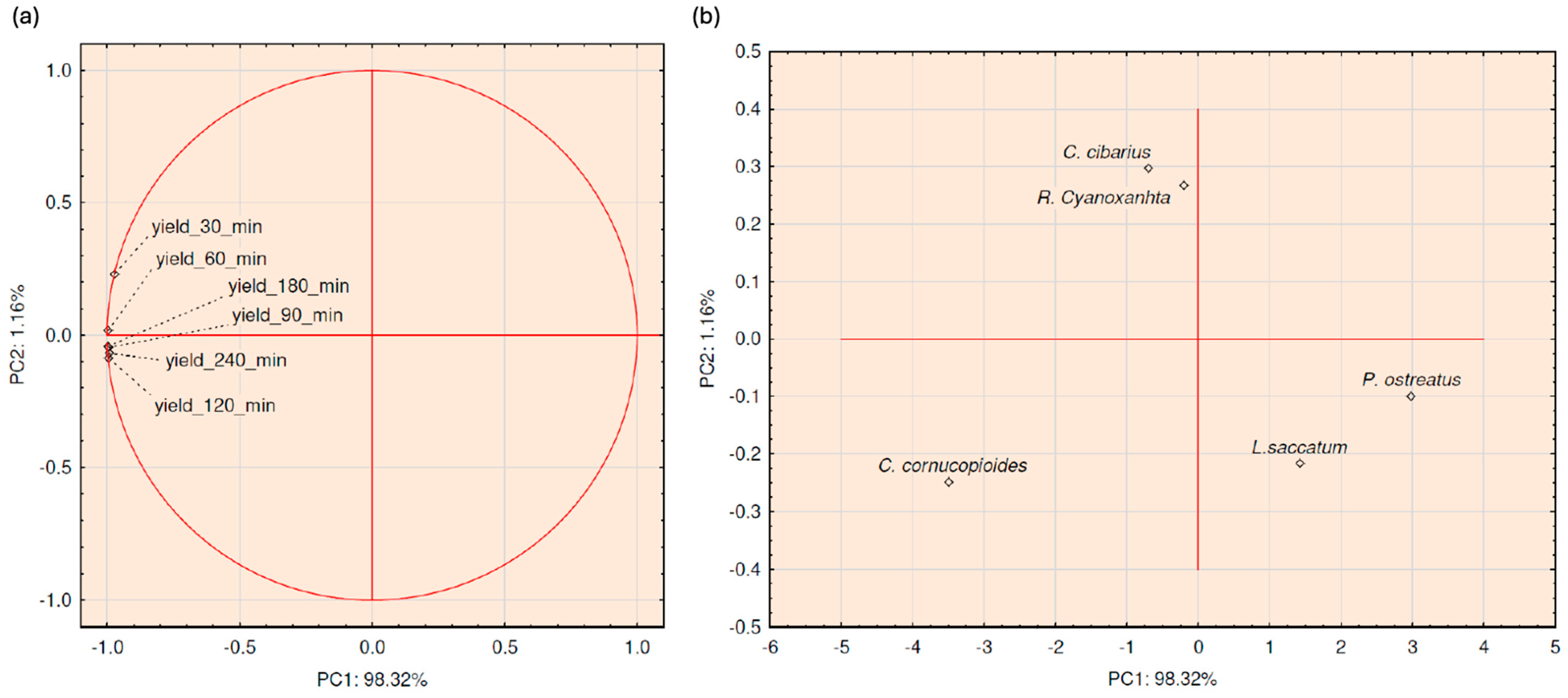
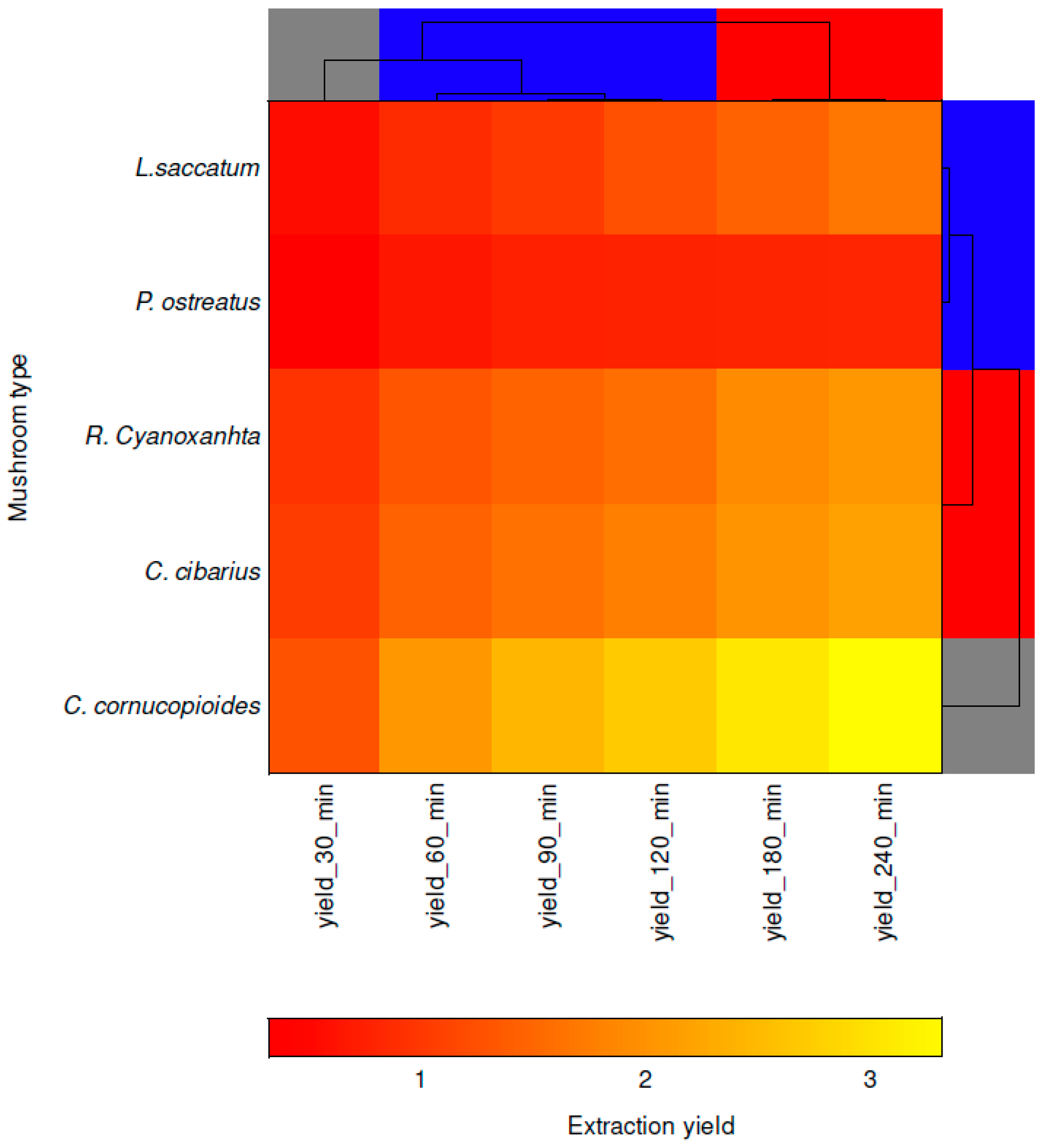
| Type of Extraction | Extraction Time [min] | Extraction Yield [%] | ||||
|---|---|---|---|---|---|---|
| Sample | ||||||
| L. saccatum | P. ostreatus | C. cornucopioides | R. Cyanoxantha | C. cibarius | ||
| Supercritical extraction | 30 | 0.554 ± 0.03 pq | 0.330 ± 0.10 q | 1.272 ± 0.01 j–n | 0.949 ± 0.21 m–p | 1.035 ± 0.11 l–o |
| 60 | 0.870 ± 0.06 nop | 0.646 ± 0.08 opq | 2.077 ± 0.06 def | 1.309 ± 0.19 j–m | 1.443 ± 0.16 i–l | |
| 90 | 1.010 ± 0.14 mno | 0.747 ± 0.23 op | 2.443 ± 0.11 cd | 1.459 ± 0.04 ijk | 1.608 ± 0.08 h–k | |
| 120 | 1.245 ± 0.27 k–n | 0.769 ± 0.71 op | 2.694 ± 0.03 bc | 1.582 ± 0.07 h–k | 1.775 ± 0.03 e–i | |
| 180 | 1.450 ± 0.16 ijk | 0.792 ± 0.85 op | 3.055± 0.05 ab | 1.915 ± 0.12 e–h | 2.028 ± 0.56 efg | |
| 240 | 1.664 ± 0.25 g–j | 0.807 ± 0.61 op | 3.316 ± 0.05 a | 2.061 ± 0.02 d–g | 2.180 ± 0.48 de | |
| Soxhlet extraction | 480 | 1.75 ± 0.20 f–i | 0.83 ± 0.17 op | 3.38 ± 0.06 a | 2.11 ± 0.11 def | 2.47 ± 0.33 cd |
| No. | Compound | Rt | L. saccatum [%] | P. ostreatus [%] | C. cornucopioides [%] | R. Cyanoxantha [%] | C. cibarius [%] |
|---|---|---|---|---|---|---|---|
| 1. | 3-methyl-butanoic acid | 3.866 | traces | - | - | traces | - |
| 2. | hexanoic acid (caproic acid) | 7.300 | traces | - | - | - | 0.338 |
| 3. | pantolactone | 10.281 | - | - | - | 0.184 | - |
| 4. | phenylethyl alcohol | 11.983 | - | - | - | 0.203 | - |
| 5. | succinimide | 12.709 | - | - | - | - | 0.298 |
| 6. | 2,4-dimethyl penthanal | 14.322 | - | 1.087 | - | - | - |
| 7. | benzoic acid | 14.738 | traces | - | - | 0.540 | - |
| 8. | 6,6-dimethyl 2,4-heptanedione | 15.565 | - | 0.322 | - | - | - |
| 9. | isothiocyanato cyclohexane | 17.107 | traces | - | - | traces | traces |
| 10. | benzeneacetic acid | 17.981 | traces | - | - | 1.077 | traces |
| 11. | 2-methyl-3,5-dodecadiyne | 18.608 | - | - | 0.154 | - | - |
| 12. | benzamide | 21.575 | 0.237 | - | - | - | - |
| 13. | 2-methyl crotonic acid | 22.212 | - | 5.234 | - | - | - |
| 14. | 2-methyl-2-penten-1-ol | 23.248 | - | 1.139 | - | - | - |
| 15. | decanoic acid (capric acid) | 23.307 | - | - | - | 0.164 | - |
| 16. | 2-methyl-1-penten-3-ol | 24.232 | - | 0.213 | - | - | - |
| 17. | cinnamic acid | 25.827 | traces | - | - | 0.160 | - |
| 18. | 5-pentyl resorcinol | 29.886 | - | 0.332 | - | - | - |
| 19. | dodecanoic acid (lauric acid) | 31.555 | 0.188 | - | - | 0.375 | 0.200 |
| 20. | tetradecanoic acid (myristic acid) | 39.142 | 0.253 | - | - | 0.349 | - |
| 21. | pentadecanoic acid | 42.771 | 1.323 | 1.012 | 0.479 | 0.487 | 0.298 |
| 22. | palmitoleic acid | 45.801 | - | - | - | 3.517 | - |
| 23. | palmitic acid | 46.816 | 8.398 | 6.736 | 12.259 | 8.615 | 15.613 |
| 24. | oleic acid + linoleic acid | 53.066 | 64.796 | 57.544 | 47.848 | 61.891 | 55.581 |
| 25. | stearic acid | 53.440 | 3.345 | 2.354 | 6.281 | 2.033 | 4.740 |
| 26. | 10,13-octadecadiynoic acid | 54.143 | - | - | 24.214 | - | 3.436 |
| 27. | 6-oxo-octadecanoic acid | 58.495 | - | - | - | 5.096 | - |
| 28. | methyl 10,13-octadecadiynoate | 61.366 | - | - | 0.554 | - | 0.492 |
| 29. | α- glyceryl linoleate | 74.650 | - | 0.321 | - | - | - |
| 30. | cis permethrin * | 67.628 | - | 0.300 | - | - | traces |
| 31. | trans permethrin * | 68.077 | - | 0.643 | - | - | 0.285 |
| 32. | anthraergostateraenol | 78.660 | - | 0.297 | 0.103 | - | - |
| 33. | ergosterol | 79.519 | 1.707 | 2.412 | 0.318 | 1.319 | 0.717 |
| 34. | 7,22-ergostadienol | 79.763 | 2.162 | - | - | - | - |
| 35. | ergosta-4,6,8(14),22-tetraen-3-one | 83.266 | - | - | - | - | 0.173 |
| 36. | 7,22-ergostadienone | 80.463 | 3.580 | - | - | - | 0.299 |
| No. | Compound | Rt | L. saccatum [%] | P. ostreatus [%] | C. cornucopioides [%] | R. Cyanoxantha [%] | C. cibarius [%] |
|---|---|---|---|---|---|---|---|
| 1. | 12:0 | 6.00 | 0.30 | - | 0.13 | 0.73 | - |
| 2. | 14:0 | 8.07 | 0.32 | 0.12 | 0.11 | - | - |
| 3. | 15:0 | 9.36 | 1.55 | 0.31 | 0.43 | 0.89 | - |
| 4. | 16:0 | 10.76 | 9.57 | 10.21 | 10.48 | 12.29 | 15.22 |
| 5. | 16:1 t11 | 11.21 | - | 0.25 | - | - | - |
| 6. | 16:1 c9 | 11.33 | 0.40 | 0.61 | 0.41 | 3.25 | 0.66 |
| 7. | 17:0 | 11.60 | 0.77 | - | - | - | 0.80 |
| 8. | 18:0 | 13.89 | 6.16 | 2.96 | 5.76 | 2.99 | 4.51 |
| 9. | 18:1 t9 a | 14.20 | - | 0.74 | - | - | - |
| 10. | 18:1 c9 | 14.36 | 6.64 | 40.25 | 26.44 | 27.99 | 9.80 |
| 11. | 18:1 b | 14.54 | 1.22 | 1.50 | 0.36 | 1.93 | 22.23 |
| 12. | 18:2 c10c13 | 15.09 | - | - | 0.34 | - | - |
| 13. | 18:2 c9c12 c | 15.24 | 67.69 | 40.37 | 25.41 | 41.61 | 34.61 |
| 14. | 18:3 c6c9c12 | 16.00 | - | 0.27 | - | - | - |
| 15. | 20:0 | 17.20 | - | - | 0.11 | - | - |
| 16. | 20:1 c11 | 17.70 | - | 0.31 | 0.25 | - | - |
| 17. | A d | 18.78 | - | - | 24.05 | - | 5.29 |
| 18. | 22:0 | 20.29 | 0.36 | 0.13 | - | - | - |
| 19. | 23:0 | 21.83 | - | - | - | 0.66 | - |
| 20. | 24:0 | 23.33 | - | - | 0.18 | 1.65 | 4.47 |
| 21. | B e | 24.44 | - | - | - | 3.33 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivošija, S.; Nastić, N.; Karadžić Banjac, M.; Kovačević, S.; Podunavac-Kuzmanović, S.; Vidović, S. Supercritical Extraction and Compound Profiling of Diverse Edible Mushroom Species. Foods 2025, 14, 107. https://doi.org/10.3390/foods14010107
Krivošija S, Nastić N, Karadžić Banjac M, Kovačević S, Podunavac-Kuzmanović S, Vidović S. Supercritical Extraction and Compound Profiling of Diverse Edible Mushroom Species. Foods. 2025; 14(1):107. https://doi.org/10.3390/foods14010107
Chicago/Turabian StyleKrivošija, Slađana, Nataša Nastić, Milica Karadžić Banjac, Strahinja Kovačević, Sanja Podunavac-Kuzmanović, and Senka Vidović. 2025. "Supercritical Extraction and Compound Profiling of Diverse Edible Mushroom Species" Foods 14, no. 1: 107. https://doi.org/10.3390/foods14010107
APA StyleKrivošija, S., Nastić, N., Karadžić Banjac, M., Kovačević, S., Podunavac-Kuzmanović, S., & Vidović, S. (2025). Supercritical Extraction and Compound Profiling of Diverse Edible Mushroom Species. Foods, 14(1), 107. https://doi.org/10.3390/foods14010107











