Factors Affecting Growth and Survival of Salmonella in Onion Extracts and Onion Bulbs
Abstract
1. Introduction
2. Materials and Methods
2.1. Antimicrobial Effect of Onion Extracts on Salmonella Survival and Growth
2.1.1. Salmonella Strains and Inoculum Preparation
2.1.2. Onion Extract Preparation
2.1.3. Microtiter Broth Dilution Method
2.2. Fate of Salmonella Serotype Newport on Red, White, and Yellow Onion Varieties
2.2.1. Salmonella Newport Strains and Inoculum Preparation
2.2.2. Procurement and Inoculation of Onion Bulbs
2.2.3. Sample Collection and Salmonella Enumeration
2.3. Measurement of Water Activity
2.4. Statistical Analysis
3. Results
3.1. Antimicrobial Effect of Onion Extracts on Salmonella Survival and Growth
3.1.1. Effect of Various Exposed Lights During Onion Growth on the Inhibition of Salmonella by Extracts of Red, White, and Yellow Onion Varieties
3.1.2. Impact of Various Onion Genotypes and Their Layers on Salmonella Growth
3.2. The Fate of Salmonella Serotype Newport on Yellow, White, and Red Onion Bulbs During Storage at Room Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brummell, D.A.; Toivonen, P.M.A. Postharvest Physiology of Vegetables. In Handbook of Vegetables and Vegetable Processing; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 223–245. [Google Scholar]
- Nile, S.H.; Nile, A.S.; Keum, Y.S.; Sharma, K. Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease, and xanthine oxidase inhibitors. Food Chem. 2017, 235, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Majid, I.; Hussain, S.; Nanda, V. Impact of sprouting on the degradation kinetics of color and vitamin C of onion powder packaged in different packaging materials. JFPP 2018, 43, e138. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones and flavanols—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Islam, M.; Doyle, M.P.; Phatak, S.C.; Millner, P.; Jiang, X. Survival of Escherichia coli O157:H7 in soil and on carrots and onions grown in fields treated with contaminated manure composts or irrigation water. Food Microbiol. 2005, 22, 63–70. [Google Scholar] [CrossRef]
- Emch, A.W.; Waite-Cusic, J.G. Conventional curing practices reduce generic Escherichia coli and Salmonella spp. on dry bulb onions produced with contaminated irrigation water. Food Microiol. 2016, 53, 41–47. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial Contamination of Fresh Produce: What, Where, and How? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef]
- Kianian, F.; Marefati, N.; Boskabady, M.; Ghasemi, S.Z.; Boskabady, M.H. Pharmacological properties of Allium cepa, preclinical and clinical evidences; A review. Iran J. Pharm. Res. 2021, 20, 107–134. [Google Scholar] [CrossRef]
- Prakash, D.; Singh, B.N. Upadhyay, Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chem. 2007, 102, 1389–1393. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Gonzalez-Aguilar, G.A. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: A comparative study of fifteen Indian cultivars. J. Food Sci. Technol. 2020, 57, 2423–2432. [Google Scholar] [CrossRef]
- Lombard, K.; Peffley, E.; Geoffriau, E.; Thompson, L.; Herring, A. Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation. J. Food Compos. Anal. 2005, 18, 571–581. [Google Scholar] [CrossRef]
- Jang, M.; Asnin, L.; Nile, S.H.; Keum, Y.S.; Kim, H.Y.; Park, S.W. Ultrasound-assisted extraction of quercetin from onion solid wastes. Int. J. Food Sci. Tech. 2013, 48, 246–252. [Google Scholar] [CrossRef]
- Ioku, K.; Aoyama, Y.; Tokuno, A.; Terao, J.; Nakatani, N.; Takei, Y. Various cooking methods and the flavonoid content in onion. J. Nutr. Sci. Vitaminol. 2001, 47, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Islek, M.; Nilufer-Erdil, D.; Knuthsen, P. Changes in flavonoids of sliced and fried yellow onions (Allium cepa L. var. zittauer) during storage at different atmospheric, temperature, and light conditions. J. Food Proc. Preserv. 2015, 39, 357–368. [Google Scholar] [CrossRef]
- Benett, S.D.; Sodha, S.V.; Ayers, T.L.; Lynch, M.F.; Gould, L.H.; Tauxe, R.V. Produce-associated foodborne disease outbreaks, USA, 1998–2013. Epidemiol. Infect. 2018, 146, 1397–1406. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Lee, O.-H.; Lee, B.-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.R.; Kirchner, M.; Schneider, B.; McClure, M.; Neil, K.P.; Madad, A.; Jemaneh, T.; Tijerina, M.; Nolte, K.; Wellman, A.; et al. Multistate outbreak of Salmonella Oranienburg infections linked to bulb onions imported from Mexico—United States, 2021. Food Cont. 2024, 160, 110325. [Google Scholar] [CrossRef]
- Sandquist, E.L. Effect of Simulated Storage and Distribution on Listeria innocua Growth in Non-traditional Salad Ingredients. Master’s Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, January 2021. [Google Scholar]
- McCormic, Z.D.; Patel, K.; Higa, J.; Bancroft, J.; Donovan, D.; Edwards, L.; Cheng, J.; Adcock, B.; Bond, C.; Pereira, E.; et al. Bi-national outbreak of Salmonella Newport infections linked to onions: The United States experience. Epidemiol. Infect. 2022, 150, e199. [Google Scholar] [CrossRef] [PubMed]
- Commichaux, S.; Rand, H.; Javkar, K.; Molloy, E.K.; Pettengill, J.B.; Pightling, A.; Hoffmann, M.; Pop, M.; Jayeola, V.; Foley, S.; et al. Assessment of plasmids for relating the 2020 Salmonella enterica serovar Newport onion outbreak to farms implicated by the outbreak investigation. BMC Genom. 2023, 24, 165. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions. J. Food Drug Anal. 2018, 26, 518–528. [Google Scholar] [CrossRef]
- Brandt, A.L.; Castillo, A.; Harris, K.B.; Keeton, J.T.; Hardin, M.D.; Taylor, T.M. Inhibition of Listeria monocytogenes by food antimicrobials applied singly and in combination. J. Food Sci. 2010, 75, M557–M563. [Google Scholar] [CrossRef]
- Branen, J.K.; Davidson, P.M. Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int. J. Food Microbiol. 2004, 90, 63–74. [Google Scholar] [CrossRef] [PubMed]
- USDA Food Safety and Inspection Service. Isolation and Identification of Salmonella from Meat, Poultry, Pasteurized Egg, Siluriformes (Fish) Products and Carcass and Environmental Sponges. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/documents/MLG-4.14.pdf (accessed on 12 March 2024).
- Herrmann, K. Flavonols and flavones in food plants: A review. Int. Food Sci. Technol. 1976, 11, 433–448. [Google Scholar] [CrossRef]
- Haytowitz, D.B.; Bhagwat, S.; Holden, J.M. Sources of variability in the flavonoid content of foods. Procedia Food Sci. 2013, 2, 46–51. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of quercetin rich onion extracts on bacterial quorum sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Xu Shen, X. d-Alanine:d-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef]
- Roso, R.; Nunes, U.R.; Müller, C.A.; Paranhos, J.T.; Lopes, S.J.; Dornelles, S.H.B.; Bertagnolli, C.M.; Huth, C.; Forte, C.T.; Menegaes, J.F. Light quality and dormancy overcoming in seed germination of Echium plantagineum L. (Boraginaceae). Braz. J. Biol. 2021, 81, 650–656. [Google Scholar] [CrossRef]
- Ko, E.Y.; Nile, S.H.; Sharma, K.; Li, G.H.; Park, S.W. Effect of different exposed lights on quercetin and quercetin glucoside content in onion (Allium cepa L.). Saudi J. Biol. Sci. 2015, 22, 398–403. [Google Scholar] [CrossRef]
- Grace, S.C.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Phil. Trans. R. Soc. Lond. 2000, 355, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Bumah, V.V.; Masson-Meyers, D.S.; Enwemeka, C.S. Blue 470 nm light suppresses the growth of Salmonella enterica and methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg. Med. 2015, 47, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, Y.; Moraru, C.I. Blue 405 nm LED light effectively inactivates bacterial pathogens on substrates and packaging materials used in food processing. Sci. Rep. 2023, 13, 15472. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, D.; Lucarelli, V.; De Palma, I.; Puccio, A.; Nante, N.; Cevenini, G.; Messina, G. Efficacy of violet–blue light to inactive microbial growth. Sci. Rep. 2022, 12, 20179. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Luo, S.; Wu, J.; Sun, X.; Liu, M.; Wang, X.; Wang, X. Enhanced sensitivity of Salmonella to antimicrobial blue light caused by inactivating rfaC gene involved in lipopolysaccharide biosynthesis. Foodborne Pathog. Dis. 2021, 18, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Mengarda Buosi, D.T.; de Moraes, J.O.; Cheng, Y.; Cheng, R.A.; Moraru, C.I.; Carciofi, B.A.M. Effective pulsed light treatments for inactivating Salmonella enterica serotypes. Food Cont. 2022, 135, 108776. [Google Scholar] [CrossRef]
- Prasad, A.; Gänzle, M.; Roopesh, M.S. Understanding the Salmonella inactivation mechanisms of 365, 395 and 455 nm light pulses emitted from light-emitting Diodes. Appl. Sci. 2023, 13, 1501. [Google Scholar] [CrossRef]
- Ramos, F.A.; Takaishi, Y.; Shirotori, M.; Kawaguchi, Y.; Tsuchiya, K.; Shibata, H.; Higuti, T.; Tadokoro, T.; Takeuchi, M. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) Skin. J. Agric. Food Chem. 2006, 54, 3551–3557. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Park, S.Y. Impact of quercetin against Salmonella Typhimurium biofilm formation on food-contact surfaces and molecular mechanism pattern. Foods 2022, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Chadorshabi, S.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red onion skin active ingredients, extraction and biological properties for functional food applications. Food Chem. 2022, 386, 132737. [Google Scholar] [CrossRef]
- Brandl, M. Plant lesions promote the rapid multiplication of Escherichia coli O157: H7 on postharvest lettuce. Appl. Environ. Microbiol. 2008, 74, 5285–5289. [Google Scholar] [CrossRef]
- Semenov, A.M.; Kuprianov, A.A.; Van Bruggen, A.H. Transfer of enteric pathogens to successive habitats as part of microbial cycles. Microb. Ecol. 2010, 60, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.A.; Lee, D.H.; Heu, S. The interaction of human enteric pathogens with plants. Plant Pathol. J. 2014, 30, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Belo, T.; Du Toit, L.J.; Lahue, G.T. Reducing the risk of onion bacterial diseases: A review of cultural management strategies. Agron. J. 2023, 115, 459–473. [Google Scholar] [CrossRef]
- Gorshkov, V.; Tsers, I. Plant susceptible responses: The underestimated side of plant-pathogen interactions. Biol. Rev. Camb. Philos. Soc. 2022, 97, 45–66. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef]
- Lieberman, V.M.; Zhao, I.Y.; Schaffner, D.W.; Danyluk, M.D.; Harris, L.J. Survival or growth of inoculated Escherichia coli O157:H7 and Salmonella on yellow onions (Allium cepa) under conditions simulating food service and consumer handling and storage. J. Food Prot. 2015, 78, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, V.M.; Harris, L.J. Fate of inoculated Listeria monocytogenes on yellow onions (Allium cepa) under conditions simulating food service and consumer handling and storage. Food Cont. 2019, 96, 375–382. [Google Scholar] [CrossRef]
- Masfria, G.H.; Mierza, V. Analysis chemical compounds and antimicrobial activity of red onion (Allium cepa L.) bulb skin extract. RASAYAN J. Chem. 2019, 12, 1002–1010. [Google Scholar] [CrossRef]
- Mardani, N.; Jahadi, M.; Sadeghian, M.; Keighobadi, K.; Khosravi-Darani, K. Antimicrobial activities, phenolic and flavonoid contents, antioxidant and DNA protection of the internal and outer layers of Allium cepa L. from Iran. NFS J. 2023, 31, 93–101. [Google Scholar] [CrossRef]
- Khiari, Z.; Makris, D.P. Stability and transformation of major flavonols in onion (Allium cepa) solid wastes. J. Food Sci. Technol. 2012, 49, 489–494. [Google Scholar] [CrossRef]
- Santiago, B.; Arias Calvo, A.; Gullón, B.; Feijoo, G.; Moreira, M.T.; González-García, S. Production of flavonol quercetin and fructooligosaccharides from onion (Allium cepa L.) waste: An environmental life cycle approach. Chem. Eng. J. 2020, 392, 123772. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Benkeblia, N.; Xiao, J. Onion (Allium cepa L.) bioactives: Chemistry, pharmacotherapeutic functions, and industrial applications. Food Front. 2022, 3, 380–412. [Google Scholar] [CrossRef]
- Škerget, M.; Majhenič, L.; Bezjak, M.; Knez, Ž. Antioxidant, radical scavenging and antimicrobial activities of red onion (Allium cepa L) skin and edible part extracts. Chem. Biochem. Eng. Q. 2009, 23, 435–444. Available online: https://hrcak.srce.hr/en/45385 (accessed on 12 November 2024).
- Kim, S.; Kim, G.-H. Quantification of quercetin in different parts of onion and its DPPH radical scavenging and antibacterial activity. Food Sci. Biotechnol. 2006, 15, 39–43. [Google Scholar]
- Mark, G.L.; Gitaitis, R.D.; Lorbeer, J.W. Bacterial Diseases of Onion. In Allium Crop Science: Recent Advances; Rabinowitch, H.D., Currah, L., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 267–292. [Google Scholar]
- Van Linden, V.; Scheerlinck, N.; Desmet, M.; De Baerdemaeker, J. Factors that affect tomato bruise development as a result of mechanical impact. Postharvest Biol. Technol. 2006, 42, 260–270. [Google Scholar] [CrossRef]
- Aliasgarian, S.; Ghassemzadeh, S.H.R.; Moghaddam, H.R.M.; Ghaffari, H. Mechanical damage of strawberry during harvest and postharvest operations. World Appl. Sci. J. 2013, 22, 969–974. [Google Scholar] [CrossRef]
- Tokarskyy, O.; De, J.; Fatica, M.K.; Brecht, J.; Schneider, K.R. Survival of Escherichia coli O157:H7 and Salmonella on bruised and unbruised tomatoes from three ripeness stages at two temperatures. J. Food Prot. 2018, 81, 2028–2033. [Google Scholar] [CrossRef]
- Wells, J.M.; Butterfield, J.E. Incidence of Salmonella on fresh fruits and vegetables affected by fungal rots or physical injury. Plant Dis. 1999, 83, 722–726. [Google Scholar] [CrossRef]
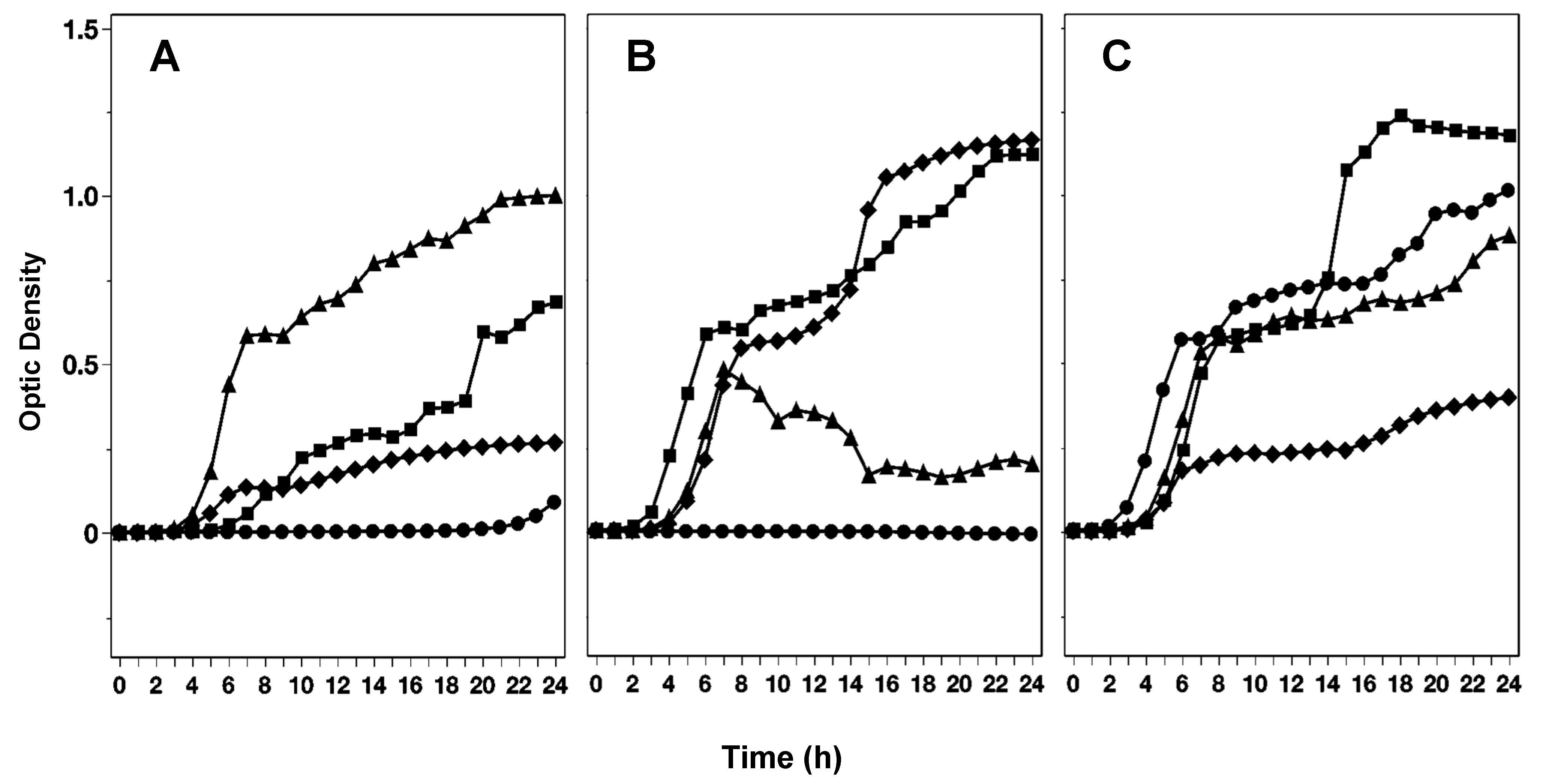
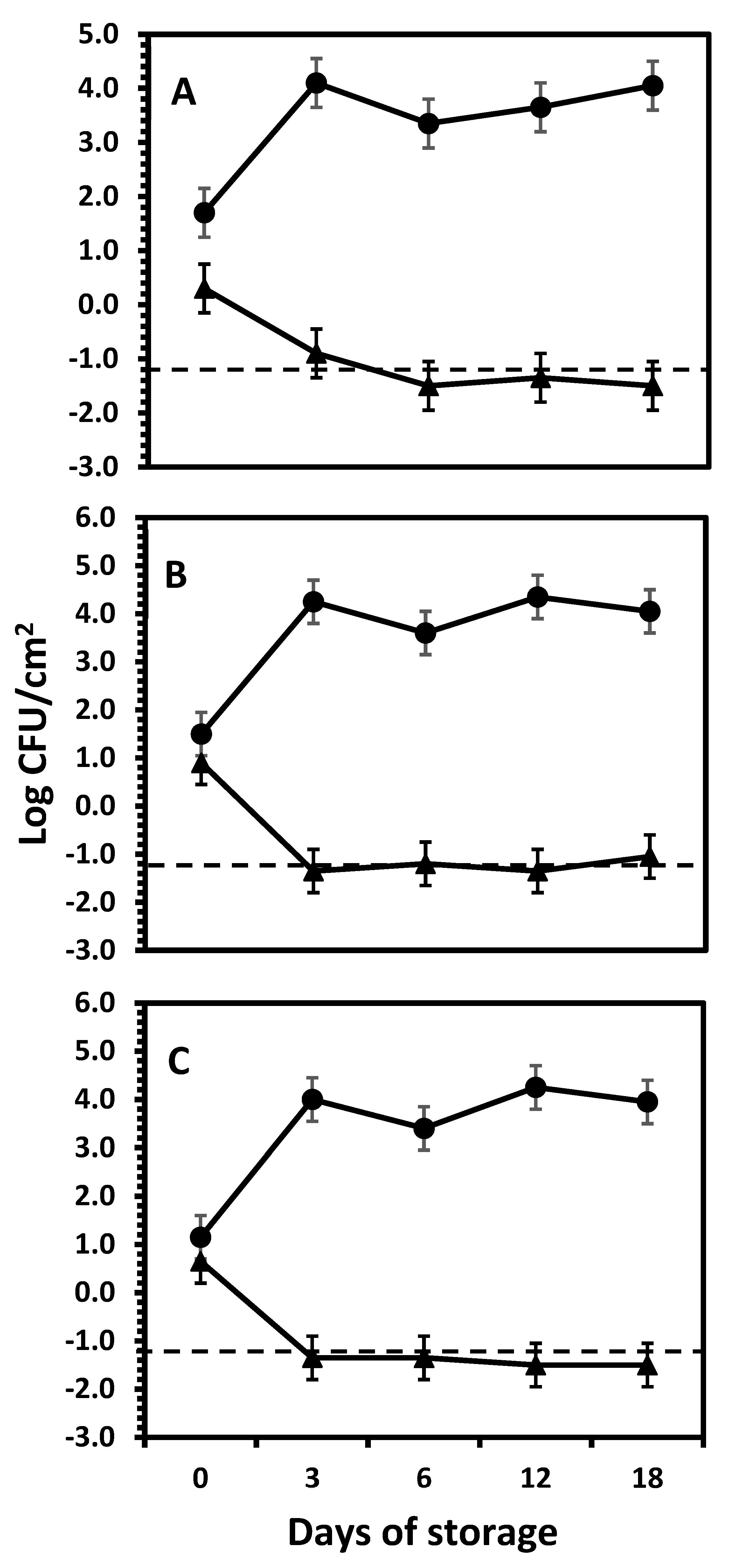
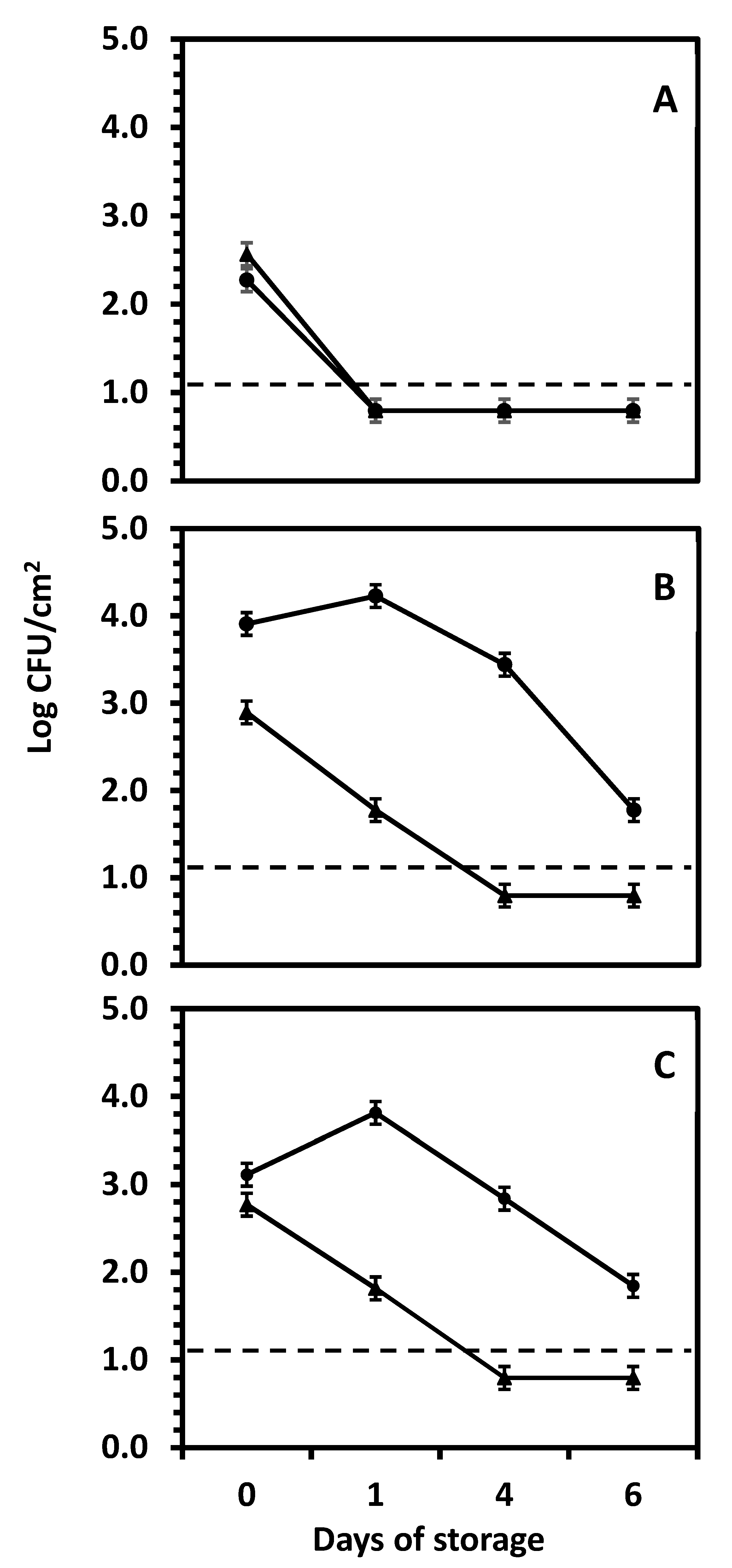
| Mean ∆OD a ± STDEV by Location of Scales Used for Extracts | ||
|---|---|---|
| Genotype | Outer | Inner |
| 25X | 0.62 ± 0.11 AB b | 0.97 ± 0.35 AB |
| 218Y | 0.85 ± 0.38 AB | 0.67 ± 0.02 AB |
| 231Y | 0.60 ± 0.02 AB | 0.70 ± 0.22 AB |
| 541X | 0.76 ± 0.20 AB | 0.68 ± 0.04 AB |
| 542 | 0.93 ± 0.38 AB | 0.33 ± 0.47 AB |
| 544X | 0.82 ± 0.01 AB | 0.80 ± 0.01 AB |
| 545X | 0.88 ± 0.43 A | 1.14 ± 0.03 AB |
| 1102 | 0.83 ± 0.37 AB | 1.08 ± 0.02 A |
| 1104 | −0.0025 ± 0.01 B | 0.26 ± 0.36 AB |
| 1196 | 0.64 ± 0.03 AB | 0.92 ± 0.34 AB |
| 1197 | 0.87 ± 0.35 AB | 0.58 ± 0.06 AB |
| YELLOW H6 | 0.67 ± 0.03 A | 1.17 ± 0.03 AB |
| Layer | |||||
|---|---|---|---|---|---|
| Variety | Outer Papery | 1st After Outer | 2nd After Outer | 3rd After Outer | 4th After Outer |
| Red | 0.509 ± 0.032 A a | 0.764 ± 0.023 C | 0.836 ± 0.023 G | 0.897 ± 0.024 E | 0.961 ± 0.011 F b |
| White | 0.583 ± 0.003 B | 0.904 ± 0.042 E | 0.909 ± 0.001 E | 0.928 ± 0.003 E | 0.997 ± 0.003 G |
| Yellow | 0.525 ± 0.011 A | 0.863 ± 0.027 D | 0.895 ± 0.017 E | 0.921 ± 0.015 E | 0.972 ± 0.001 F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiplagat, E.; Ramezani, M.; Malla, S.; Cisneros-Zevallos, L.; Joshi, V.; Castillo, A. Factors Affecting Growth and Survival of Salmonella in Onion Extracts and Onion Bulbs. Foods 2025, 14, 1. https://doi.org/10.3390/foods14010001
Kiplagat E, Ramezani M, Malla S, Cisneros-Zevallos L, Joshi V, Castillo A. Factors Affecting Growth and Survival of Salmonella in Onion Extracts and Onion Bulbs. Foods. 2025; 14(1):1. https://doi.org/10.3390/foods14010001
Chicago/Turabian StyleKiplagat, Emmanuel, Moazzameh Ramezani, Subas Malla, Luis Cisneros-Zevallos, Vijay Joshi, and Alejandro Castillo. 2025. "Factors Affecting Growth and Survival of Salmonella in Onion Extracts and Onion Bulbs" Foods 14, no. 1: 1. https://doi.org/10.3390/foods14010001
APA StyleKiplagat, E., Ramezani, M., Malla, S., Cisneros-Zevallos, L., Joshi, V., & Castillo, A. (2025). Factors Affecting Growth and Survival of Salmonella in Onion Extracts and Onion Bulbs. Foods, 14(1), 1. https://doi.org/10.3390/foods14010001







