Identifying Variables Influencing Traditional Food Solid-State Fermentation by Statistical Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation, Online Measurements and Chemical Analyses
2.2. Parameter Definitions
2.3. Statistical Analysis
2.3.1. Multiple Regression
2.3.2. Classifier Model
3. Results and Discussion
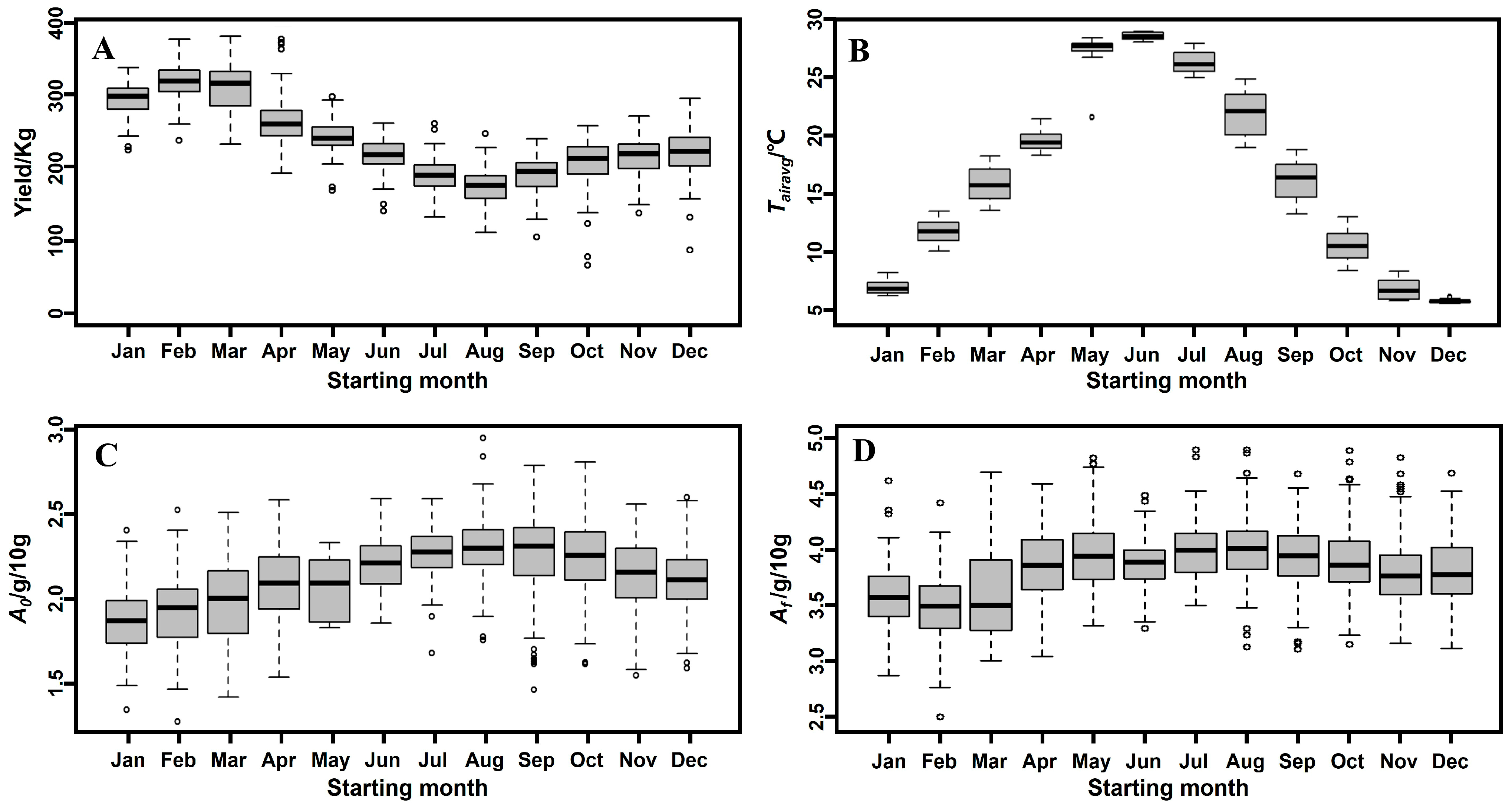
3.1. Data Distribution
3.2. Descriptive Statistics
3.3. Correlation of Yield with All Variables except Final Contents
- -
- The initial acid content ( ) has a very significant negative effect (p < 0.001) in combination with other variables.
- -
- The maximum substrate temperature has a significant positive coefficient. This finding indicates that no overheating occurred. As discussed earlier, this result does not indicate that heating stimulates ethanol production.
3.4. Model Based on Initial Conditions
3.5. Model Based on Temperature Variables
3.6. Model Based on Starting Month
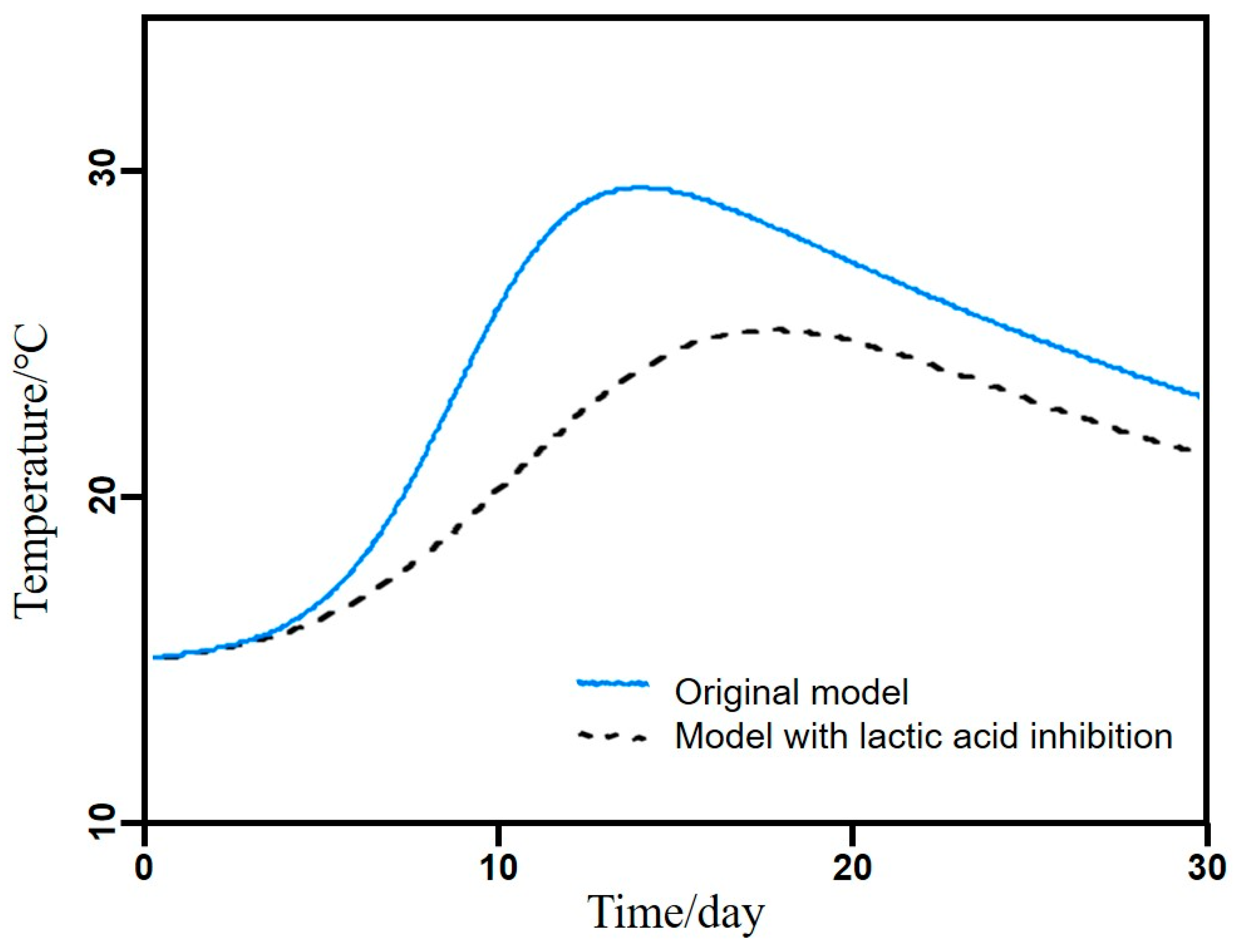
3.7. Explaining the Seasonal Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karabulut, G.; Kapoor, R.; Feng, H. Biotransformation approaches using solid-state fermentation and germination with high-intensity ultrasound to produce added-value hemp protein nanoaggregates. Food Biosci. 2023, 56, 13. [Google Scholar] [CrossRef]
- Holker, U.; Lenz, J. Solid-state fermentation—Are there any biotechnological advantages? Curr. Opin. Microbiol. 2005, 8, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Liu, J.L.; Li, P.L.; Zhao, W.; Zhang, A.X.; Liu, S.Y.; Wang, Z.X.; Liu, J.K. Flavor production driven by microbial community dynamics and their interactions during two-stage fermentation of Ziziphus jujuba vinegar. Food Biosci. 2024, 57, 13. [Google Scholar] [CrossRef]
- Pang, Z.M.; Hao, J.; Li, W.W.; Du, B.H.; Guo, C.H.; Li, X.T.; Sun, B.G. Investigation into spatial profile of microbial community dynamics and flavor metabolites during the bioaugmented solid-state fermentation of Baijiu. Food Biosci. 2023, 56, 11. [Google Scholar] [CrossRef]
- Jin, G.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Fan, J.Y.; Qu, G.Y.; Wang, D.T.; Chen, J.; Du, G.C.; Fang, F. Synergistic Fermentation with Functional Microorganisms Improves Safety and Quality of Traditional Chinese Fermented Foods. Foods 2023, 12, 2892. [Google Scholar] [CrossRef]
- Jin, G.; Zhu, Y.; Rinzema, A.; Wijffels, R.H.; Ge, X.Y.; Xu, Y. Water dynamics during solid-state fermentation by Aspergillus oryzae YH6. Bioresour. Technol. 2019, 277, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Zhu, J.; Wu, B.; Zhao, C.; Sun, J.; Dai, C.X. Discrimination of Chinese Liquors Based on Electronic Nose and Fuzzy Discriminant Principal Component Analysis. Foods 2019, 8, 38. [Google Scholar] [CrossRef]
- Jin, G.; Uhl, P.; Zhu, Y.; Wijffels, R.H.; Xu, Y.; Rinzema, A. Modeling of industrial-scale anaerobic solid-state fermentation for Chinese liquor production. Chem. Eng. J. 2020, 394, 124942. [Google Scholar] [CrossRef]
- Laluce, C.; Igbojionu, L.I.; Silva, J.L.; Ribeiro, C.A. Statistical prediction of interactions between low concentrations of inhibitors on yeast cells responses added to the SD-medium at low pH values. Biotechnol. Biofuels 2019, 12, 10. [Google Scholar] [CrossRef]
- Bicciato, S.; Bagno, A.; Solda, M.; Manfredini, R.; Di Bello, C. Fermentation diagnosis by multivariate statistical analysis. Appl. Biochem. Biotechnol. 2002, 102, 49–62. [Google Scholar] [CrossRef]
- Kennedy, M.; Krouse, D. Strategies for improving fermentation medium performance: A review. J. Ind. Microbiol. Biotechnol. 1999, 23, 456–475. [Google Scholar] [CrossRef]
- Doan, X.T.; Srinivasan, R.; Bapat, P.M.; Wangikar, P.P. Detection of phase shifts in batch fermentation via statistical analysis of the online measurements: A case study with rifamycin B fermentation. J. Biotechnol. 2007, 132, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Besenhard, M.O.; Scheibelhofer, O.; Francois, K.; Joksch, M.; Kavsek, B. A multivariate process monitoring strategy and control concept for a small-scale fermenter in a PAT environment. J. Intell. Manuf. 2018, 29, 1501–1514. [Google Scholar] [CrossRef]
- Wang, K.; Rippon, L.; Chen, J.H.; Song, Z.H.; Gopaluni, R.B. Data-Driven Dynamic Modeling and Online Monitoring for Multiphase and Multimode Batch Processes with Uneven Batch Durations. Ind. Eng. Chem. Res. 2019, 58, 13628–13641. [Google Scholar] [CrossRef]
- Luo, L.J.; Bao, S.Y. Knowledge-data-integrated sparse modeling for batch process monitoring. Chem. Eng. Sci. 2018, 189, 221–232. [Google Scholar] [CrossRef]
- Zheng, X.W.; Tabrizi, M.R.; Nout, M.J.R.; Han, B.Z. Daqu—A Traditional Chinese Liquor Fermentation Starter. J. Inst. Brew. 2011, 117, 82–90. [Google Scholar] [CrossRef]
- Hageman, J.A.; Engel, B.; de Vos, R.C.H.; Mumm, R.; Hall, R.D.; Jwanro, H.; Crouzillat, D.; Spadone, J.C.; van Eeuwijk, F.A. Robust and Confident Predictor Selection in Metabolomics. In Statistical Analysis of Proteomics, Metabolomics, and Lipidomics Data Using Mass Spectrometry; Datta, S., Mertens, B.J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 239–257. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Wu, H.X.; Deng, Z.H.; Zhang, B.J.; Liu, Q.Y.; Chen, J.Y. Classifier Model Based on Machine Learning Algorithms: Application to Differential Diagnosis of Suspicious Thyroid Nodules via Sonography. Am. J. Roentgenol. 2016, 207, 859–864. [Google Scholar] [CrossRef]
- Driehuis, F.; Elferink, S.; Spoelstra, S.F. Anaerobic lactic acid degradation during ensilage of whole crop maize inoculated with Lactobacillus buchneri inhibits yeast growth and improves aerobic stability. J. Appl. Microbiol. 1999, 87, 583–594. [Google Scholar] [CrossRef]
- Marquez, J.M.A.; Bohorquez, M.A.M.; Melgar, S.G. Ground Thermal Diffusivity Calculation by Direct Soil Temperature Measurement. Application to very Low Enthalpy Geothermal Energy Systems. Sensors 2016, 16, 306. [Google Scholar] [CrossRef]
- Liu, H.L.; Sun, B.G. Effect of fermentation processing on the flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, L.Q.; Liu, Y.F.; Li, J.H.; Wang, L.; Chen, J. Identification of microorganisms producing lactic acid during solid-state fermentation of Maotai flavour liquor. J. Inst. Brew. 2019, 125, 171–177. [Google Scholar] [CrossRef]
- Tao, Y.; Li, J.B.; Rui, J.P.; Xu, Z.C.; Zhou, Y.; Hu, X.H.; Wang, X.; Liu, M.H.; Li, D.P.; Li, X.Z. Prokaryotic Communities in Pit Mud from Different-Aged Cellars Used for the Production of Chinese Strong-Flavored Liquor. Appl. Environ. Microbiol. 2014, 80, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.S.; Yang, J.G.; Zhang, K.Z.; Wang, M.Y.; Zhao, X.X.; Su, C.; Cao, X.Z. Lactic acid bacteria in the brewing of traditional Daqu liquor. J. Inst. Brew. 2020, 126, 14–23. [Google Scholar] [CrossRef]
- Zhang, B.; Li, R.Q.; Yu, L.H.; Wu, C.C.; Liu, Z.; Bai, F.Y.; Yu, B.; Wang, L.M. l-Lactic Acid Production via Sustainable Neutralizer-Free Route by Engineering Acid-Tolerant Yeast Pichia kudriavzevii. J. Agric. Food Chem. 2023, 71, 11131–11140. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xiao, H.; Peng, Q.; Zhang, Q.; Li, X.; Han, Y. Analysis of bacterial diversity of Chinese Luzhou-flavor liquor brewed in different seasons by Illumina Miseq sequencing. Ann. Microbiol. 2016, 66, 1293–1301. [Google Scholar] [CrossRef]
- Xu, Y.; Qiao, X.; He, L.; Wan, W.J.; Xu, Z.J.; Shu, X.; Yang, C.; Tang, Y. Airborne microbes in five important regions of Chinese traditional distilled liquor (Baijiu) brewing: Regional and seasonal variations. Front. Microbiol. 2024, 14, 1324722. [Google Scholar] [CrossRef]
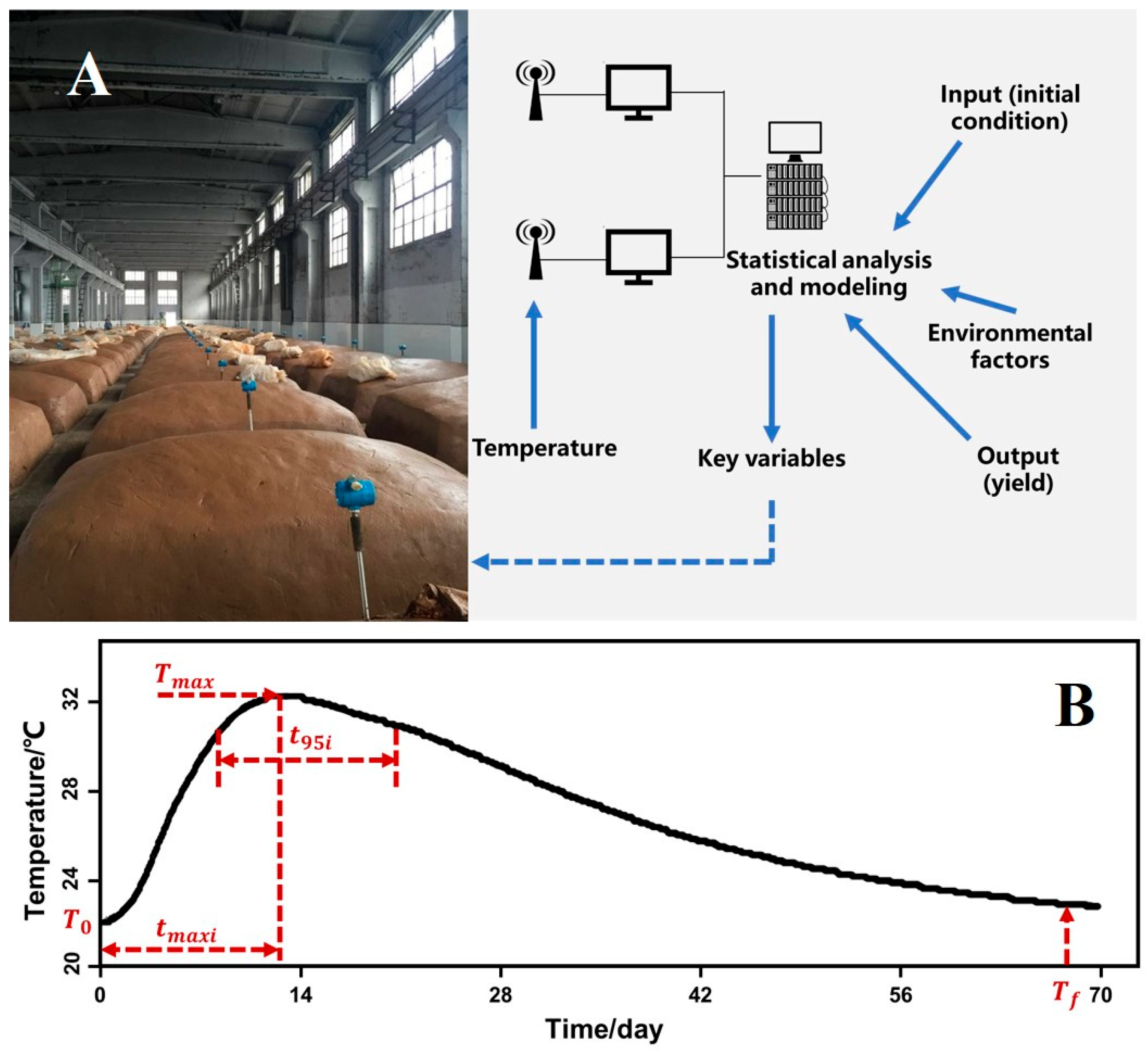
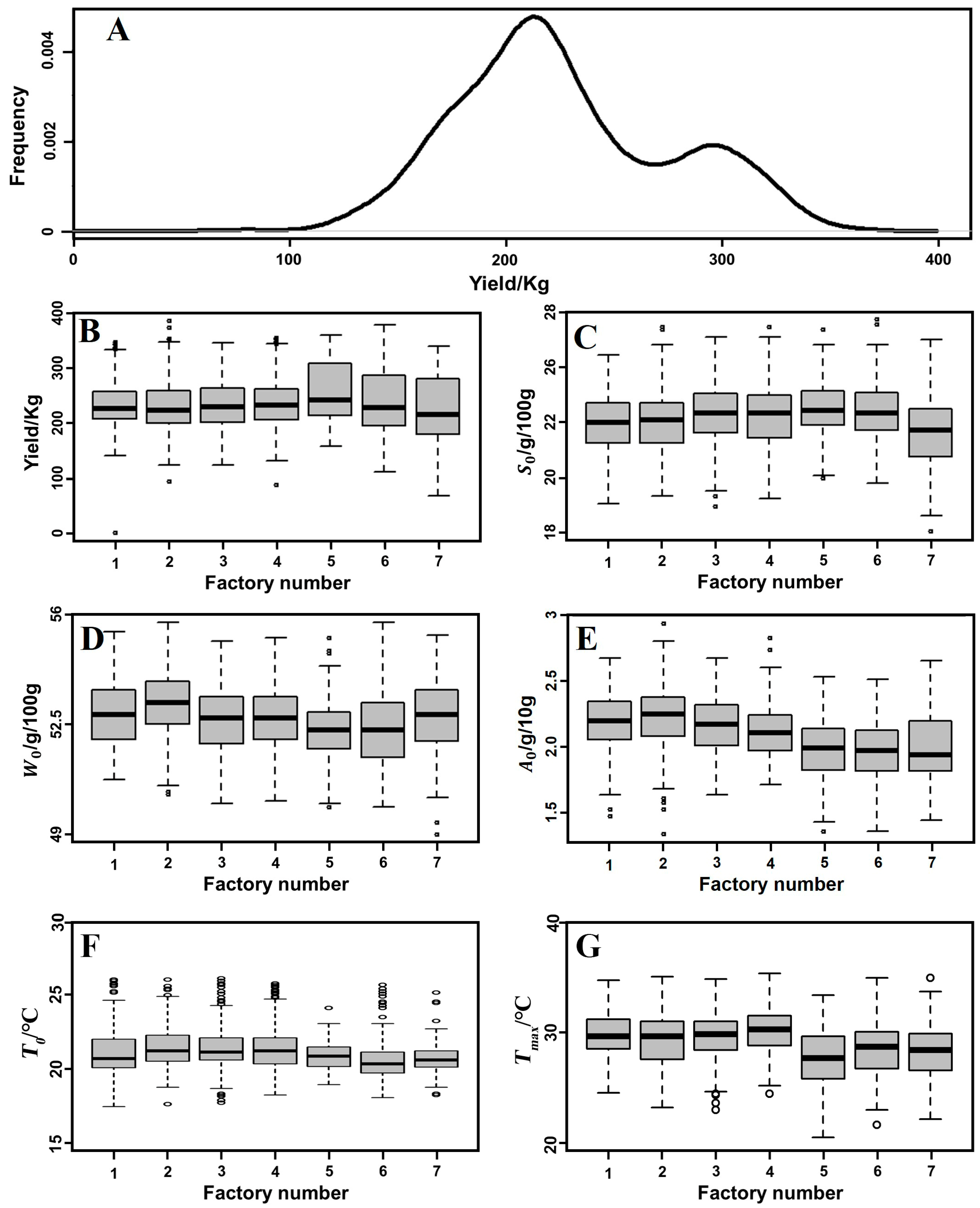
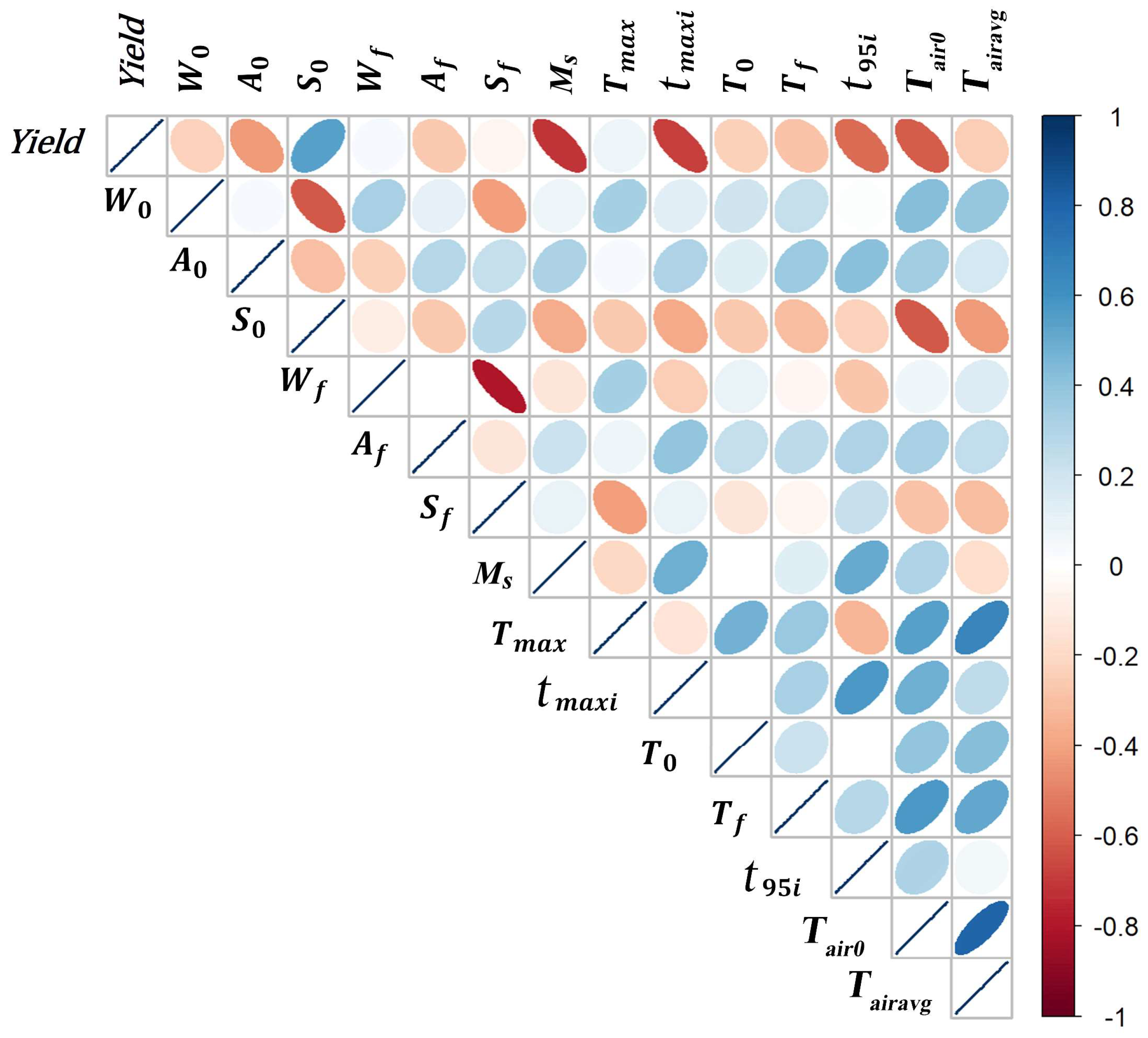
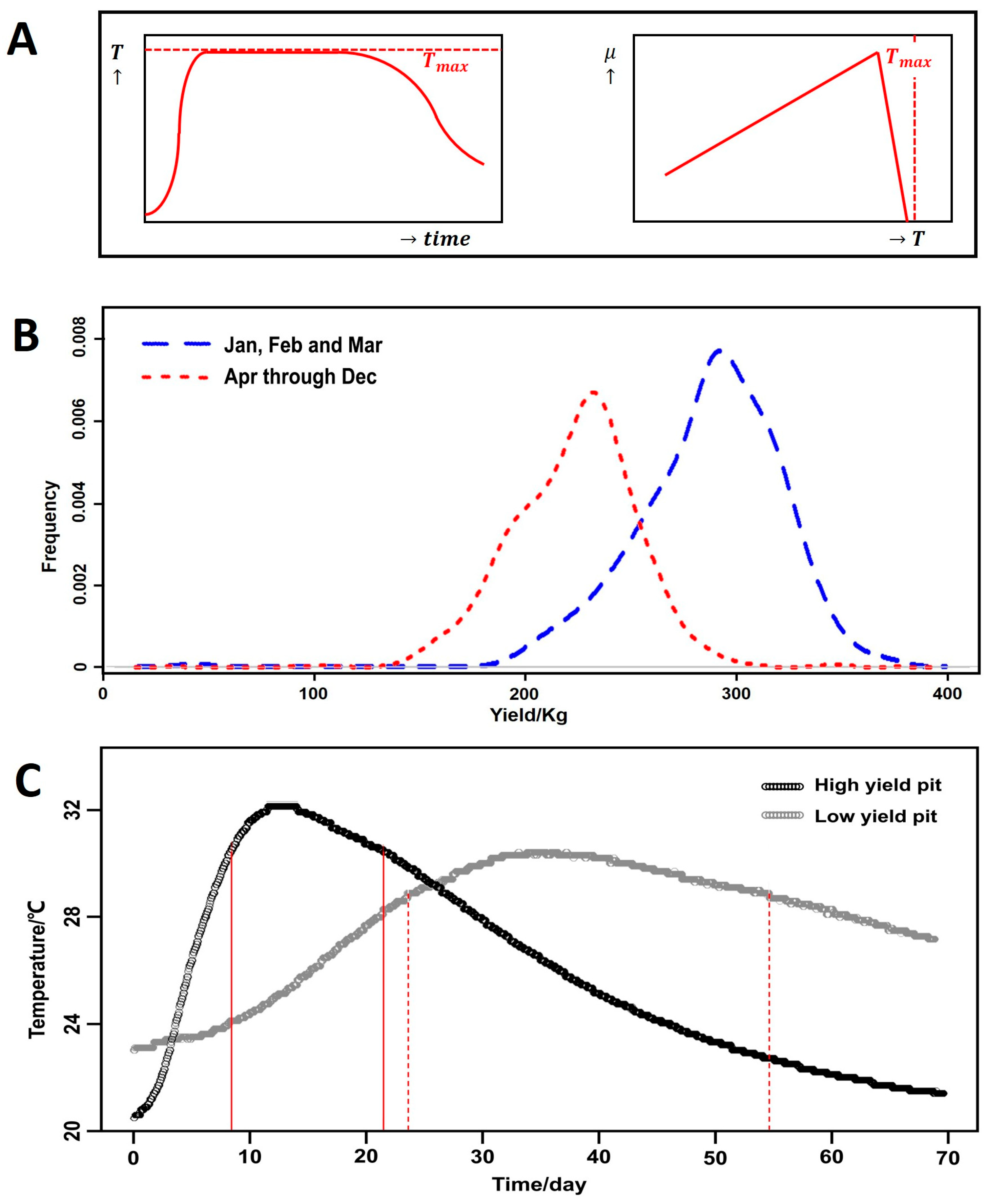
| Parameter | Description | Unit |
|---|---|---|
| A0 | Starting concentration of acid | g/10 g wet substrate |
| Af | Final concentration of acid | g/10 g wet substrate |
| S0 | Starting concentration of starch | g/100 g wet substrate |
| Sf | Final concentration of starch | g/100 g wet substrate |
| W0 | Starting content of water | g/100 g wet substrate |
| Wf | Final content of water | g/100 g wet substrate |
| smn | Starting month | NA |
| swk | Starting week | NA |
| T0 | Starting temperature | °C |
| Tf | Final temperature | °C |
| Tmax | Maximum temperature | °C |
| tmaxi | Time to maximum temperature | h |
| t95i | Time at 95% of the maximum temperature | h |
| at | Total area under temperature curve | NA |
| ap | Area under temperature curve before maximum temperature is reached | NA |
| Tsoil | Soil temperature | °C |
| Parameters | Coefficient ± Standard Error | ||||||
|---|---|---|---|---|---|---|---|
| β0 | 114.2 ± 5.0 *** | −540.7 ± 92.8 *** | 526.5 ± 13.5 *** | −56.3 ± 13.0 * | 290.9 ± 2.3 *** | 273.2 ± 3.8 *** | −46.6 ± 6.2 * |
| W0 | 1.6 ± 0.3 | 8.2 ± 1.3 * | 0 | 1.4 ± 1.1 | 0 | 0 | 2.9 ± 1.0 |
| A0 | −9.5 ± 0.6 *** | −42.5 ± 2.3 *** | 0 | −10.0 ± 0.9 *** | 0 | 0 | −8.5 ± 0.7 *** |
| S0 | 4.3 ± 0.3 *** | 23.2 ± 1.2 *** | 0 | 14 ± 0.9 *** | 0 | 0 | 14 ± 0.9 *** |
| T0 | −5.0 ± 0.5 * | −3.7 ± 0.8 *** | −11.0 ± 0.6 *** | −7.0 ± 0.3 *** | 0 | 0 | −7.0 ± 0.5 *** |
| tmaxi | −0.1 ± 0.0 *** | 0 | −0.2 ± 0.0 *** | −0.1 ± 0.0 *** | 0 | 0 | −0.1 ± 0.0 *** |
| Tmax | 4.1 ± 0.5 * | 0 | 1.2 ± 0.3 * | 2.5 ± 0.6 * | 0 | 0 | 2.1 ± 0.6 * |
| t95i | −0.1 ± 0.0 *** | 0 | −0.1 ± 0.0 *** | −0.1 ± 0.0 *** | 0 | 0 | −0.1 ± 0.0 * |
| Tf | −0.5 ± 0.11 | 0 | 0.6 ± 0.3 | 0.48 ± 0.2 | 0 | 0 | 0.2 ± 0.1 |
| Ms | −37.3 ± 2.0 *** | 0 | 0 | 0 | −66.0 ± 1.9 *** | 0 | 0 |
| Tair0 | −1.3 ± 0.1 * | 0 | 0 | 0 | 0 | −7.8 ± 0.4 ** | −1.6 ± 0.1 * |
| Tairavg | −0.3 ± 0.1 | 0 | 0 | 0 | 0 | −1.6 ± 0.2 | 0.4 ± 0.2 |
| R2 | 0.82 | 0.37 | 0.56 | 0.63 | 0.72 | 0.55 | 0.71 |
| Q2 | 0.81 | 0.37 | 0.56 | 0.63 | 0.72 | 0.55 | 0.71 |
| VIF | 3.05 | 1.16 | 1.46 | 1.66 | 2.08 | 1.00 | 2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, G.; Boeschoten, S.; Hageman, J.; Zhu, Y.; Wijffels, R.; Rinzema, A.; Xu, Y. Identifying Variables Influencing Traditional Food Solid-State Fermentation by Statistical Modeling. Foods 2024, 13, 1317. https://doi.org/10.3390/foods13091317
Jin G, Boeschoten S, Hageman J, Zhu Y, Wijffels R, Rinzema A, Xu Y. Identifying Variables Influencing Traditional Food Solid-State Fermentation by Statistical Modeling. Foods. 2024; 13(9):1317. https://doi.org/10.3390/foods13091317
Chicago/Turabian StyleJin, Guangyuan, Sjoerd Boeschoten, Jos Hageman, Yang Zhu, René Wijffels, Arjen Rinzema, and Yan Xu. 2024. "Identifying Variables Influencing Traditional Food Solid-State Fermentation by Statistical Modeling" Foods 13, no. 9: 1317. https://doi.org/10.3390/foods13091317
APA StyleJin, G., Boeschoten, S., Hageman, J., Zhu, Y., Wijffels, R., Rinzema, A., & Xu, Y. (2024). Identifying Variables Influencing Traditional Food Solid-State Fermentation by Statistical Modeling. Foods, 13(9), 1317. https://doi.org/10.3390/foods13091317







