Widely Targeted Metabolomics and Network Pharmacology Reveal the Nutritional Potential of Yellowhorn (Xanthoceras sorbifolium Bunge) Leaves and Flowers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Yellowhorn Flower and Leaf Sample Pretreatment
2.2.1. UPLC-MS/MS Sample Pretreatment
2.2.2. GC-MS Sample Pretreatment
2.3. UPLC-MS/MS Analysis
2.4. GC-MS Analysis
2.5. Data Processing and Analysis
2.6. Network Pharmacological Analysis
3. Results and Discussion
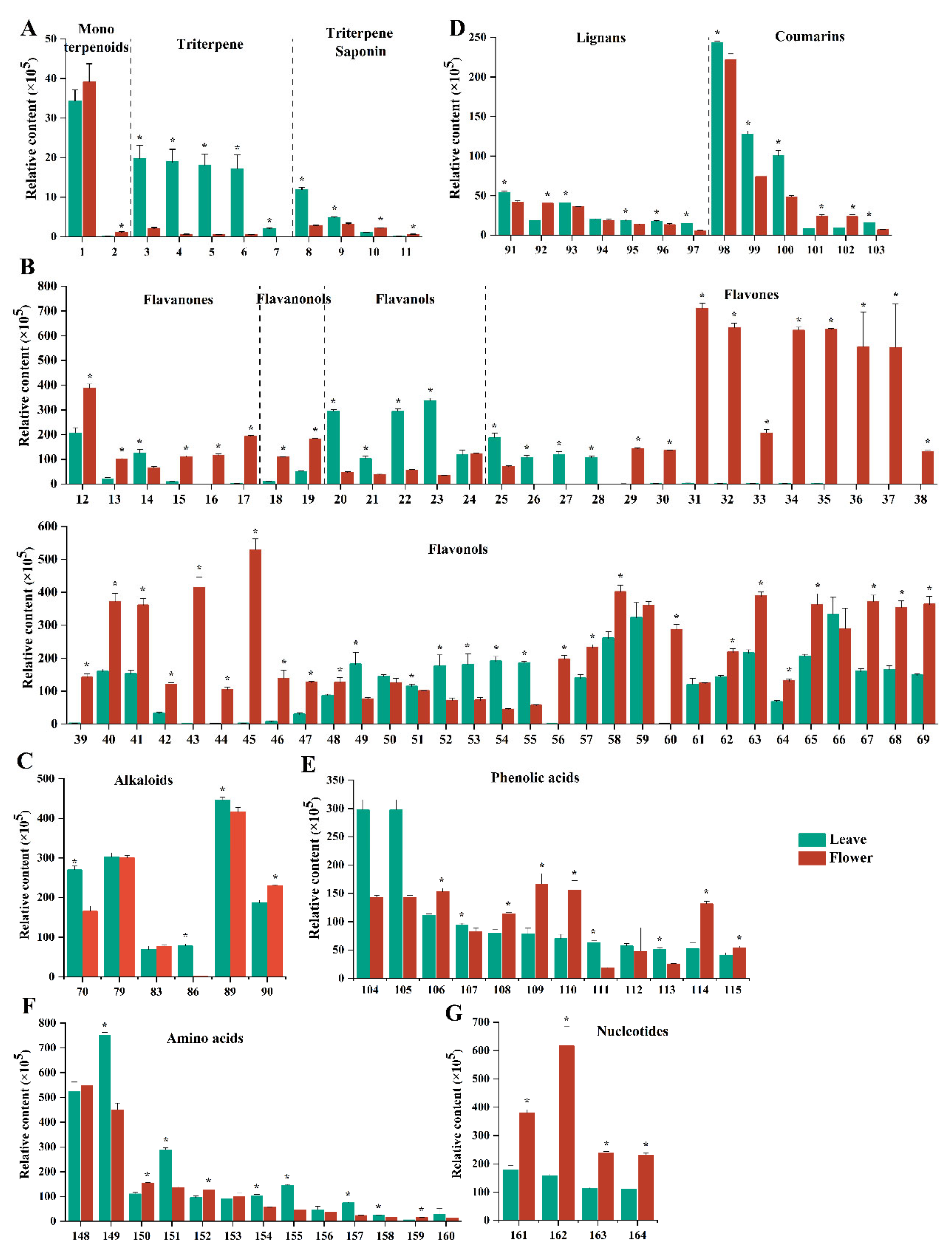
3.1. Overview of Non-Volatile Metabolites in Leaves and Flowers
3.2. Identification of Differential Non-Volatile Metabolites
3.3. Analysis of Non-Volatile Metabolites in the Leaves and Flowers of Yellowhorn
3.3.1. Terpenoids
3.3.2. Flavonoids
3.3.3. Alkaloids
3.3.4. Lignans and Coumarins
3.3.5. Phenolic Acids
3.3.6. Amino Acids
3.3.7. Nucleotides
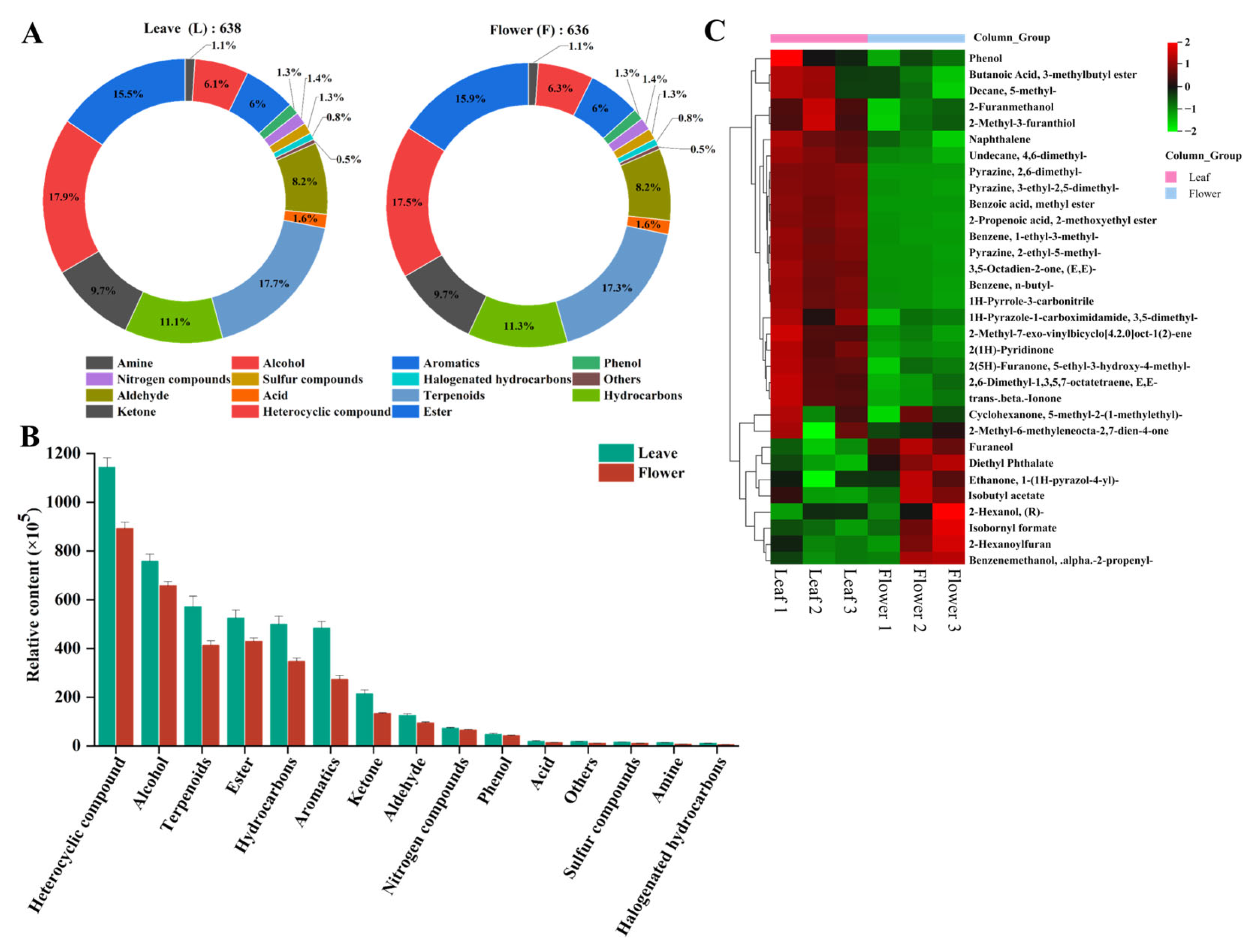
3.4. Overview of Volatile Metabolites in the Leaf and Flower
3.5. Identification of Differential Volatile Metabolites
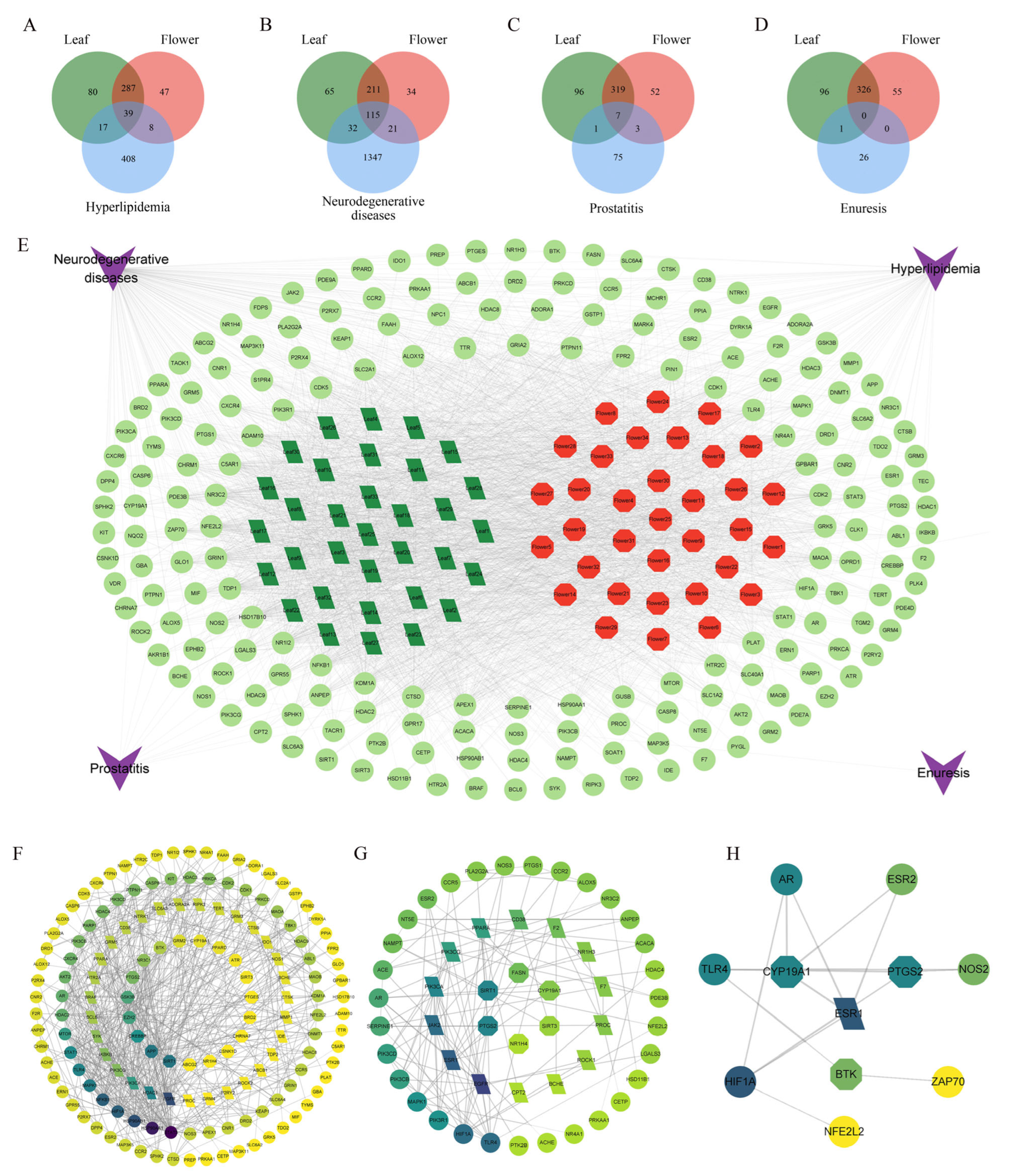
3.6. Network Pharmacology of the Leaf and Flower of Yellowhorn
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.-H.; Wang, T.-T.; Li, Q.-Q.; Zhao, N.; Zhang, Y.; Liu, D.; Hu, Q.; Li, F.-L. Two Novel Diacylglycerol Acyltransferase Genes from Xanthoceras Sorbifolia Are Responsible for Its Seed Oil Content. Gene 2013, 527, 266–274. [Google Scholar] [CrossRef]
- Venegas-Calerón, M.; Ruíz-Méndez, M.V.; Martínez-Force, E.; Garcés, R.; Salas, J.J. Characterization of Xanthoceras Sorbifolium Bunge Seeds: Lipids, Proteins and Saponins Content. Ind. Crops Prod. 2017, 109, 192–198. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Ao, Y.; Saunders, M.R.; Wang, X. Diversity of Seed and Seed Oil Physicochemical Traits of Xanthoceras Sorbifolium Bunge. J. Food Compos. Anal. 2021, 96, 103705. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Y.; Wang, Z.; El-Kassaby, Y.; Guan, W. Fruit Shape and Reproductive Self and Cross Compatibility for the Performance of Fruit Set in an Andromonoecious Species: Xanthoceras Sorbifolium Bunge. Tree Genet. Genomes 2017, 13, 116. [Google Scholar] [CrossRef]
- Xiao, J.; Zou, Y.; Wen, X.; Guo, Y.; Hu, F.; Chen, G.; Wu, Z.; Lin, Y.; Wang, Z.; Sun, L.; et al. Functional Contents and Antioxidant Potency of Chinese Wenguan Flower Tea. Food Control 2022, 138, 109002. [Google Scholar] [CrossRef]
- Zang, E.; Qiu, B.; Chen, N.; Li, C.; Liu, Q.; Zhang, M.; Liu, Y.; Li, M. Xanthoceras Sorbifolium Bunge: A Review on Botany, Phytochemistry, Pharmacology, and Applications. Front. Pharmacol. 2021, 12, 708549. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Zhang, P.; Li, N.; Jiang, S.; Wang, J.; Huang, J.; Li, X. Bioactive Barrigenol Type Triterpenoids from the Leaves of Xanthoceras Sorbifolia Bunge. Eur. J. Med. Chem. 2013, 60, 263–270. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Li, X.; Zhang, H.; Zhou, D.; Wang, W.; Li, W.; Zhang, X.; Li, X.; Hou, Y.; et al. Bioactive Phenols as Potential Neuroinflammation Inhibitors from the Leaves of Xanthoceras Sorbifolia Bunge. Bioorg. Med. Chem. Lett. 2016, 26, 5018–5023. [Google Scholar] [CrossRef]
- Lee, D.E.; Shin, G.R.; Lee, S.; Jang, E.S.; Shin, H.W.; Moon, B.S.; Lee, C.H. Metabolomics Reveal That Amino Acids Are the Main Contributors to Antioxidant Activity in Wheat and Rice Gochujangs (Korean Fermented Red Pepper Paste). Food Res. Int. 2016, 87, 10–17. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Wei, G.; Tian, P.; Zhang, F.; Qin, H.; Miao, H.; Chen, Q.; Hu, Z.; Cao, L.; Wang, M.; Gu, X.; et al. Integrative Analyses of Nontargeted Volatile Profiling and Transcriptome Data Provide Molecular Insight into VOC Diversity in Cucumber Plants (Cucumis Sativus). Plant Physiol. 2016, 172, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lei, Z.; Cao, J.; Zhang, W.; Wu, R.; Cao, F.; Guo, Q.; Wang, J. Traditional Uses, Phytochemistry, Pharmacology and Current Uses of Underutilized Xanthoceras Sorbifolium Bunge: A Review. J. Ethnopharmacol. 2022, 283, 114747. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Huang, M.-Q.; Bao, J.-L.; Chen, X.-P.; Wang, Y.-T. Terpenoids: Natural Products for Cancer Therapy. Expert Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Yoon, J.; Park, B. Pomolic Acid Suppresses HIF1α/VEGF-Mediated Angiogenesis by Targeting P38-MAPK and mTOR Signaling Cascades. Phytomed. Int. J. Phytother. Phytopharm. 2016, 23, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.-P.; Zhang, X.-H.; Sun, L.-N.; Xing, W.-F.; Wang, Y.; Sun, S.-Y.; Ma, M.-Y.; Cheng, Z.-P.; Wu, Z.-D.; Xing, C.; et al. Corosolic Acid and Its Structural Analogs: A Systematic Review of Their Biological Activities and Underlying Mechanism of Action. Phytomed. Int. J. Phytother. Phytopharm. 2021, 91, 153696. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, T.; Wieland, L.S.; Miller, L.E.; Munir, K.; Pollin, T.I.; Shuldiner, A.R.; Amoils, S.; Gallagher, L.; Bahr-Robertson, M.; D’Adamo, C.R. Polyherbal Dietary Supplementation for Prediabetic Adults: Study Protocol for a Randomized Controlled Trial. Trials 2019, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Huang, T.; Xue, M.; Chen, J.; Feng, L.; Du, R.; Feng, Y. Current Knowledge and Development of Hederagenin as a Promising Medicinal Agent: A Comprehensive Review. RSC Adv. 2018, 8, 24188–24202. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, A.; Itokawa, H. Triterpenoids of Paeonia Japonica Callus Tissue. Phytochemistry 1988, 27, 2813–2815. [Google Scholar] [CrossRef]
- Wu, K.-C.; Lee, D.-Y.; Hsu, J.-T.; Cheng, C.-F.; Lan, J.-L.; Chiu, S.-C.; Cho, D.-Y.; Hsu, J.-L. Evaluations and Mechanistic Interrogation of Natural Products Isolated From Paeonia Suffruticosa for the Treatment of Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 696158. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Martins, A.; Susano, P.; Simões, M.; Guedes, M.; Rehfeldt, S.; Pinteus, S.; Gaspar, H.; Rodrigues, A.; et al. Loliolide, a New Therapeutic Option for Neurological Diseases? In Vitro Neuroprotective and Anti-Inflammatory Activities of a Monoterpenoid Lactone Isolated from Codium Tomentosum. Int. J. Mol. Sci. 2021, 22, 1888. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Kim, H.-S.; Asanka Sanjeewa, K.K.; Han, E.J.; Jee, Y.; Ahn, G.; Rho, J.-R.; Jeon, Y.-J. Loliolide, Isolated from Sargassum Horneri; Abate LPS-Induced Inflammation via TLR Mediated NF-κB, MAPK Pathways in Macrophages. Algal Res. 2021, 56, 102297. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic Potential of Flavonoids in Cancer: ROS-Mediated Mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and Gut Health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Chaudhary, S.K.; Goyal, A.; Sarup, P.; Kumari, S.; Garg, N.; Vaid, L.; Shiveena, B. Comprehensive Review on Therapeutic and Phytochemical Exploration of Diosmetin: A Promising Moiety. Phytomedicine Plus 2022, 2, 100179. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, L.; Yang, B. Diosmetin Alleviates S. Aureus-Induced Mastitis by Inhibiting SIRT1/GPX4 Mediated Ferroptosis. Life Sci. 2023, 331, 122060. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, F.; Yan, J.; Xia, Z.; Jiang, D.; Ma, P. Hispidulin: A Promising Flavonoid with Diverse Anti-Cancer Properties. Life Sci. 2020, 259, 118395. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhang, L.; Wang, L. Kaempferol, a Potential Neuroprotective Agent in Neurodegenerative Diseases: From Chemistry to Medicine. Biomed. Pharmacother. 2023, 165, 115215. [Google Scholar] [CrossRef]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The Relationship between the Structure and Biological Actions of Green Tea Catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef]
- Cavalier, A.N.; Clayton, Z.S.; Wahl, D.; Hutton, D.A.; McEntee, C.M.; Seals, D.R.; LaRocca, T.J. Protective Effects of Apigenin on the Brain Transcriptome with Aging. Mech. Ageing Dev. 2023, 217, 111889. [Google Scholar] [CrossRef]
- Javanbakht, P.; Yazdi, F.R.; Taghizadeh, F.; Khadivi, F.; Hamidabadi, H.G.; Kashani, I.R.; Zarini, D.; Mojaverrostami, S. Quercetin as a Possible Complementary Therapy in Multiple Sclerosis: Anti-Oxidative, Anti-Inflammatory and Remyelination Potential Properties. Heliyon 2023, 9, e21741. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for Cancer Prevention and Therapy: Current Progress and Future Perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, K.; Jiang, C.; Su, Y.; Fan, X.; Li, J.; Xue, S.; Yao, L. Trigonelline Protects Hippocampal Neurons from Oxygen-Glucose Deprivation-Induced Injury through Activating the PI3K/Akt Pathway. Chem. Biol. Interact. 2020, 317, 108946. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of Polyamines and Their Metabolic Products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Sakuragi, M.; Takao, K.; Lefor, A.; Konishi, F. Long-Term Oral Polyamine Intake Increases Blood Polyamine Concentrations. J. Nutr. Sci. Vitaminol. 2009, 55, 361–366. [Google Scholar] [CrossRef]
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Spermine and Spermidine Are Cytotoxic towards Intestinal Cell Cultures, but Are They a Health Hazard at Concentrations Found in Foods? Food Chem. 2018, 269, 321–326. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Yu, L.; Chen, L.; Qiu, F. Chemical Constituents of Seed Oil Leavings of Xanthoceras Sorbifolia. Chem. Nat. Compd. 2018, 54, 769–771. [Google Scholar] [CrossRef]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a Neurotransmitter: The Role of Serotonin in Inflammation and Immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Habtemariam, S.; Rahimi, R.; Nabavi, S.M. The What and Who of Dietary Lignans in Human Health: Special Focus on Prooxidant and Antioxidant Effects. Trends Food Sci. Technol. 2020, 106, 382–390. [Google Scholar] [CrossRef]
- Wang, L.-X.; Wang, H.-L.; Huang, J.; Chu, T.-Z.; Peng, C.; Zhang, H.; Chen, H.-L.; Xiong, Y.-A.; Tan, Y.-Z. Review of Lignans from 2019 to 2021: Newly Reported Compounds, Diverse Activities, Structure-Activity Relationships and Clinical Applications. Phytochemistry 2022, 202, 113326. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kwon, H.; Cho, E.; Jeon, J.; Kang, R.H.; Youn, K.; Jun, M.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. The Effects of Pinoresinol on Cholinergic Dysfunction-Induced Memory Impairments and Synaptic Plasticity in Mice. Food Chem. Toxicol. 2019, 125, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, F.; Chen, Y.; Li, F.; Han, B.; Liu, D. The Antithrombotic Activity of Natural and Synthetic Coumarins. Fitoterapia 2021, 154, 104947. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. Amst. Neth. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remón, A.; Martínez-González, M.A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Inverse Association between Habitual Polyphenol Intake and Incidence of Cardiovascular Events in the PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. NMCD 2014, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Deters, B.J.; Saleem, M. The Role of Glutamine in Supporting Gut Health and Neuropsychiatric Factors. Food Sci. Hum. Wellness 2021, 10, 149–154. [Google Scholar] [CrossRef]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as Optimal Candidates for Essential Nutrients in Living Organisms: A Review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Weng, D.; Chen, J.-X.; Li, H.-H.; Liu, F.; Zhou, L.-D.; Liu, H.-P.; Zheng, R.-J.; Jiang, Y.; Liu, Z.-H.; Ge, B. 2-Aminopurine Suppresses the TGF-Β1-Induced Epithelial-Mesenchymal Transition and Attenuates Bleomycin-Induced Pulmonary Fibrosis. Cell Death Discov. 2018, 4, 17. [Google Scholar] [CrossRef]
- Lanznaster, D.; Dal-Cim, T.; Piermartiri, T.C.B.; Tasca, C.I. Guanosine: A Neuromodulator with Therapeutic Potential in Brain Disorders. Aging Dis. 2016, 7, 657–679. [Google Scholar] [CrossRef]
- Suzuki, M.; Okuda, T.; Shiraki, K. Synergistic Antiviral Activity of Acyclovir and Vidarabine against Herpes Simplex Virus Types 1 and 2 and Varicella-Zoster Virus. Antiviral Res. 2006, 72, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Eissa, I.H.; Khalifa, M.M.; Elkaeed, E.B.; Hafez, E.E.; Alsfouk, A.A.; Metwaly, A.M. In Silico Exploration of Potential Natural Inhibitors against SARS-Cov-2 Nsp10. Molecules 2021, 26, 6151. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Chen, J.; Li, X.; Zhou, X.; Hu, Y.-M.; Chu, S.-F.; Peng, Y.; Chen, N.-H. Research Progress on Adenosine in Central Nervous System Diseases. CNS Neurosci. Ther. 2019, 25, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Mura, K.; Shibamoto, T. Antioxidative Activity of Volatile Chemicals Extracted from Beer. J. Agric. Food Chem. 2001, 49, 4097–4101. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Reina, R.; Segura-Borrego, M.P.; Morales, M.L.; Callejón, R.M. Characterization of the Aroma Profile and Key Odorants of the Spanish PDO Wine Vinegars. Food Chem. 2020, 311, 126012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lv, H.-P.; Shao, C.-Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.-D.; Tan, J.-F.; Peng, Q.-H.; Lin, Z. Identification of Key Odorants Responsible for Chestnut-like Aroma Quality of Green Teas. Food Res. Int. Ott. Ont. 2018, 108, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-C.; Zhu, Y.; Yan, H.; Chen, M.; Xie, D.-C.; Wang, M.-Q.; Ni, D.-J.; Lin, Z. Identification of Aroma Composition and Key Odorants Contributing to Aroma Characteristics of White Teas. Molecules 2020, 25, 6050. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, Y.; Wang, K.; Niu, Y.; Xiao, Z. Characterization of Key Aroma Compounds and Enantiomer Distribution in Longjing Tea. Food Chem. 2021, 361, 130096. [Google Scholar] [CrossRef] [PubMed]
- Revel, G.; Marchand, S.; Bertrand, A. Identification of Maillard-Type Aroma Compounds in Winelike Model Systems of Cysteine—Carbonyls: Occurrence in Wine. In Nutraceutical Beverages; ACS Publications: Washington, DC, USA, 2003; Volume 222, pp. 353–364. ISBN 0-8412-3823-5. [Google Scholar] [CrossRef]
- Ho, C.-T.; Zheng, X.; Li, S. Tea Aroma Formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Kanasawud, P.; Crouzet, J.C. Mechanism of Formation of Volatile Compounds by Thermal Degradation of Carotenoids in Aqueous Medium. 1. .Beta.-Carotene Degradation. J. Agric. Food Chem. 1990, 38, 237–243. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Shibamoto, T. Volatile Constituents of the Chestnut Flower. J. Agric. Food Chem. 1980, 28, 82–84. [Google Scholar] [CrossRef]
- Baydar, H.; Schulz, H.; Krüger, H.; Erbas, S.; Kineci, S. Influences of Fermentation Time, Hydro-Distillation Time and Fractions on Essential Oil Composition of Damask Rose (Rosa damascena Mill.). J. Essent. Oil Bear. Plants 2008, 11, 224–232. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Johnson, S.; Luo, J.; Fang, Z. Comparison of the Phenolic Contents, Antioxidant Activity and Volatile Compounds of Different Sorghum Varieties during Tea Processing. J. Sci. Food Agric. 2020, 100, 978–985. [Google Scholar] [CrossRef]
- Lv, S.; Wu, Y.; Wei, J.; Lian, M.; Wang, C.; Gao, X.; Meng, Q. Application of Gas Chromatography-Mass Spectrometry and Chemometrics Methods for Assessing Volatile Profiles of Pu-Erh Tea with Different Processing Methods and Ageing Years. RSC Adv. 2015, 5, 87806–87817. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Kong, Y.; Peng, X.; Li, C.; Liu, S.; Du, L.; Xiao, D.; Xu, Y. A Comparative Study of Volatile Components in Dianhong Teas from Fresh Leaves of Four Tea Cultivars by Using Chromatography-Mass Spectrometry, Multivariate Data Analysis, and Descriptive Sensory Analysis. Food Res. Int. Ott. Ont. 2017, 100, 267–275. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Wang, H.; Huang, W.; Li, F.; Wang, L.; Ho, C.-T.; Zhang, Y.; Zhang, L.; Zhai, X.; et al. Decoding the Specific Roasty Aroma Wuyi Rock Tea (Camellia Sinensis: Dahongpao) by the Sensomics Approach. J. Agric. Food Chem. 2022, 70, 10571–10583. [Google Scholar] [CrossRef]
- Ruan, C.-J.; Yan, R.; Wang, B.-X.; Mopper, S.; Guan, W.-K.; Zhang, J. The Importance of Yellow Horn (Xanthoceras Sorbifolia) for Restoration of Arid Habitats and Production of Bioactive Seed Oils. Ecol. Eng. 2017, 99, 504–512. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Yang, J.-L.; Ha, W.; Shi, Y.-P. Advances in Studies on Chemical Constituents from Husks of Xanthoceras Sorbifolia and Their Biological Activities. Chin. Tradit. Herb. Drugs 2016, 47, 1418–1424. [Google Scholar] [CrossRef]
- Ji, X.-F.; Chi, T.-Y.; Xu, Q.; He, X.-L.; Zhou, X.-Y.; Zhang, R.; Zou, L.-B. Xanthoceraside Ameliorates Mitochondrial Dysfunction Contributing to the Improvement of Learning and Memory Impairment in Mice with Intracerebroventricular Injection of aβ1-42. Evid. Based Complement. Alternat. Med. 2014, 2014, 969342. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, J.; Wang, J.; Duan, X.; Hui, S. Key Secondary Metabolite Markers for Wuchang Daohuaxiang Rice Discrimination in China. Food Res. Int. 2023, 169, 112943. [Google Scholar] [CrossRef] [PubMed]
- Punia Bangar, S.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A Flavone with Myriads of Bioactivities and Food Applications. Food Biosci. 2023, 52, 102366. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, M.; Jin, C.; Sun, X.; Feng, F.; Niu, X.; Wang, B.; Zhang, Y.; Wang, J. Naringenin Confers Protection against Experimental Autoimmune Encephalomyelitis through Modulating the Gut-Brain Axis: A Multiomics Analysis. J. Nutr. Biochem. 2023, 122, 109448. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, D.; Ji, Q.; Li, Y.; Cai, Z.; Fang, L.; Huo, H.; Zhou, G.; Yan, X.; Shen, L.; et al. Jujuboside A Attenuates Sepsis-Induced Cardiomyopathy by Inhibiting Inflammation and Regulating Autophagy. Eur. J. Pharmacol. 2023, 947, 175451. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, H.; Li, S.; Li, J.; Zhao, J.; Su, D. Widely Targeted Metabolomics and Network Pharmacology Reveal the Nutritional Potential of Yellowhorn (Xanthoceras sorbifolium Bunge) Leaves and Flowers. Foods 2024, 13, 1274. https://doi.org/10.3390/foods13081274
Sha H, Li S, Li J, Zhao J, Su D. Widely Targeted Metabolomics and Network Pharmacology Reveal the Nutritional Potential of Yellowhorn (Xanthoceras sorbifolium Bunge) Leaves and Flowers. Foods. 2024; 13(8):1274. https://doi.org/10.3390/foods13081274
Chicago/Turabian StyleSha, Haojie, Shouke Li, Jiaxing Li, Junying Zhao, and Dingding Su. 2024. "Widely Targeted Metabolomics and Network Pharmacology Reveal the Nutritional Potential of Yellowhorn (Xanthoceras sorbifolium Bunge) Leaves and Flowers" Foods 13, no. 8: 1274. https://doi.org/10.3390/foods13081274
APA StyleSha, H., Li, S., Li, J., Zhao, J., & Su, D. (2024). Widely Targeted Metabolomics and Network Pharmacology Reveal the Nutritional Potential of Yellowhorn (Xanthoceras sorbifolium Bunge) Leaves and Flowers. Foods, 13(8), 1274. https://doi.org/10.3390/foods13081274




