3.1. Chemical Composition of А. altissima Seeds
The isolated
А. altissima seed oil was liquid with a light yellow color. The results for the content (%) of the oil, proteins, carbohydrates, fiber, ash, and moisture in the seeds are presented in
Table 1. As can be seen from the table, the analyzed plant species is characterized by a relatively high content (30.7%) of oil. The amount of carbohydrates prevailed as well (38.9%), of which the fibers were the most represented. The quantity of starch is significantly low, followed by invert sugar and reducing sugars. Protein content was two times lower than the carbohydrate one. Moisture and ash content were 6.0% and 5.7%, respectively.
The oil content of
A. altissima seeds was almost twice as high as that determined in the studies of El Ayeb-Zakhama et al. [
3] (17.32%) and relatively close to that reported by the authors of [
20] (38%) and [
22] (40%). A lower yield of multiple folds (0.2–2.3%) is reported for
A. altissima wood oil, which is indicated as suitable for obtaining biodiesel [
21]. Our data support the statement of other authors that the species has a high oil yield. The amount of oil extracted is close to or higher than that reported for black cumin, hemp, evening primrose, and milk thistle seed oils, which are known for their medicinal and nutritional value [
2]. According to the researchers who worked on
A. altissima seed oil, it even exceeds the oil yield obtained from some edible and non-edible seeds [
20,
22]. The content of cellulose and, respectively, fiber, in the studied seeds of
A. altissima was in lower values than those in wood (46.7%), while that of ash was significantly higher compared to the 0.5% found for this indicator by the authors of [
21].
The quantitative data on fiber give us reason to believe that the seeds of
A. аltissima would be an excellent source of dietary fiber. Various health effects and benefits for the human body have been documented for the latter [
49,
50,
51]. People consuming larger amounts of dietary fiber are exposed to a lower risk of developing gastrointestinal and cardiovascular diseases, obesity, hypertension, diabetes, etc. Supplements containing them improve blood sugar control, and lower blood pressure and serum lipoprotein levels [
50]. Through various mechanisms, they limit the development of cancer cells and participate in the prevention of various forms of cancer—colorectal, ovarian, pancreatic, prostate, breast, and head and neck cancer [
51]. Fibers have a healing and protective effect against constipation, hemorrhoids, gastroesophageal reflux, duodenal ulcer, hypertension, diverticulitis, and other diseases [
50]. They are part of the nutrients in a healthy diet [
49].
Another group of macronutrients present in significant amounts in ailanthus seeds and performing a constructive role in the human body is proteins. Incorporating them into the food diet is essential for wellbeing. Their sources are mainly animal-based products (dairy and meat products), but some negative factors in their production (cruelty to animals, pollution of the environment, negative effect of animal food on health, etc.) are reasons to look for their vegan substitutes such as plant-based proteins. The main sources of such plant-based proteins are cereals (6–15%), nuts (18–38%), and seeds (9–30%), groups of which are shown to be superior in protein content to milk (3–5%), and come close to that of meat (23%) [
52]. Plant alternatives to animal proteins are preferred in the vegan diet because they contain bioactive molecules such as polyphenols, vitamins, antioxidants, dietary fiber, etc., which are beneficial compounds that have a positive effect on human health.
3.2. Physicochemical Properties of А. altissima Seed Oil
The measured values of the physicochemical parameters of the oil are shown in
Table 2.
The peroxide value indicates the amount of primary oxidation products in the oils: peroxides and hydroperoxides. The peroxide value of the studied oil from ailanthus seeds indicates that no oxidation processes occurred during its extraction. An indicator of the content of free fatty acids in the oil is the acid value; for the degree of unsaturation, it is the iodine value; and the saponification value reflects the content of ester bonds and free fatty acids. The obtained results for the acid value of the oil are slightly higher than the requirements of [
53] for glyceride oils (up to 4 mg KOH/g). More than six times lower is the acid value (0.64 mg KOH/g) obtained by the authors of [
20]. According to the iodine value data (129.4 gI
2/100 g),
A. altissima seed oil is characterized as a semi-dry oil, the value of which is within the limits of the widely used soybean (124–139 gI
2/100 g) and sunflower (118–141 gI
2/100 g) seed oil [
53]. The saponification value corresponds to that established for palm oil (190–209 mg KOH/g) [
53],
A. altissima wood oil (206.3 mg KOH/g) [
21], and that reported by the authors of [
3] for
A. altissima oil (192.60 mg KOH/g). It can be seen from
Table 2 that the iodine values were lower than those found by other authors—132.11 gI
2/100 g [
20]; 136.77 gI
2/100 g [
3].
A. altissima wood oil has a lower iodine value (107.2 g I
2/100 g) compared to seed oil [
21]. The refractive index values correspond to those for cottonseed oil (1.458–1.466), grapeseed oil (1.467–1.477), maize oil (1.465–1.468), mustard seed oil (1.461–1.469) [
53], as well as sesame seed oil (1.47) [
54]. Also, close to these values was the measured relative density, which matches that reported for palm oil [
53]. The oxidative stability of ailanthus oil was relatively low (5.0 h) and was similar to that of refined grapeseed oil (3.7 h) [
55]. The team of [
3] reported similar to our results for refractive index (1.451) and different for the peroxide value (9.4 meqO
2/kg), which we would attribute to the autoxidation processes that occurred in them. The latter authors have also published the oxidative stability (4.00 h) of the seed oil of
A. altissima since these values are close to those obtained in the present work.
3.3. Lipid Composition of the Seeds and Seed Oil
The content of the determined lipid-soluble compounds (some of which were biologically active) in the seeds and oil from the “tree of heaven” are presented in
Table 3.
Based on the percentage of the lipid-soluble components (in the oil), the best represented were the unsaponifiable matter in oil (3.3%) (which included sterols and tocopherols), followed by phospholipids. Calculations for the seeds showed that these components were as follows: unsaponifiable matter (1.0%); phospholipids (0.3%); and sterols (0.2%). Relatively high were the levels of total tocopherols, whose content in the oil was 414 mg/kg and 127 mg/kg in the seeds.
The total content of sterols in
A. altissima seed oil is close to the values for sterols in widely used vegetable oils such as soybean (0.18–0.41%), cottonseed (0.27–0.64%), and sunflower (0.24–0.46%) [
53]. The total tocopherols in the studied oil are similar to those indicated in grapeseed oil (240–410 mg/kg) and safflower oil (240–670 mg/kg) according to [
53]. By this indicator, it surpasses some of the most used oils such as walnut oil (209.4 mg/kg), peanut oil (259.6 mg/kg), olive oil (311.0 mg/kg), tomato seed oil (345.8 mg/kg), and evening primrose oil (391.9 mg/kg), and has a similar quantitative value to that reported for
Camellia oil (416.0 mg/kg) [
1]. The higher number of total tocopherols in the oil contributes to its higher resistance to oxidation, which is important to keep its qualities unchanged for a longer time.
A. altissima seed oil is superior in unsaponifiable matter content to other species of the genus. In
А. Exelsa, the amount of this group of bioactive components is 2.0% in the refined oil and 1.2% after its refining, respectively [
23].
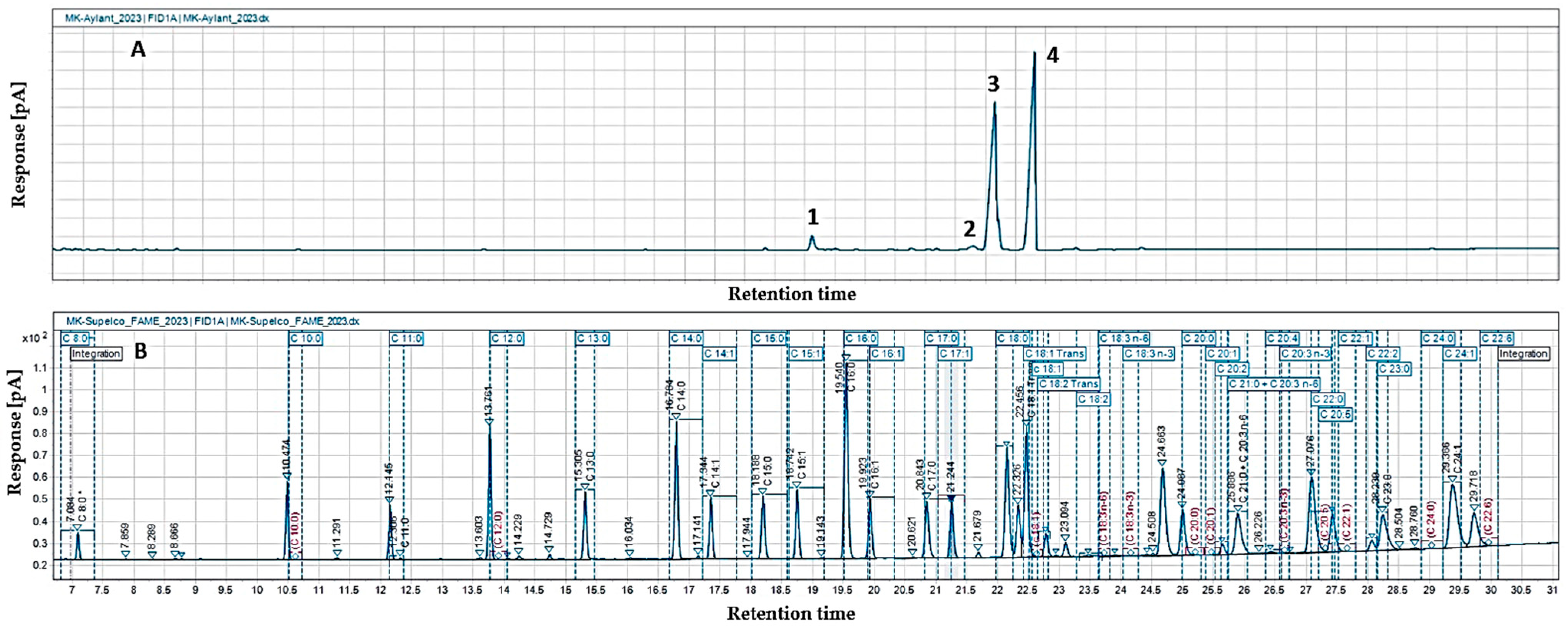
The gas chromatographic analysis of the fatty acid composition of
A. altissima seed oil showed the presence of eleven fatty acids (
Table 4,
Figure 2), among which the compositions determined were unsaturated linoleic (48.6%) and oleic (44.8%) acids. The content of saturated palmitic and stearic acids was below 3%, and the other identified fatty acids were present in significantly smaller amounts (0.2–0.6%). The lowest percentage was recorded for saturated margaric acid. The total content of saturated (SFAs), mono- (MUFAs), and polyunsaturated fatty acids (UFAs) in the oil, presented in
Table 4, showed that unsaturated fatty acids (UFAs) predominated in
A. altissima seed oil (95.3%), and a more even distribution of mono- and polyunsaturated fatty acids was observed (46.3% and 49.0%, respectively). Saturated fatty acids in the lipid fraction were only 4.7%.
The total content of saturated and unsaturated fatty acids in ailanthus seed oil was similar to the results obtained by the authors of [
3], 4.74 and 95.37%, respectively, but they established more polyunsaturated fatty acids (56.15%). In the present study, the measured amounts of total PUFAs in ailanthus seed oil were similar to corn oil (49.74%), cress oil (48.86%), milk thistle seed oil (48.81%), pumpkin seed oil (48.14%), and jackfruit seed oil (46.72%), while that of MUFAs corresponded to palm oil (46.3%), rice bran oil (43.7%), sesame oil (42.0%), and date palm seed oil (49.59%). The low SFA content corresponds to pomegranate seed oil (5.35%), with values lower even than those reported in various beneficial vegetable oils [
1].
The descending order found In the study (linoleic acid > oleic acid > palmitic acid > stearic acid) is also reported in [
3], with the best represented fatty acids in amounts similar to ours (55.76% and 38.35% for linoleic acid and oleic acid, respectively). For palmitic and stearic acid, the authors established the same quantities as we found. Other authors’ research confirms the above arrangement, with some variation in percentage content (linoleic (56.2–37.35%), oleic (23.1–25.53%), and palmitic acids (11.1–2.01%)) [
19,
20]. A similar fatty acid composition, with linoleic acid predominating (50.8%), followed by palmitic acid (30.5%), oleic acid (8.1%), and
α-linolenic acid (7.3%) is reported by the authors of [
21] for oil obtained from the wood of
A. altissima. Studies on the fatty acid composition of
Ailanthus exelsa seed oil showed a higher percentage of palmitic (12.6%) and stearic acids (7.6%) [
23]. The authors indicated that oleic acid was predominant in the fraction (65.6%) and linoleic acid content was 11.7% (more than four times lower than that found in the present study for
A. altissima.
Taking into account the fatty acid composition found, we can consider that the species is suitable for filling deficiencies related to the levels of linoleic and oleic acids. As an essential fatty acid, linoleic acid is obtained with food and it is responsible for the proper functioning of the nervous system. It participates in the construction and strength of the cell membrane, the synthesis of some hormones (thyroid and adrenal glands), and has healing properties for human hair and skin [
56]. In the review article by the aforementioned authors, healthy oils are commented on, among which pumpkin seed oil is indicated as a source of unsaturated fatty acids (73.1–73.7% total unsaturated fatty acid), in which linoleic acid (39.5–47.5%) dominates, followed by oleic acid (16.6–25.5%). For comparison, the seed kernels of custard apple contain oleic acid 47.4% (close to that found in ailanthus oil) and twice as low linoleic acid (22.9%) [
56]. The
A. altissima seed oil has a similar composition to sesame oil, which is known for its health effects, due to the strong presence of oleic 38.84% and linoleic 46.26% acids [
54]. Oils with a high content of monounsaturated fatty acids, in combination with linoleic acid, up to 45% (for better taste), and saturated fatty acids, which give them stability, are suitable for frying [
57]. An additional requirement for cooking oils is that they must be liquid, which makes high-oleic oils very suitable (such as sunflower oil, canola oil, and safflower oil). The similar composition of
A. altissima seed oil suggests similarity in biological activity, nutritional, and other beneficial properties.
3.4. Individual Sterol, Tocopherol, and Phospholipid Composition of A. altissima Seed Oil
The good results obtained for the total biologically active components of the oil from
A. altissima are a reason to deepen our research on its individual sterol, tocopherol, and phospholipid compositions (
Table 5). The main component in the sterol composition was
β-sitosterol (72.6%), followed by stigmasterol (14.0%) and campesterol (10.3%). A certain amount of Δ
7—stigmasterol (2.1%)—was also found, while the rest of the identified compounds from this group were in significantly smaller amounts (0.3% brassicasterol and cholesterol 0.7%).
El Ayeb-Zakhama et al. [
3] also found
β-sitosterol (70.30%) as the main component of the sterol fraction in ailanthus seed oil. On the other hand, they determined that the second most abundant was campesterol (13.03%), followed by Δ
5—avenasterol (6.75%) and stigmasterol (6.24%). The individual sterol composition of
A. altissima seed oil is similar to that of palm kernel oil, where
β-sitosterol content is the highest (62.6–73.1%), followed by stigmasterol (12.0–16.6%) and campesterol (8.4–12.7%) [
53]. Beta-sitosterol is also best represented in wheat germ oil, corn oil, rice bran oil, chili seed oil, and sesame oils (from 400 to 1060 mg/100 g) [
58]. The multifunctional role and great pharmacological potential are the reasons for the increased scientific interest in this food component. Pharmacological screening of
β-sitosterol revealed various antioxidant, anti-inflammatory, anticancer (against breast, prostate, colon, lung, stomach, leukemia, etc.), antimicrobial, immunomodulating, hepatoprotective, and antidiabetic effects. It also helps in the recovery processes of the human body and has a beneficial effect on the cardiovascular system (prevents heart attack and atherosclerosis), and its use as an antihyperlipidemic agent is recommended. It is proven to be harmless, which is a very important characteristic [
59,
60,
61,
62]. The second most abundant sterol in the studied oil—stigmasterol—is reported in the same levels in coconut, palm, and sesame oils used for different purposes (food, cosmetic, and pharmaceutical). Campesterol corresponds to amounts measured in coconut, grapeseed, cottonseed, grapeseed, palm, safflower, sunflower, and sesame oils (5–20%) [
53]. The presence of stigmasterols in
A. altissima fruits (ethanol extracts) is associated with the demonstrated antibacterial activity against
Escherichia coli,
Staphylococcus aureus,
Pseudomonas aeruginosa, and
Salmonella typhimurium [
13].
The individual tocopherol composition of the studied oil identified two components:
γ-tocopherol (74.6%) and
α-tocopherol, whose content is about three times lower (
Table 5). The tocopherol composition of the seed oil from
A. altissima comes close to that of peanut oil [
53] and sesame oil [
54], where
γ-tocopherol is predominant over
α-tocopherol. According to recent authors, sesame oil is oxidatively stable, which is due to the tocopherols contained in it. Tocopherols are mainly present in vegetable oils and in the form of various isomers, together with tocotrienols, which make up the vital vitamin E. The antioxidant properties of vit. E give reason to regard it as a natural antioxidant that protects the oil from oxidation and preserves its qualities [
54,
63]. It is an important nutritional component necessary in the human diet, which participates in the disposal of free radicals, the protection of the cell membrane and the prevention of cancer, cardiovascular diseases, Alzheimer’s disease, etc. [
63].
From the individual phospholipid composition, four phospholipid classes were identified, the content of which was relatively evenly distributed, varying between 19.8 and 29.5% (
Table 5). The highest was the amount of phosphatidylinositol (29.5%), followed by phosphatidic acids and phosphatidylcholine, and the lowest percentage was of phosphatidylethanolamine. Phosphatidic acids were determined for the first time for
A. altissima seed oil in the present study (25.7%).
A single publication by the authors of [
19] reported some phospholipid compounds (phosphatidylinositol, phosphatidylethanolamine, and phosphatidylcholine) in cotyledons and endosperm ailanthus. Of the three phospholipids identified, phosphatidylinositol was equally abundant in endosperm and cotyledons (2.6% in cotyledons, 2.3% in endosperm), while phosphatidylethanolamine predominated in endosperm (3.0%) and phosphatidylcholine in cotyledons (7.0%). The data in the present work are significantly higher than reported.
The phospholipid composition is close to that of linseed oil [
1]. Some of the representatives have higher concentrations in ailanthus oil: phosphatidic acids from 2.8 to 25.7 times more, and phosphatidylcholine—1.3–3.6 times more. The percentage content of phosphatidylinositol is the same as that of linseed oil. The phosphatidylcholine and phosphatidylethanolamine present in the oil of ailanthus are the two most common components in eukaryotic cell membranes, with the first occupying the largest share in their composition. The two key macromolecules are found in a certain ratio, the violation of which is associated with the progression of diseases such as diabetes and atherosclerosis [
64]. Various biological manifestations are attributed to them, and their presence in food is of particular importance for human health—they exhibit antioxidant properties, improve memory and immunity, and participate in the prevention of cardiovascular diseases [
65]. According to recent studies, an important feature of phospholipids is their ability to form complexes with phytoactive compounds or plant extracts. Phospholipid complexation promotes the solubility of the drug, and facilitates its penetration into the body, as well as its sustained release. One of the most important advantages of the formed phospholipid complexes is that they are biocompatible (their main ingredient is usually phosphatidylcholine); therefore, their side effects are weak or absent [
66].
3.5. In Vitro Cytotoxicity
Cytotoxicity was presented as mean values of CC
50 (50% cytotoxic concentration) for each tested substance based on the obtained sigmoidal curves (
Table 6,
Figure 3), and cisplatin is the compound applied as a standard in the present study. In the cytotoxic experiment at an initial concentration of 1 mg/mL of glyceride oil, no effect was recorded. Therefore, the in vitro test continued using higher concentrations in the order of 0.04 to 10 mg/mL on the selected BALB/c 3T3, clone A31 cell line (
Figure 3). In the case of cisplatin, the initial cytotoxicity was observed at a concentration from 2.5 to 600 µg/mL. The sigmoidal curves of the graph show significantly increased cytotoxicity of cisplatin over
A. altissima seed oil.
This is also shown in
Table 6—the cytotoxicity exhibited by cisplatin is at a lower concentration of over 173 times, compared to that of the oil tested.
Based on the results obtained, we can summarize that the tested oil from the tree of heaven does not exhibit a cytotoxic effect on mouse embryonic fibroblast, i.e., it is harmless (non-toxic substance).
The lack of cytotoxicity in ailanthus oil has also been reported by previous authors [
3]. They indicate the LD
50 of seed oil at more than 2 g/kg, which proves its potential safe use below this concentration. What they observed is confirmed by the fact that so far no side effects have been reported when using it for treatment. Some studies have focused on investigating the cytotoxicity of ailanthus fruit extracts, but no such data are available for its glyceride oil. The crude extract (methanolic from fruits) and fractions showed good cytotoxic activity attributed to the isolated
β-sitosterol glucoside [
12].
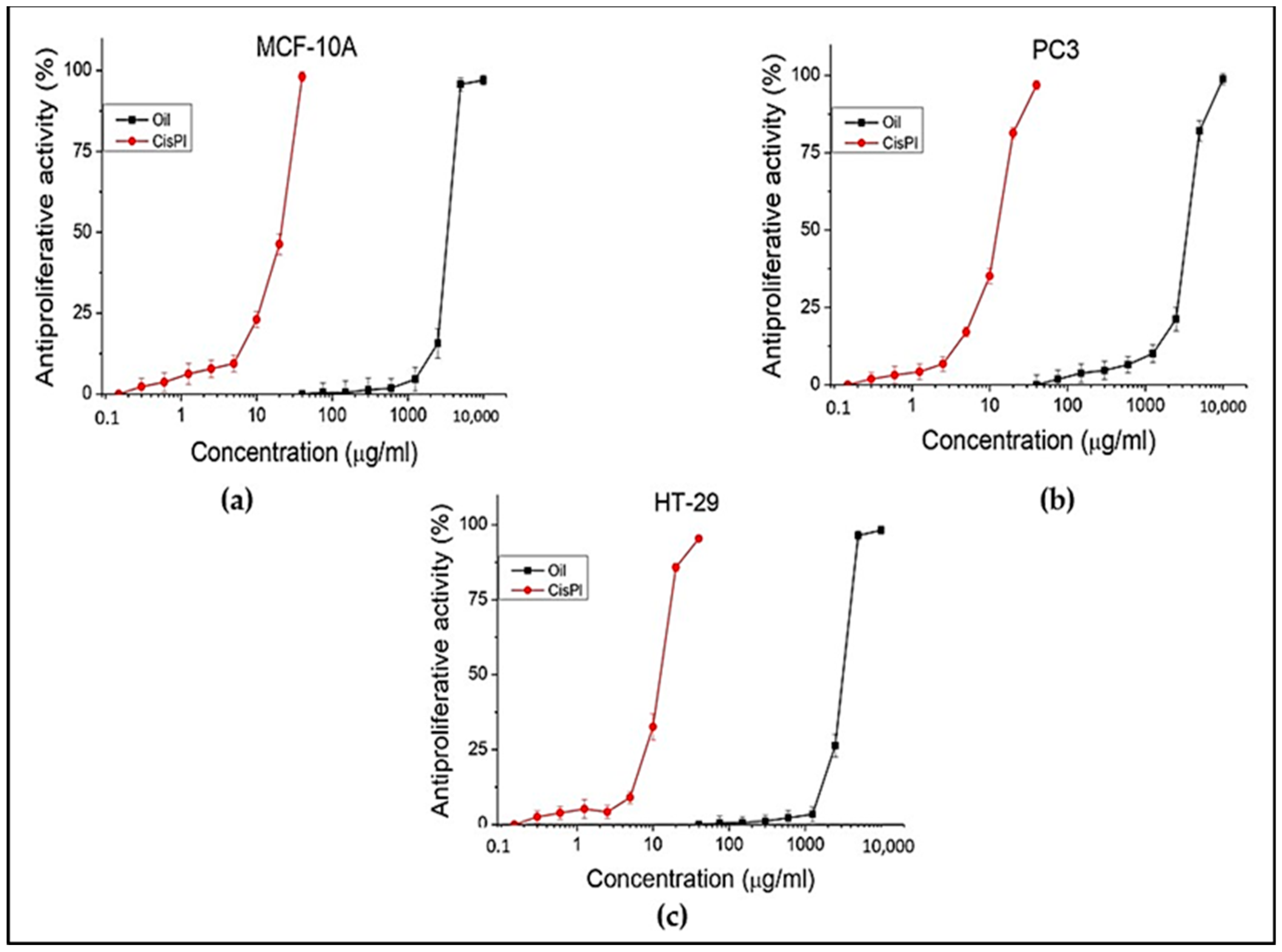
3.6. In vitro Antiproliferative Capacity
The antiproliferative activity of the oil was evaluated against the following cell lines: HT-29 (colorectal adenocarcinoma), PC3 (prostate carcinoma), and MCF-10A (normal human epithelial) cell lines. The standard cisplatin that was used for the cytotoxic test was also used for the antiproliferative test. The results of the research are presented in
Table 7 and
Figure 4.
Different concentrations (from 40 to 10,000 µg/mL) of the oil were tested on all three selected cell lines. No activity was found below 1000 µg/mL concentration for HT-29 and PC3 cell lines. Positive results were observed from 2500 µg/mL to 10,000 µg/mL concentrations for tumor cell lines. The effect and concentrations of oil observed on tumor cell lines are similar to those on the normal human cell line (MCF-10A). An indicator of the ratio that exists between the test substance and the particular cell line (normal or tumor) is the selectivity index (SI). In this case, its value in both tumor lines is low (about 1), indicating that the oil lacks selectivity toward them. The taller than 1.5 values for cisplatin are an expression of greater selectivity.
The present experiment demonstrated a weak antiproliferative effect of ailanthus oil on HT-29 and PC3 cell lines.
The present good antiproliferative results bring new information about the antitumor potential of the species, namely that both its extracts and its seed oil possess such capacity. Until now, antiproliferative activity has been reported only for its extracts (from stem barks, leaves, and roots) and for individual isolated compounds, among which ailanthone is the most significant [
6,
7]. Ailanthone can be used as an effective anticancer agent, inducing apoptosis in gastrointestinal cancer cells (damages cancer DNA and inhibits its repair [
67]. Anticancer effects of
A. altissima extracts have been described against carcinoma of the thyroid gland, breast, bladder, melanoma, hepatocellular carcinoma, acute myeloid leukemia, human histiocytic lymphoma, osteosarcoma, prostate cancer, lung, liver, etc. [
6,
7,
13]. There are data on potent cytotoxic activity against hepatocarcinoma cells from the leaf extracts, but there is no evidence of similar activity from the oil of the plant species [
68].
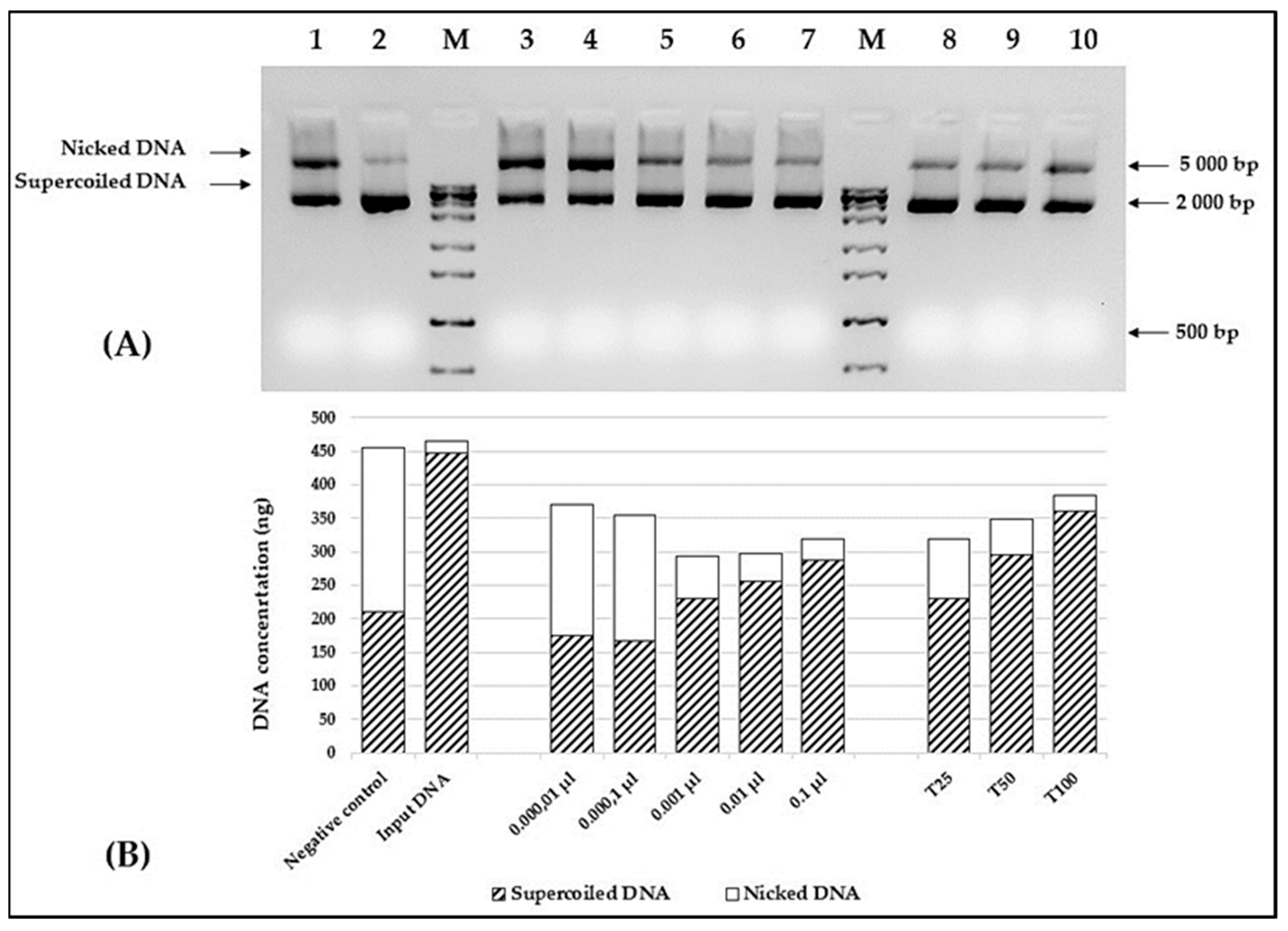
3.7. In Vitro DNA Protective Capacity of the A. altissima Seed Oil
The protective capacity of the
A. altissima seed oil was assessed in the in vitro DNA nicking assay experiment. To obtain better dispersion of the oil in an aqueous solution, all reactions were set in the presence of 6% methylated cyclodextrin. The results are shown in
Figure 5A. Despite the limited extract solubility in aqueous solutions, a clear correlation between the oil concentration and the amount of supercoiled plasmid DNA was observed (
Figure 5B). As depicted in
Figure 5, the presence of 0.01 μL of the tested oil is sufficient to significantly reduce the amount of nicked DNA. As expected, a similar correlation was found, when different concentrations of Trolox were used as a positive control.
In vitro DNA nicking protection of ethanol leaf, flower, and stem bark extracts of
A. altissima is reported in our previous study. At concentration of 5.25–10 μg/mL of the extracts a complete protection of plasmid DNA from oxidative damage caused by hydrogen peroxide was found [
26].
Other authors also reported a protective potential of ailanthus stem bark extracts on DNA damage caused by zeocin [
69]. The latter compared the activity of methanolic and hexane extracts, establishing a higher degree of protection on the tested DNA from yeast cells of
Saccharomyces cerevisiae of the first extract, whose difference is explained by the better solubility of the bioactive compounds in the hydrophilic agent. The present study adds valuable information on the observed biological activity of another plant product from
A. altissima, such as its glyceride oil, which has been missing until now.