Reactive Carbonyl Species Scavenger: Epigallocatechin-3-Gallate
Abstract
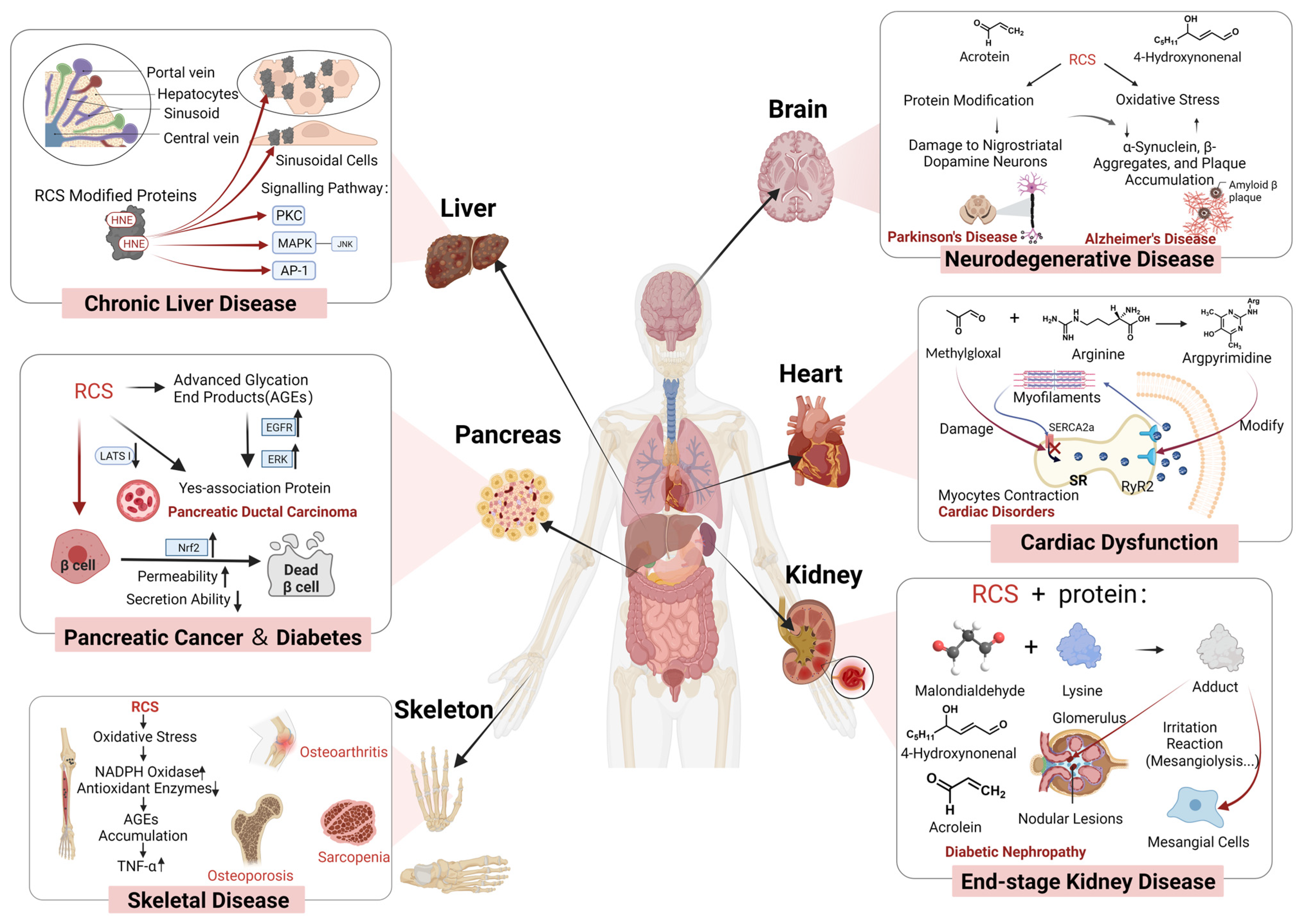
1. Introduction
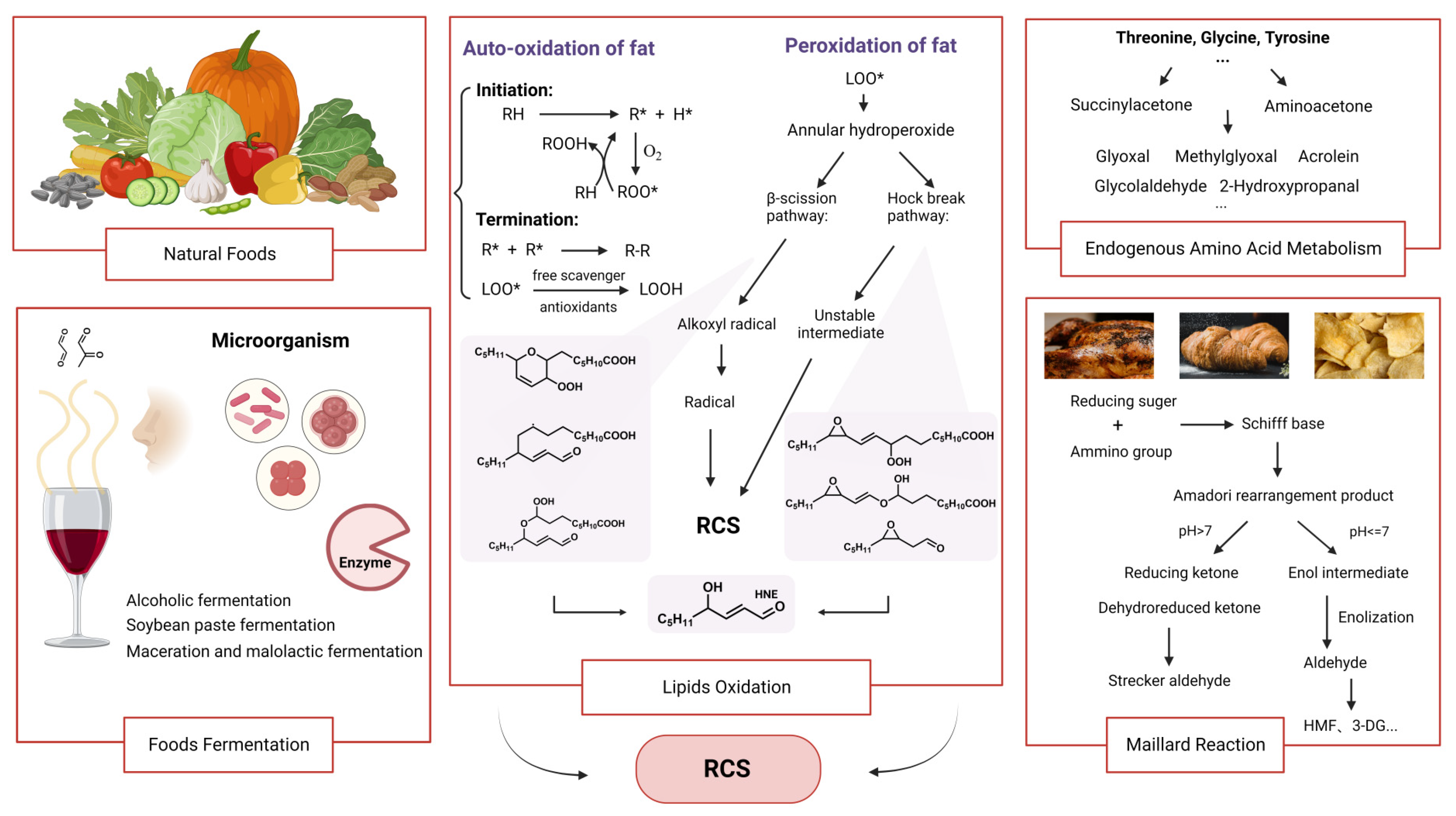
2. RCS in Food Processing
2.1. Species and Toxicity
2.2. Protein Oxidation
2.3. Lipid Oxidation
2.4. Maillard Reaction
2.5. Fermentation
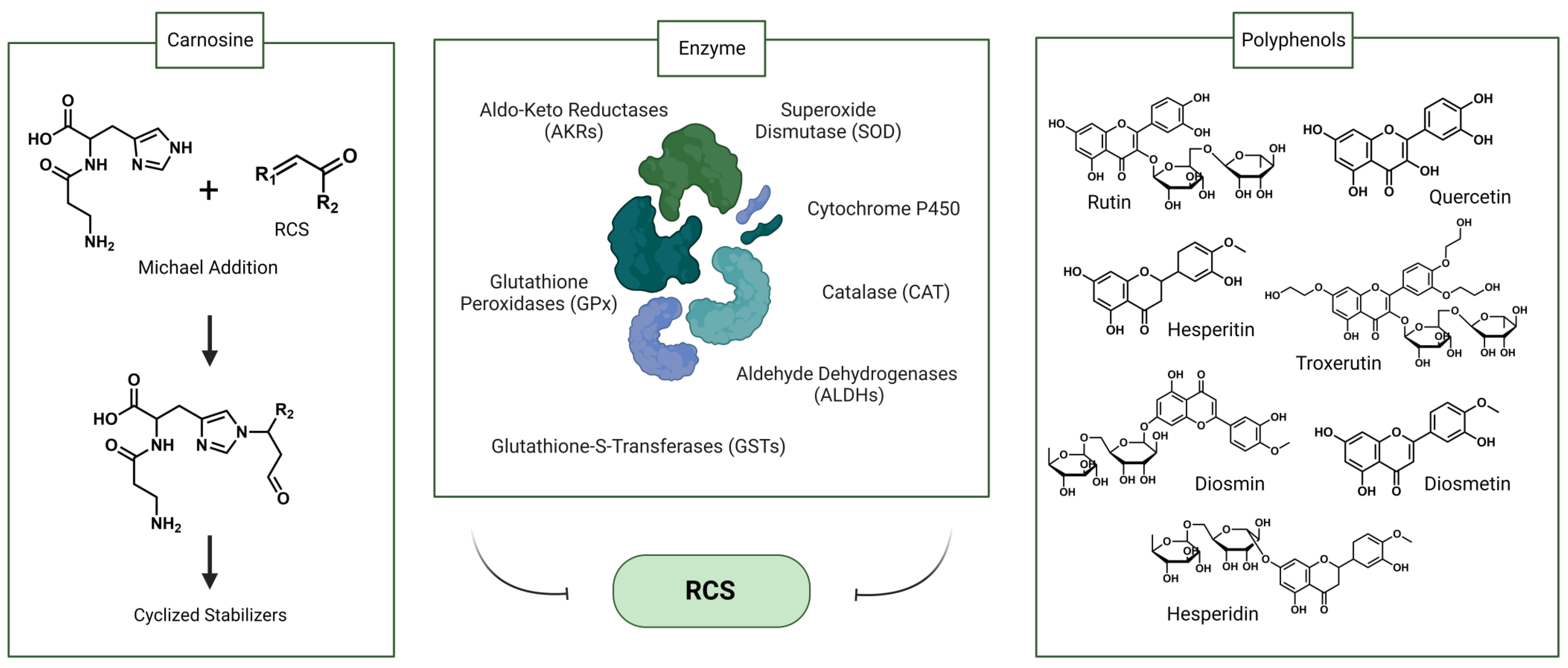
3. Metabolic Processes and Influencing Factors of RCS Elimination
3.1. Endogenous Factors
3.2. Exogenous Factors
4. Clearance Pathways of the RCS by EGCG
4.1. Source of EGCG
4.2. Radical Capture
4.3. RCS Inhibition in Maillard Reaction
4.4. Direct RCS Capture
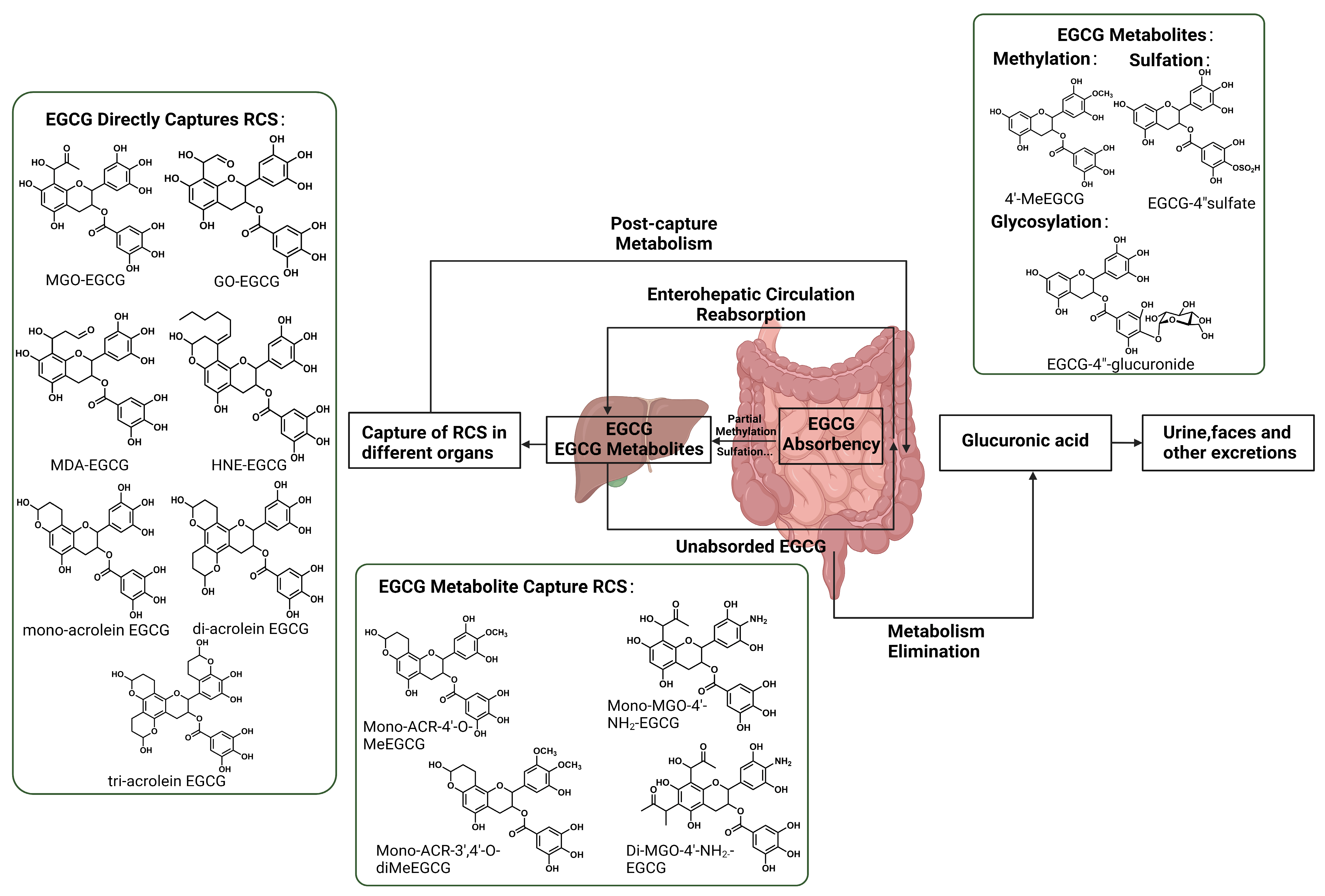
5. EGCG-RCS Adducts Metabolism
5.1. Metabolites of EGCG Capture RCS
5.2. EGCG-RCS Adducts Metabolism
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1-DG | 1-deoxyglucosone |
| ECGT | 1-o-gallacyl-β-D-glucose o-galloyltransferase |
| ABTS | 2,2′-azinobis(3-ethylbenzothiaziline-6-sulfonate); |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| 3-DG | 3-deoxyglucosone |
| DGO | 3-deoxyglucosone |
| 4CL | 4-coumarin-Coenzyme A |
| HHE | 4-hydroxy-2-hexenal |
| HNE | 4-hydroxy-2-nonenal |
| HMF | 5-hydroxymethylfurfural |
| 6′-OH-DMA | 6′-hydroxy-O-demethylangolensin |
| APAO | acetylpolyamine oxidase |
| GA | acid-derived gallic acid |
| ACR-Gen | ACR adduct of genistein |
| ACR | acrolein; NAD-binding, adenine dinucleotide -binding |
| AGEs | advanced glycation end products |
| ALEs | advanced lipoxidation end products |
| ALDH1A1 | aldehyde dehydrogenase 1 family member A1 |
| ALDH1A3 | aldehyde dehydrogenase 1 family member A3 |
| ALDH4 | aldehyde dehydrogenase 4 |
| ALDHs | aldehyde dehydrogenases |
| AKR1B1 | aldo-keto reductase family 1 member B1 |
| AKR7A2 | aldo-keto reductase family 7 member A2 |
| AKRs | aldo-keto reductases |
| ARP | Amadori rearrangement products |
| AA | arachidonic acid |
| Arg | arginine |
| CO | carbon monoxide |
| CML | carboxymethyl lysine |
| CAT | catalase |
| C4H | cinnamate 4-hydroxylase |
| DGEN | dehydrogenistein |
| DP | deoxypentosone |
| DMS | dimethyl sulfide |
| EGCG | epigallocatechin-3-gallate |
| FA | formaldehyde |
| GLA | gamma-linolenic acid |
| GSH | glutathione |
| GPx | glutathione peroxidases |
| GSTs | glutathione-s-transferases |
| GS-DHN | glutathionyl-1,4-dihydroxynonane |
| GO | glyoxal |
| GOLD | glyoxal lysine dimer |
| H2S | hydrogen sulfide |
| LA | linoleic acid |
| LOOH | lipid hydroperoxides |
| Lys | lysine |
| MDA | malondialdehyde |
| MGO | methylglyoxal |
| MOLD | methylglyoxal lysine dimer |
| MFN2 | mitofusin-2 |
| MAPK | mitogen-activated protein kinase |
| MeEGCG | mono-4”-O-methyl-EGCG |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NHBE | normal human bronchial epithelial |
| NF-κB | nuclear factor κB |
| OPA1 | optic atrophy-1 |
| PAL | phenylalanine ammonia-lyase |
| Pro | proline |
| PKC | protein kinase C |
| RCS | reactive carbonyl species |
| ROS | reactive oxygen species |
| RAGE | receptor for advanced glycation end products |
| RCS-BSA | reduced, carboxamidomethylated, and succinylated bovine serum albumin; RyR2, ryanodine receptor 2 |
| SSAO | Semicarbazide-sensitive amine oxidase |
| SSAT | spermidine/spermine N1-acetyltransferase |
| SMO | spermine oxidase |
| SOD | superoxide dismutase |
| Trx | thioredoxin |
| TrxR | thioredoxin reductase |
| Thr | threonine |
| MUC | trans-muconaldehyde |
| TRAF6 | tumor necrosis factor receptor-associated factor 6 |
| CsSCPL1A | type 1A serine carboxypeptidase-like acyltransferases |
| VAP-1 | vascular adhesion protein 1 |
| β-G | β-glucose gallate |
References
- Zhai, X.; Zhang, L.; Granvogl, M.; Ho, C.T.; Wan, X. Flavor of tea (Camellia sinensis): A review on odorants and analytical techniques. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3867–3909. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Karim, M.R.; Wang, B.H.; Peng, J.N. Effects of Green Tea (-)-Epigallocatechin-3-Gallate (EGCG) on Cardiac Function-A Review of the Therapeutic Mechanism and Potentials. Mini-Rev. Med. Chem. 2022, 22, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Wu, C.J.; Chien, C.Y.; Chien, C.T. Green Tea Polyphenol Catechins Inhibit Coronavirus Replication and Potentiate the Adaptive Immunity and Autophagy-Dependent Protective Mechanism to Improve Acute Lung Injury in Mice. Antioxidants 2021, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Karami, E.; Esfahrood, Z.R.; Mansouri, R.; Haerian, A.; Abdian-Asl, A. Effect of epigallocatechin-3-gallate on tumor necrosis factor-alpha production by human gingival fibroblasts stimulated with bacterial lipopolysaccharide: An in vitro study. J. Indian. Soc. Periodontol. 2021, 25, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.-C.; Li, S.; Zhan, J.; Ho, C.-T. Immunomodulatory effects of green tea polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Ho, C.-T.; Jun, M. Multifaceted neuroprotective effects of (-)-epigallocatechin-3-gallate (EGCG) in Alzheimer’s disease: An overview of pre-clinical studies focused on β-amyloid peptide. Food Sci. Hum. Wellness 2022, 11, 483–493. [Google Scholar] [CrossRef]
- Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Liu, Q.; Mao, Q.Q.; Shang, A.; Li, H.B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Fuloria, S.; Subramaniyan, V.; Karupiah, S.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Guad, R.M.; Udupa, K.; Fuloria, N.K. A comprehensive review on source, types, effects, nanotechnology, detection, and therapeutic management of reactive carbonyl species associated with various chronic diseases. Antioxidants 2020, 9, 1075. [Google Scholar] [CrossRef]
- Semchyshyn, H.M. Reactive Carbonyl Species In Vivo: Generation and Dual Biological Effects. Sci. World J. 2014, 2014, 417842. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, C.; Liu, F.; Zheng, J.; Ou, J.; Zhao, D.; Ou, S. Origin and fate of acrolein in foods. Foods 2022, 11, 1976. [Google Scholar] [CrossRef]
- Iacobini, C.; Vitale, M.; Haxhi, J.; Pesce, C.; Pugliese, G.; Menini, S. Food-related carbonyl stress in cardiometabolic and cancer risk linked to unhealthy modern diet. Nutrients 2022, 14, 1061. [Google Scholar] [CrossRef]
- Amslinger, S. The Tunable Functionality of α,β-Unsaturated Carbonyl Compounds Enables Their Differential Application in Biological Systems. Chemmedchem 2010, 5, 351–356. [Google Scholar] [CrossRef]
- Henning, R.J.; Johnson, G.T.; Coyle, J.P.; Harbison, R.D. Acrolein Can Cause Cardiovascular Disease: A Review. Cardiovasc. Toxicol. 2017, 17, 227–236. [Google Scholar] [CrossRef]
- Dham, D.; Roy, B.; Gowda, A.; Pan, G.D.; Sridhar, A.; Zeng, X.Q.; Thandavarayan, R.A.; Palaniyandi, S.S. 4-Hydroxy-2-nonenal, a lipid peroxidation product, as a biomarker in diabetes and its complications: Challenges and opportunities. Free Radic. Res. 2021, 55, 547–561. [Google Scholar] [CrossRef]
- Huang, Y.J.; Jin, M.H.; Pi, R.B.; Zhang, J.J.; Ouyang, Y.; Chao, X.J.; Chen, M.H.; Liu, P.Q.; Yu, J.C.; Ramassamy, C.; et al. Acrolein induces Alzheimer’s disease-like pathologies in vitro and in vivo. Toxicol. Lett. 2013, 217, 184–191. [Google Scholar] [CrossRef]
- Bein, K.; Leikauf, G.D. Acrolein—A pulmonary hazard. Mol. Nutr. Food Res. 2011, 55, 1342–1360. [Google Scholar] [CrossRef]
- Mure, K.; Tomono, S.; Mure, M.; Horinaka, M.; Mutoh, M.; Sakai, T.; Ishikawa, H.; Wakabayashi, K. The Combination of Cigarette Smoking and Alcohol Consumption Synergistically Increases Reactive Carbonyl Species in Human Male Plasma. Int. J. Mol. Sci. 2021, 22, 9043. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.S.; Mano, J.i. Lipid Peroxide-Derived Reactive Carbonyl Species as Mediators of Oxidative Stress and Signaling. Front. Plant Sci. 2021, 12, 720867. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.J.; Zhen, Z. Reactive Carbonyl Species: Diabetic Complication in the Heart and Lungs. Trends Endocrinol. Metab. 2019, 30, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jin, W.; Wu, Q.; Zhou, Q. Acrolein: Formation, health hazards and its controlling by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2023, 1–14. [Google Scholar] [CrossRef]
- Ansari, M.A.; Keller, J.N.; Scheff, S.W. Protective effect of Pycnogenol in human neuroblastoma SH-SY5Y cells following acrolein-induced cytotoxicity. Free Radic. Biol. Med. 2008, 45, 1510–1519. [Google Scholar] [CrossRef]
- Tulen, C.B.M.; Snow, S.J.; Leermakers, P.A.; Kodavanti, U.P.; van Schooten, F.J.; Opperhuizen, A.; Remels, A.H.V. Acrolein inhalation acutely affects the regulation of mitochondrial metabolism in rat lung. Toxicology 2022, 469, 153129. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, P.; Bailey, T.; Bhattarai, S.; Subedi, U.; Miller, C.; Ara, H.; Kidambi, S.; Sun, H.; Panchatcharam, M.; et al. Electrophilic Aldehyde 4-Hydroxy-2-Nonenal Mediated Signaling and Mitochondrial Dysfunction. Biomolecules 2022, 12, 1555. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Morton, J.D.; Clerens, S.; Dyer, J.M. Proteomic investigation of protein profile changes and amino acid residue-level modification in cooked lamb longissimus thoracis et lumborum: The effect of roasting. Meat Sci. 2016, 119, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Unzeta, M.; Hernàndez-Guillamon, M.; Sun, P.; Solé, M. SSAO/VAP-1 in cerebrovascular disorders: A potential therapeutic target for stroke and Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 3365. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.M.; Hazen, S.L.; Hsu, F.F.; Heinecke, J.W. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha, beta-unsaturated aldehydes by phagocytes at sites of inflammation. J. Clin. Investig. 1997, 99, 424–432. [Google Scholar]
- Li, P.; He, W.; Wu, G. Composition of amino acids in foodstuffs for humans and animals. In Amino Acids in Nutrition and Health: Amino Acids in Gene Expression, Metabolic Regulation, and Exercising Performance; Springer: Cham, Switzerland, 2021; pp. 189–210. [Google Scholar]
- Iguacel, I.; Schmidt, J.A.; Perez-Cornago, A.; Van Puyvelde, H.; Travis, R.; Stepien, M.; Scalbert, A.; Casagrande, C.; Weiderpass, E.; Riboli, E. Associations between dietary amino acid intakes and blood concentration levels. Clin. Nutr. 2021, 40, 3772–3779. [Google Scholar] [CrossRef]
- Uemura, T.; Nakamura, M.; Sakamoto, A.; Suzuki, T.; Dohmae, N.; Terui, Y.; Tomitori, H.; Casero Jr, R.A.; Kashiwagi, K.; Igarashi, K. Decrease in acrolein toxicity based on the decline of polyamine oxidases. Int. J. Biochem. Cell Biol. 2016, 79, 151–157. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Igarashi, K. Molecular Characteristics of Toxicity of Acrolein Produced from Spermine. Biomolecules 2023, 13, 298. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef]
- Xiang, Z.Y.; Wang, H.L.; Stevanovic, S.; Jing, S.G.; Lou, S.; Tao, S.; Li, L.; Liu, J.; Yu, M.Z.; Wang, L.N. Assessing impacts of factors on carbonyl compounds emissions produced from several typical Chinese cooking. Build. Environ. 2017, 125, 348–355. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Liu, X.; Wu, D.; Ding, Y.; Li, G.; Wu, Y. Typical reactive carbonyl compounds in food products: Formation, influence on food quality, and detection methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 503–529. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Z.; He, Z.; Wang, Z.; Qin, F.; Zeng, M.; Chen, J. Effect of Freeze-Thaw Cycles on the Oxidation of Protein and Fat and Its Relationship with the Formation of Heterocyclic Aromatic Amines and Advanced Glycation End Products in Raw Meat. Molecules 2021, 26, 1264. [Google Scholar] [CrossRef]
- Hodge, J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Cha, J.; Debnath, T.; Lee, K.-G. Analysis of α-dicarbonyl compounds and volatiles formed in Maillard reaction model systems. Sci. Rep. 2019, 9, 5325. [Google Scholar] [CrossRef]
- Lee, J.; Roux, S.; Le Roux, E.; Keller, S.; Rega, B.; Bonazzi, C. Unravelling caramelization and Maillard reactions in glucose and glucose+ leucine model cakes: Formation and degradation kinetics of precursors, α-dicarbonyl intermediates and furanic compounds during baking. Food Chem. 2022, 376, 131917. [Google Scholar] [CrossRef]
- Li, M.; Shen, M.; Lu, J.; Yang, J.; Huang, Y.; Liu, L.; Fan, H.; Xie, J.; Xie, M. Maillard reaction harmful products in dairy products: Formation, occurrence, analysis, and mitigation strategies. Food Res. Int. 2022, 151, 110839. [Google Scholar] [CrossRef] [PubMed]
- Tavares, W.P.S.; Dong, S.; Jin, W.; Yang, Y.; Han, K.; Zha, F.; Zhao, Y.; Zeng, M. Effect of different cooking conditions on the profiles of Maillard reaction products and nutrient composition of hairtail (Thichiurus lepturus) fillets. Food Res. Int. 2018, 103, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Wang, A.; Lao, F.; Shen, Q.; Liao, X.; Zhang, P.; Wu, J. Effects of frying, roasting and boiling on aroma profiles of adzuki beans (Vigna angularis) and potential of adzuki bean and millet flours to improve flavor and sensory characteristics of biscuits. Food Chem. 2021, 339, 127878. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Shah, A.M.; Tarfeen, N.; Mohamed, H.; Song, Y. Fermented Foods: Their Health-Promoting Components and Potential Effects on Gut Microbiota. Fermentation 2023, 9, 118. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic fermentation of polyphenols: Potential sources of novel functional foods. Food Prod. Process. Nutr. 2022, 4, 21. [Google Scholar] [CrossRef]
- Gammacurta, M.; Marchand, S.; Moine, V.; de Revel, G. Influence of different yeast/lactic acid bacteria combinations on the aromatic profile of red Bordeaux wine. J. Sci. Food Agric. 2017, 97, 4046–4057. [Google Scholar] [CrossRef] [PubMed]
- Lonvaud-Funel, A. Microbiology of the malolactic fermentation: Molecular aspects. FEMS Microbiol. Lett. 1995, 126, 209–214. [Google Scholar] [CrossRef]
- An, F.; Li, M.; Zhao, Y.; Zhang, Y.; Mu, D.; Hu, X.; You, S.; Wu, J.; Wu, R. Metatranscriptome-based investigation of flavor-producing core microbiota in different fermentation stages of dajiang, a traditional fermented soybean paste of Northeast China. Food Chem. 2021, 343, 128509. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilicstress. Free Radic. Biol. Med. 2013, 56, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Laskar, A.; Younus, H. Aldehyde toxicity and metabolism: The role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab. Rev. 2019, 51, 42–64. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Amunom, I.; Dieter, L.J.; Tamasi, V.; Cai, J.; Conklin, D.J.; Srivastava, S.; Martin, M.V.; Guengerich, F.P.; Prough, R.A. Cytochromes P450 catalyze the reduction of alpha,beta-unsaturated aldehydes. Chem. Res. Toxicol. 2011, 24, 1223–1230. [Google Scholar] [CrossRef]
- Li, D.; Ferrari, M.; Ellis, E.M. Human aldo-keto reductase AKR7A2 protects against the cytotoxicity and mutagenicity of reactive aldehydes and lowers intracellular reactive oxygen species in hamster V79-4 cells. Chem. Biol. Interact. 2012, 195, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, Y.C.; Ramana, K.V.; Chaudhary, P.; Srivastava, S.K.; Awasthi, S. Regulatory roles of glutathione-S-transferases and 4-hydroxynonenal in stress-mediated signaling and toxicity. Free Radic. Biol. Med. 2017, 111, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.J.; Witmer, C.M.; Witz, G. Nephrotoxicity of the 1:1 acrolein-glutathione adduct in the rat. Toxicol. Appl. Pharmacol. 1992, 117, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Bhatnagar, A.; Srivastava, S.; Yadav, U.C.; Awasthi, S.; Awasthi, Y.C.; Srivastava, S.K. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): Role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J. Biol. Chem. 2006, 281, 17652–17660. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.D.; Thomas, P.D.; Lopaschuk, G.D.; Poznansky, M.J. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J. Biol. Chem. 1993, 268, 416–420. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Regazzoni, L.; de Courten, B.; Garzon, D.; Altomare, A.; Marinello, C.; Jakubova, M.; Vallova, S.; Krumpolec, P.; Carini, M.; Ukropec, J.; et al. A carnosine intervention study in overweight human volunteers: Bioavailability and reactive carbonyl species sequestering effect. Sci. Rep. 2016, 6, 27224. [Google Scholar] [CrossRef] [PubMed]
- Bispo, V.S.; de Arruda Campos, I.P.; Di Mascio, P.; Medeiros, M.H. Structural Elucidation of a Carnosine-Acrolein Adduct and its Quantification in Human Urine Samples. Sci. Rep. 2016, 6, 19348. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I. Potential of Vasoprotectives to Inhibit Non-Enzymatic Protein Glycation, and Reactive Carbonyl and Oxygen Species Uptake. Int. J. Mol. Sci. 2021, 22, 10026. [Google Scholar] [CrossRef]
- Li, D.X.; Mitsuhashi, S.; Ubukata, M. Protective effects of hesperidin derivatives and their stereoisomers against advanced glycation end-products formation. Pharm. Biol. 2012, 50, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, J.; Andersen, M.L.; Peters, G.H.; Lund, M.N. Predicting the reaction rates between flavonoids and methylglyoxal by combining molecular properties and machine learning. Food Biosci. 2023, 54, 102890. [Google Scholar] [CrossRef]
- Zhu, Q.; Liang, C.P.; Cheng, K.W.; Peng, X.; Lo, C.Y.; Shahidi, F.; Chen, F.; Ho, C.T.; Wang, M. Trapping effects of green and black tea extracts on peroxidation-derived carbonyl substances of seal blubber oil. J. Agric. Food Chem. 2009, 57, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Additives, E.P.o.F.; Food, N.S.a.t.; Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar]
- Huang, M.; Han, Y.; Li, L.; Rakariyatham, K.; Wu, X.; Gao, Z.; Xiao, H. Protective effects of non-extractable phenolics from strawberry against inflammation and colon cancer in vitro. Food Chem. 2022, 374, 131759. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; van de Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Hudthagosol, C.; Haddad, E.H.; McCarthy, K.; Wang, P.; Oda, K.; Sabate, J. Pecans acutely increase plasma postprandial antioxidant capacity and catechins and decrease LDL oxidation in humans. J. Nutr. 2011, 141, 56–62. [Google Scholar] [CrossRef]
- Shahidi, F.; Alasalvar, C.; Liyana-Pathirana, C.M. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J. Agric. Food Chem. 2007, 55, 1212–1220. [Google Scholar] [CrossRef]
- Xiang, P.; Zhu, Q.; Zhang, L.; Xu, P.; Liu, L.; Li, Y.; Cheng, B.; Wang, X.; Liu, J.; Shi, Y.; et al. Integrative analyses of transcriptome and metabolome reveal comprehensive mechanisms of Epigallocatechin-3-gallate (EGCG) biosynthesis in response to ecological factors in tea plant (Camellia sinensis). Food Res. Int. 2023, 166, 112591. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, M.; Wan, J.; Zheng, X.; Teng, C.; Xie, X.; Pan, W.; Hu, B.; Huang, J.; Liu, Z.; et al. Research progress of epigallocatechin-3-gallate (EGCG) on anti-pathogenic microbes and immune regulation activities. Food Funct. 2021, 12, 9607–9619. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, Z.; Huang, Z.; Zhang, X.; Zhou, Y.; Luo, Z.; Zeng, W.; Su, J.; Peng, C.; Chen, X. Epigallocatechin-3-gallate(EGCG) suppresses melanoma cell growth and metastasis by targeting TRAF6 activity. Oncotarget 2016, 7, 79557–79571. [Google Scholar] [CrossRef]
- Mandel, S.; Amit, T.; Reznichenko, L.; Weinreb, O.; Youdim, M.B.H. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 2006, 50, 229–234. [Google Scholar] [CrossRef]
- Boulmokh, Y.; Belguidoum, K.; Meddour, F.; Amira-Guebailia, H. Investigation of antioxidant activity of epigallocatechin gallate and epicatechin as compared to resveratrol and ascorbic acid: Experimental and theoretical insights. Struct. Chem. 2021, 32, 1907–1923. [Google Scholar] [CrossRef]
- Sang, S.; Cheng, X.; Stark, R.E.; Rosen, R.T.; Yang, C.S.; Ho, C.T. Chemical studies on antioxidant mechanism of tea catechins: Analysis of radical reaction products of catechin and epicatechin with 2,2-diphenyl-1-picrylhydrazyl. Bioorg. Med. Chem. 2002, 10, 2233–2237. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Okuno, K.; Yoshioka, H.; Yoshioka, H. Characteristics of the OH radical scavenging activity of tea catechins. J. Radioanal. Nucl. Chem. 2007, 272, 455–459. [Google Scholar] [CrossRef]
- Elbling, L.; Weiss, R.-M.; Teufelhofer, O.; Uhl, M.; Knasmueller, S.; Schulte-Hermann, R.; Berger, W.; Micksche, M. Green tea extract and (−)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB J. 2005, 19, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bin, Q.; Peterson, D.G.; Elias, R.J. Influence of phenolic compounds on the mechanisms of pyrazinium radical generation in the Maillard reaction. J. Agric. Food Chem. 2012, 60, 5482–5490. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Mujumdar, A.S.; Liu, Y. Combination of epigallocatechin gallate with l-cysteine in inhibiting Maillard browning of concentrated orange juice during storage. LWT 2022, 154, 112604. [Google Scholar] [CrossRef]
- Liao, X.; Yang, C.; Lin, H.; Li, B. Quenching effects of (-)-Epigallocatechin gallate for singlet oxygen production and its protection against oxidative damage induced by Ce6-mediated photodynamic therapy in vitro. Photodiagnosis Photodyn. Ther. 2021, 36, 102467. [Google Scholar] [CrossRef]
- Yu, X.; Cui, H.; Hayat, K.; Hussain, S.; Jia, C.; Zhang, S.L.; Tahir, M.U.; Zhang, X.; Ho, C.T. Effective Mechanism of (-)-Epigallocatechin Gallate Indicating the Critical Formation Conditions of Amadori Compound during an Aqueous Maillard Reaction. J. Agric. Food Chem. 2019, 67, 3412–3422. [Google Scholar] [CrossRef]
- Yu, J.H.; Cui, H.P.; Zhang, Q.; Hayat, K.; Zhan, H.; Yu, J.Y.; Jia, C.S.; Zhang, X.M.; Ho, C.T. Adducts Derived from (-)-Epigallocatechin Gallate-Amadori Rearrangement Products in Aqueous Reaction Systems: Characterization, Formation, and Thermolysis. J. Agric. Food Chem. 2020, 68, 10902–10911. [Google Scholar] [CrossRef]
- Yu, J.; Cui, H.; Tang, W.; Hayat, K.; Hussain, S.; Tahir, M.U.; Gao, Y.; Zhang, X.; Ho, C.T. Interaction of (-)-Epigallocatechin Gallate and Deoxyosones Blocking the Subsequent Maillard Reaction and Improving the Yield of N-(1-Deoxy-d-xylulos-1-yl)alanine. J. Agric. Food Chem. 2020, 68, 1714–1724. [Google Scholar] [CrossRef]
- Wei, R.; Hackman, R.M.; Wang, Y.; Mackenzie, G.G. Targeting Glycolysis with Epigallocatechin-3-Gallate Enhances the Efficacy of Chemotherapeutics in Pancreatic Cancer Cells and Xenografts. Cancers 2019, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Tu, A.-T.; Lin, J.-A.; Lee, C.-H.; Chen, Y.-A.; Wu, J.-T.; Tsai, M.-S.; Cheng, K.-C.; Hsieh, C.-W. Reduction of 3-deoxyglucosone by epigallocatechin gallate results partially from an addition reaction: The possible mechanism of decreased 5-hydroxymethylfurfural in epigallocatechin gallate-treated black garlic. Molecules 2021, 26, 4746. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, Z.; Qin, C.; Lai, G.; Wen, M.; Ho, C.-T.; Zhang, L.; Wan, X. Model studies on the reaction products formed at roasting temperatures from either catechin or tea powder in the presence of glucose. J. Agric. Food Chem. 2021, 69, 11417–11426. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Zhang, L.; Qiao, X.; Xu, Z. Tea polyphenols as inhibitors of furan formed in the Maillard model system and canned coffee model. J. Food Sci. 2017, 82, 1271–1277. [Google Scholar] [CrossRef]
- Ho, C.-T.; Wang, M. Dietary phenolics as reactive carbonyl scavengers: Potential impact on human health and mechanism of action. J. Tradit. Complement. Med. 2013, 3, 139–141. [Google Scholar] [CrossRef]
- Zhang, S.W.; Zhao, Y.T.; Ohland, C.; Jobin, C.; Sang, S. Microbiota facilitates the formation of the aminated metabolite of green tea polyphenol (-)-epigallocatechin-3-gallate which trap deleterious reactive endogenous metabolites. Free Radic. Biol. Med. 2019, 131, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Furlanetto, S.; Regazzoni, L.; Zarrella, M.; Facino, R.M. Quenching of alpha,beta-unsaturated aldehydes by green tea polyphenols: HPLC-ESI-MS/MS studies. J. Pharm. Biomed. Anal. 2008, 48, 606–611. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, H.; Ou, J.; Liu, P.; Huang, C.; Wang, M.; Simal-Gandara, J.; Battino, M.; Jafari, S.M.; Zou, L. Benefits, deleterious effects and mitigation of methylglyoxal in foods: A critical review. Trends Food Sci. Technol. 2021, 107, 201–212. [Google Scholar] [CrossRef]
- Lo, C.Y.; Li, S.; Tan, D.; Pan, M.H.; Sang, S.; Ho, C.T. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol. Nutr. Food Res. 2006, 50, 1118–1128. [Google Scholar] [CrossRef]
- Li, S.M.; Zhang, L.; Wan, X.C.; Zhan, J.F.; Ho, C.T. Focusing on the recent progress of tea polyphenol chemistry and perspectives. Food Sci. Hum. Wellness 2022, 11, 437–444. [Google Scholar] [CrossRef]
- Sugimoto, K.; Matsuoka, Y.; Sakai, K.; Fujiya, N.; Fujii, H.; Mano, J. Catechins in green tea powder (matcha) are heat-stable scavengers of acrolein, a lipid peroxide-derived reactive carbonyl species. Food Chem. 2021, 355, 129403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zheng, Z.P.; Cheng, K.W.; Wu, J.J.; Zhang, S.; Tang, Y.S.; Sze, K.H.; Chen, J.; Chen, F.; Wang, M. Natural polyphenols as direct trapping agents of lipid peroxidation-derived acrolein and 4-hydroxy-trans-2-nonenal. Chem. Res. Toxicol. 2009, 22, 1721–1727. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. 2-Alkenal-scavenging ability of m-diphenols. Food. Chem. 2014, 160, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef]
- Sang, S.; Shao, X.; Bai, N.; Lo, C.-Y.; Yang, C.S.; Ho, C.-T. Tea Polyphenol (-)-epigallocatechin-3-gallate: A new trapping agent of reactive dicarbonyl species. Chem. Res. Toxicol. 2007, 20, 1862–1870. [Google Scholar] [CrossRef]
- Yang, C.S.; Chen, T.T.; Ho, C.T. Redox and Other Biological Activities of Tea Catechins That May Affect Health: Mechanisms and Unresolved Issues. J. Agric. Food Chem. 2022, 70, 7887–7899. [Google Scholar] [CrossRef]
- Ma, H.; Hu, Y.; Zhang, B.; Shao, Z.; Roura, E.; Wang, S. Tea polyphenol–gut microbiota interactions: Hints on improving the metabolic syndrome in a multi-element and multi-target manner. Food Sci. Hum. Wellness 2022, 11, 11–21. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Zhang, S.; Mao, B.; Cui, S.; Zhang, Q.; Zhao, J.; Tang, X.; Chen, W. Absorption, metabolism, bioactivity, and biotransformation of epigallocatechin gallate. Crit. Rev. Food Sci. Nutr. 2023, 1–21. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, Y.; Lv, L.; Sang, S. Translating In Vitro Acrolein-Trapping Capacities of Tea Polyphenol and Soy Genistein to In Vivo Situation is Mediated by the Bioavailability and Biotransformation of Individual Polyphenols. Mol. Nutr. Food Res. 2020, 64, 1900274. [Google Scholar] [CrossRef]
- Baba, S.P.; Hoetker, J.D.; Merchant, M.; Klein, J.B.; Cai, J.; Barski, O.A.; Conklin, D.J.; Bhatnagar, A. Role of aldose reductase in the metabolism and detoxification of carnosine-acrolein conjugates. J. Biol. Chem. 2013, 288, 28163–28179. [Google Scholar] [CrossRef]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human gut microbiota and drug metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.-P. Green and Black Tea Phenolics: Bioavailability, Transformation by Colonic Microbiota, and Modulation of Colonic Microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef]
- Chen, M.; Liu, P.Z.; Zhou, H.; Huang, C.H.; Zhai, W.Y.; Xiao, Y.T.; Ou, J.Y.; He, J.; El-Nezami, H.; Zheng, J. Formation and metabolism of 6-(1-acetol)-8-(1-acetol)-rutin in foods and their cytotoxicity. Front. Nutr. 2022, 9, 973048. [Google Scholar] [CrossRef] [PubMed]
- İğde, M.; Özhamam, E.U.; Yaşar, B.; Tapan, M. The Effects of Systemic Use of Epigallocatechin Gallate in Thermal Injury Progression. Indian J. Surg. 2021, 83, 168–176. [Google Scholar] [CrossRef]
- Nilsson, B.O. Biological effects of aminoguanidine: An update. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 1999, 48, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Hidalgo, F.J. Carbonyl-Phenol Adducts: An Alternative Sink for Reactive and Potentially Toxic Lipid Oxidation Products. J. Agric. Food Chem. 2018, 66, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Ou, J.; Huang, J. Reactive Carbonyl Species Scavenger: Epigallocatechin-3-Gallate. Foods 2024, 13, 992. https://doi.org/10.3390/foods13070992
Luo H, Ou J, Huang J. Reactive Carbonyl Species Scavenger: Epigallocatechin-3-Gallate. Foods. 2024; 13(7):992. https://doi.org/10.3390/foods13070992
Chicago/Turabian StyleLuo, Haiying, Juanying Ou, and Junqing Huang. 2024. "Reactive Carbonyl Species Scavenger: Epigallocatechin-3-Gallate" Foods 13, no. 7: 992. https://doi.org/10.3390/foods13070992
APA StyleLuo, H., Ou, J., & Huang, J. (2024). Reactive Carbonyl Species Scavenger: Epigallocatechin-3-Gallate. Foods, 13(7), 992. https://doi.org/10.3390/foods13070992




