Abstract
In view of climate change and the increasingly antagonistic wine market, the exploitation of native genetic resources is revisited in relation to sustainable wine production. ‘Sideritis’ is a late-ripening Greek grape variety, which is quite promising for counteracting wine quality issues associated with the annual temperature rise. The aim of this study was to improve the quality and to enhance the aroma of ‘Sideritis’ wine through the use of native yeasts. To improve vinification, Hanseniaspora opuntiae L1 was used along with Saccharomyces cerevisiae W7 in mixed fermentations (SQ). The addition of H. οpuntiae significantly altered the chemical profile of the wine compared to the single-inoculated fermentations with W7 (IS). H. opuntiae increased all the acetate esters, except for hexyl acetate and (Z)-3-hexen-1-ol acetate. The concentration of 2-phenylethyl acetate, which imparts flowery and sweet notes, exhibited a 2.6-fold increase in SQ as compared to IS wines. SQ also showed higher levels in several ethyl esters, including ethyl butyrate, ethyl heptanoate and ethyl 7-octenoate, which are associated with fruity notes compared to IS. H. opuntiae produced citronellol, a terpene associated with rose and green notes, and increased the overall acceptance of the wine. Present results are thus quite promising for improving ‘Sideritis’ wine quality towards a sustainable wine production in Greece in view of global warming.
1. Introduction
Wine production is currently facing emerging challenges such as climate change and an increasingly antagonistic wine market. Climate change is evident by an increase in severe weather phenomena and the elevation of the average yearly temperature to which grapevine is quite vulnerable. In response to climatic changes, the composition of the grape juice may be altered leading to a diminishment in wine quality. Global warming, in particular, is anticipated to decrease the acidity while boosting the sugar levels in berries, resulting in unbalanced wines with elevated alcohol content that are deprived of fruitiness and aromatic complexity [1]. In the long run, the sustainability of winegrowing and the suitability of international grape cultivars in traditional viticultural regions of warm climates will become questionable. It is foreseen that Mediterranean winegrowing regions will be particularly susceptible to these changes, leading to higher-altitude vineyard sites, northward migration or introduction of grape varieties better suited to warmer climates [1,2].
The exploitation of native germplasm adapted to stress conditions related to climate change, such as global warming, is currently revisited as an alternative approach to sustainable wine production. ‘Sideritis’ is a rare Greek grape variety (Vitis vinifera) that has extremely late ripening and is well-adapted to global warming [1] and is lately claiming a place in modern viticulture [3]. It is a vigorous and quite productive variety, bearing large branches of pink-coloured hardy berries. It produces cool light white or rose wines with a “crunchy” acidity. Nevertheless, it is seldom used for the production of varietal wines due to the relatively low aroma intensity and diminished complexity of the produced wines. Therefore, means for the improvement of its aroma and the overall quality would be of great interest for the sustainability of the local wine industry in view of global warming.
The exploitation of native microbial genetic resources may also provide a powerful means to address these novel challenges in winemaking. In this context, the use of novel Saccharomyces cerevisiae strains and non-Saccharomyces (NS) yeasts is being considered towards wine improvement. Although NS yeasts have been previously considered unwanted in winemaking, due to the production of off-odours and flavours, certain NS strains have been shown to enhance the complexity and the organoleptic profile of wines [4]. As more data becomes available, it appears that various NS yeasts exhibit significant technological characteristics which are to be considered for the development of new starter cultures adapted to the new challenges [5]. Examples of the beneficial activities of NS yeasts include the reduction of volatile acidity or ethanol content, the enhancement of acidity, the production of various aroma compounds, and colour stabilization [6,7]. However, most relevant studies have been conducted at a laboratory scale, including sterile or synthetic musts and small volumes of fermentations, that makes it difficult to conduct reliable sensory analysis and judge the final product in terms of real market wine.
Among the several NS yeasts encountered in grape must, Hanseniaspora opuntiae is a relatively recently described apiculate species [8]. It is quite common in Greek vineyards as part of the NS wine yeast flora [9] where it was first found to be associated with grapes and the initial stages of alcoholic fermentations [10]. Different Hanseniaspora spp., mostly H. osmophila and H. vineae, but also H. guilliermondii and H. uvarum, have been evaluated as potential partners of S. cerevisiae in winemaking [11,12]. However, there is scarce information on the technological characterization of H. opuntiae, while its potentially positive role in winemaking has not been thoroughly evaluated as yet. Certain H. opuntiae strains have been shown to decrease the ethanol content of wines, while increasing the glycerol level [13] or to increase higher alcohols (phenylethanol and 3-methylbutanol) and phenylacetaldehyde in co-culture with S. cerevisiae [14]. Recently, the aroma production profiles of seven different strains of respective Hanseniaspora species, including H. opuntiae, was assessed in simultaneous co-inoculation with S. cerevisiae in microvinifications of Gewürztraminer grape must [15]. H. opuntiae was found to increase certain terpenes, such as citronellol and β-myrcene. Yet, limited information on the influence of H. opuntiae metabolism on the resulting wine, also considering that its impact may vary among different strains, grape cultivars, geographical regions, and winemaking practices.
Here we evaluated the performance of two native yeasts, H. opuntiae and S. cerevisiae, to enhance the aroma profile of wine produced by the late ripening ‘Sideritis’ grape variety. Alcoholic fermentations were conducted outside laboratory conditions, like those encountered in a winery. Therefore, the present results are quite promising for winemaking alternatives in the era of climate change, towards the production of a new brand on the market made by solely ‘Sideritis’ grapes.
2. Materials and Methods
2.1. Alcoholic Fermentations
Yeast strains Hanseniaspora οpuntiae L1 (hereafter referred to as L1) and Saccharomyces cerevisiae W7 (hereafter referred to as W7) from our culture collection at the Institute of Technology of Agricultural Products and the commercial starter S. cerevisiae VIN13 marketed by “Oenobrands SAS, France” were used in the present study. The strains were previously isolated from spontaneously fermented grape musts from Santorini island (L1) and Achaia region in Peloponnese (W7). Fermentations were carried out in duplicate in 20-L vessels at 16 °C with unfiltered ‘Sideritis’ grape must (Vitis vinifera) originating from the Achaia region of western Peloponnese, Greece, with the following characteristics: sugars 176 g/L, pH 3.27, yeast assimilable nitrogen 246 mg/L, total SO2 30 ppm as potassium metabisulfite (K2S2O5). Yeasts were grown in yeast extract peptone dextrose (YPD) agar at 26 °C and then resuspended in 1/4 strength Ringer’s solution. The following inoculation protocols were applied: single-culture inoculation with the indigenous strain S. cerevisiae W7 (IS) or with the commercial S. cerevisiae VIN13 (SC), and sequential inoculation (SQ) with strain L1 followed by strain W7 after ca. 1% vol ethanol production (at 8 h). The size of inoculum was at 6 log CFU/mL at single-culture inoculation. In mixed-culture fermentations, S. cerevisiae and H. opuntiae were added at 5 log CFU/mL and 7 log CFU/mL, respectively. Spontaneous (un-inoculated) fermentations (SP) were performed as a reference. Fermentation progress was monitored by density measurements. Seventy ppm of the total SO2 was added to the finished wines at the end of the alcoholic fermentations. Wines were stored at 4 °C in fully filled containers for one week before being bottled and chemically analysed.
2.2. Microbiological Analysis
Grape must samples were taken at different time intervals during fermentation for estimating yeast populations. One ml of samples was serially diluted and plated on Wallerstein laboratory nutrient agar (WL), ethanol sulfite agar (ESA) or lysine medium agar (LA) for the enumeration of total yeasts, S. cerevisiae and non-Saccharomyces species, respectively. Plated samples were incubated at 28 °C for 2–5 days. Yeast colonies were isolated from the initial, middle, and final stages of fermentations and examined microscopically. For S. cerevisiae genotyping, the interdelta region analysis using the primer set delta 12/delta 21 was applied [16]. For genotyping NS yeasts, the tandem repeat-tRNA method using the primer pair TtRNASc/ISSR-MB was applied [17].
2.3. Chemical Analysis
Reducing sugars, total acidity, volatile acidity, pH, ethanol, total SO2 and free SO2 were estimated using the methods in the Compendium of International Methods of Analysis of Musts and Wines [18]. Yeast assimilable nitrogen (YAN) was determined in the grape must as described previously [19]. Malic acid, lactic acid, citric acid, glycerol, and acetaldehyde were measured in a wine automatic analyser (Miura One, TDI, Barcelona, Spain). The volatile compounds were extracted by headspace solid-phase microextraction (SPME) and analyzed by gas chromatography–mass spectrometry (GC–MS) as described [20]. Compounds were identified by comparing the following: (i) retention indices (RI) based on the homologous series of C8–C24 n-alkanes with those of authentic compounds (when available) and those of the NIST 14 library (Scientific Instrument Services, Ringoes, NJ, USA); (ii) mass spectrometry (MS) data with those of reference compounds and MS data obtained from NIST14. The concentrations of volatile compounds were calculated relative to the internal standard (1,4-dioxane) and expressed as mg/L.
2.4. Sensory Analysis
The quantitative descriptive analysis method was applied for the sensory evaluation of wines. Wines were evaluated about four months after bottling by a group of six skilled evaluators: 3 males and 3 females 30–55 years old, either members of the Institute of Technology of Agricultural Products (ITAP) or the Department of Wine, Vine and Beverage Sciences, University of West Attica), which provided consent prior to their participation. Wines were tested in duplicate in two sessions in random order and at a temperature of 12 °C. Description was based on seven aromas (tree fruits, citric fruits, tropical fruits, floral, fermentation aromas, pungent, aroma intensity) and six palate (oxidation, acidity, sweetness, bitterness, body, after-taste) attributes on a scale from 0 (not perceivable) to 10 (high intensity).
2.5. Statistical Analysis
Analysis of Variance (ANOVA) and Tukey’s HSD test were used to assess significant differences in the chemical profiles of the wine. Chemical parameters were subjected to principal component analysis (PCA) in order to analyse interactions between samples and variables. To compare inoculation protocols, a permutational multivariate analysis of variance (PERMANOVA) was applied. Pairwise distances were estimated by Jaccard metric based on 4999 permutations. PAST program version 3.11 [21] and PRIMER Version 7 software (https://www.primer-e.com (accessed on 5 March 2024)) were used for statistical analyses.
3. Results
3.1. Fermentation Kinetics and Yeast Population Dynamics
Yeast kinetics were followed in a single inoculation of grape must with S. cerevisiae W7 (IS) and mixed sequential inoculation (SQ) with H. opuntiae L1 followed by W7 (Figure 1). Fermentations were also performed with the commercial S. cerevisiae VIN13 (CS) and with non-inoculated must (SP) for comparison reasons. The highest fermentation rate was recorded in CS, followed by the IS fermentation. The SQ fermentation had a markedly lower rate compared to both CS and IS. A significantly prolonged lag phase was observed in SP; yet the rate during the log phase was higher than the one observed in SQ and comparable to both CS and IS. Concomitantly, the SQ and SP fermentations lasted longer (ca. 15 d) than IS (ca. 12 d), whereas VIN13 was the fastest fermentor (ca. 10 d).
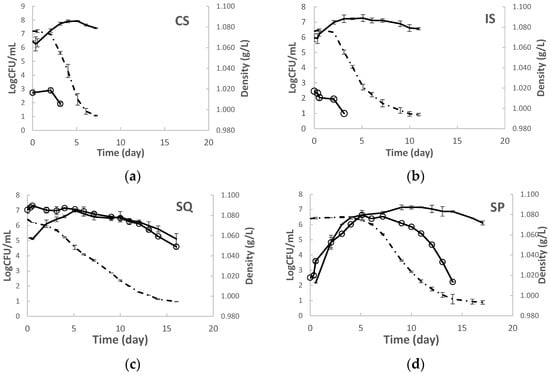
Figure 1.
Fermentation kinetics (dashed line) and yeasts population (continuous line) in grape musts inoculated with (a) S. cerevisiae VIN 13 (CS), (b) S. cerevisiae W7 (IS), (c) H. opuntiae L1 and S. cerevisiae W7 added sequentially, and (d) non-inoculated fermentation (SP). ESA agar was applied for enumeration S. cerevisiae (-), and LA (○) for non-Saccharomyces yeasts.
The strain W7 showed different kinetics in co-inoculated compared to single-inoculated fermentations (Figure 1). In IS, W7 reached a plateau at 3 d after inoculation at maximum population of 7.27 ± 0.23 Log CFU/mL. In SQ, W7 reached plateau at 5 d after inoculation and exhibited somewhat lower cell density than in IS by 0.5 Log CFU/mL till day 10. The max population of W7 was also lower in SQ than in IS (7.00 ± 0.06 Log CFU/mL). W7 achieved a lower cell density than VIN 13 (7.93 ± 0.04 Log CFU/mL) and similar to the indigenous S. cerevisiae counts in SP (7.14 ± 0.15 Log CFU/mL).
Significant differences were observed in the kinetic behaviour of the NS yeast populations in different fermentation trials. In SQ, the strain L1 retained its high population levels till day 6, after which a slow decline was observed. NS yeasts immediately declined upon the addition of S. cerevisiae in both the IS and CS fermentations. As opposed to the indigenous NS yeasts which gradually developed in SP, reaching a maximum of 6.64 ± 0.31 Log CFU/mL by date 5. The NS populations started to gradually decline by day 9.
3.2. Effect of Different Inoculation Schemes on Wine Chemical Profiles
The chemical characteristics of wines were compared by ANOVA to identify differences between the inoculation protocols applied (Table 1). Significant differences were observed in specific characteristics between the commercial and the native S. cerevisiae. The largest differences were observed in volatile acidity (VA) and acetaldehyde levels that were significantly higher in IS than in CS. CS also displayed higher levels of glycerol than IS. The use of H. οpuntiae in SQ significantly increased the acetaldehyde levels and lowered the malic acid concentration. The volatile profile of wines was also determined by GC-MS analysis (Table 2). The commercial S. cerevisiae (CS) produced higher levels of acetate esters compared to the native strain (IS), except for heptyl acetate and 2-phenylethyl acetate. The most profound differences were observed in propyl acetate, isobutyl acetate and isoamyl acetate (fold change > 1.5). On the other hand, the native S. cerevisiae generated more medium-chain fatty acid esters than the commercial strain, such as ethyl heptanoate, ethyl octanoate, ethyl decanoate, 3-methylbutyl octanoate, and ethyl 9-decenoate (fold change > 2). CS and IS wines displayed similar levels of total alcohols. IS produced higher levels (fold change > 2) of 1-decanol, 2-phenylethanol, and 1-heptanol compared to CS, while CS wines had elevated levels of 1-propanol, 2-nonanol, 1-octanol and 2-undecanol (fold change > 1.5). Regarding carboxylic acids, IS wines were characterized by significantly higher contents of acetic, hexanoic and octanoic acid. The addition of H. οpuntiae in SQ fermentation significantly altered the volatile profile of the produced wine compared to IS. In more detail, a high rise (fold change ~ 2) in the levels of acetate levels was recorded, mostly due to the production of ethyl acetate and 2-phenylethyl acetate. On the contrary, the IS wines presented a significantly higher total content of fatty acid esters, mostly due to ethyl octanoate, ethyl decanoate and ethyl 9-decenoate. However, the SQ wines presented a higher content (fold change > 1.5) of quite a few minor fatty acid esters compared to IS, such as ethyl propionate, ethyl isobutyrate, ethyl heptanoate and ethyl 7-octenoate. The total content of alcohols and acids was found higher in IS wines than SQ wines. This was due to the increased content of 3-methyl-1-butanol, 2-phenylethanol, octanoic acid and decanoic acid. There were no significant differences in the content of carbonyl compounds that was observed between samples. Citronellol levels in SQ wines were significantly higher (p < 0.05) than in IS and CS wines.

Table 1.
Chemical characteristics of wines produced by different inoculation protocols (mean ± SD). Significant differences are indicated by different letters (p < 0.05).

Table 2.
Relative content (mg/L relative to internal standard) of volatile compounds in wines produced by different inoculation protocols (mean ± SD). Significant differences are indicated by different letters (p < 0.05).
The chemical characteristics and the volatiles of wines were examined by PERMANOVA. It was observed that the inoculation protocol significantly influenced the chemical character of wines (F = 10.2, p < 0.05). According to pairwise comparisons, the SQ wine showed the highest similarity to SP (F = 1.4), while it was most dissimilar to CS (F = 35.68). PCA was applied to depict relationships between chemical profiles of wines produced by different inoculation protocols (Figure 2a). The first two principal components represented 72.9% of the overall variability (39.9% and 33.0% for PC1 and PC2, respectively). IS, CS and SQ formed distantly separated clusters on the PCA plot. SP samples were more dispersed to each other compared to other treatments. CS exhibited high values on PC1 for isoamyl acetate, glycerol, and 3-methyl-1 butanol, among other metabolites, which separated it from IS along the PC1 axis and SQ along the PC2 axis. SQ was separated from IS along the PC2 axis due to the high scores for several important metabolites such as ethyl acetate, citronellol and 2-phenylethyl acetate on PC2.
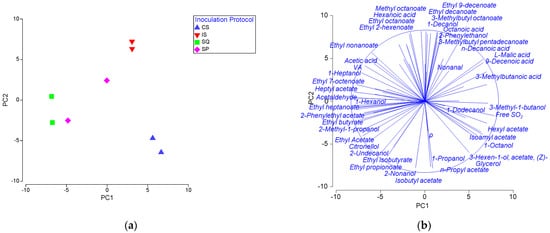
Figure 2.
PCA of the chemical profiles of wines: (a) PCA score plot; (b) PCA loading plot of the chemical characteristics. PC1 and PC2 account for 39.9% and 33.0% of the total variation, respectively. CS: commercial S. cerevisiae; IS: S. cerevisiae W7; SQ: H. opuntiae L1 and S. cerevisiae W7 added sequentially; and SP: spontaneous fermentation.
3.3. Sensory Analysis
The average values of the sensory attributes of wines are shown in Figure 3. The SQ wines showed the highest overall aroma intensity and the most notable tree-fruits and floral notes. The use of H. opuntiae was also shown to increase the tropical-fruits character in SQ compared to IS. A rose tone was identified in SQ wines, albeit at very low levels. SQ wines also showed increased acidity, viscosity, and after-taste compared to other wines. Pungent character was scored at low levels (average of 0.05) in SQ wines. Overall, the SQ wines received the highest score among all wines tested by the panel of accessors.
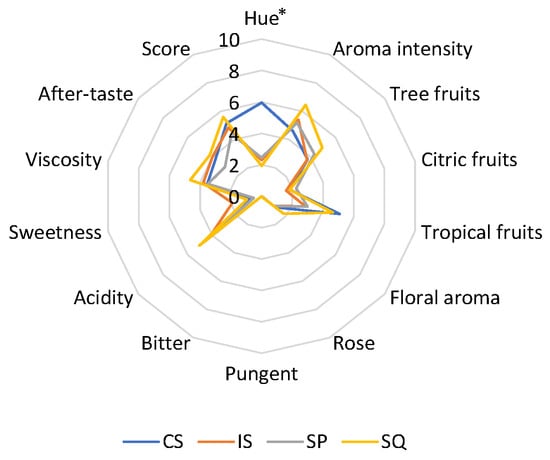
Figure 3.
Means of the sensory characteristics of wines. CS: S. cerevisiae VIN13; IS: S. cerevisiae W7; SQ: H. opuntiae L1 and S. cerevisiae W7 added sequentially; SP: spontaneous fermentation. Significant differences among samples (p < 0.05) are shown by an asterisk.
4. Discussion
The use of indigenous grape and microbial genetic resources is being reconsidered as an alternative strategy for sustainable wine production in light of the impending climate change and the wine market’s growing antagonism. A unique Greek grape variety called ‘Sideritis’, which is endemic to the Achaia area of Peloponnese, has extremely delayed ripening and thus could effectively combat the yearly rise in temperature [1,22]. Although ‘Sideritis’ c.v. may produce delicate wines, it is rarely utilized to make varietal wine due to the low aroma intensity of the final product. The purpose of this study was to investigate the use of H. opuntiae, a relatively unexplored NS wine yeast, in mixed fermentation with S. cerevisiae with the aim to enhance the aroma complexity of ‘Sideritis’ wine.
The genus Hanseniaspora is predominant in fresh grape must, producing enzymes and aroma compounds that enhance wine flavour [23]. H. opuntiae, a recently described member of the Hanseniaspora genus, has been shown repeatably to prevail over other Hanseniaspora species in grapes from Greek vineyards [9] indicating its predominance as a component of the native wine microbiota. H. opuntiae in grape must fermentation has been shown to decrease the cell growth rate of S. cerevisiae [14]. In the present study, the addition of H. opuntiae in high counts delayed both the rate of development and the maximum population reached by S. cerevisiae. Other Hanseniaspora species may also retard the growth of S. cerevisiae [24] even to a greater extent as compared to other NS yeasts [25]. For instance, H. uvarum retarded S. cerevisiae more than L. thermotolerans [25]. In line with that, H. opuntiae and H. uvarum show a better capacity to grow and persist in grape must compared to Pichia kudriavzevii and Candida flavescens [13]. Taken together, it seems that H. opuntiae is a good competitor against S. cerevisiae as is also the case for other Hanseniaspora species. Their death is principally dictated by environmental factors [25,26].
The implication of several secondary metabolic activities of non-Saccharomyces yeasts in alcoholic fermentations leads to a heightened sensory complexity in wines [27]. The alternate direction of carbon to metabolic products other than ethanol during the alcoholic fermentation may lower the final ethanol content of wines. It was previously shown that two H. opuntiae isolates produced less ethanol per gram of sugar consumed compared to S. cerevisiae and significantly reduced the ethanol levels in the final wines [13]. However, H. opuntiae did not decrease the ethanol content in favour of glycerol in our study coinciding with previous results [14,15] and suggesting that this capacity could be probably strain-dependent.
Acetaldehyde is an important constituent of wines which at low levels confers fruity notes but it can impart undesirable ‘nut-like’ odours above 125 mg/L in table white wines, or a grassy and apple-like off-flavour at even higher concentrations [28]. In the present study, it was produced in higher concentrations in SQ (100 mg/L) compared to IS fermentations, close to the threshold of perception in the wine (100–125 mg/L). Hanseniaspora is known to produce relatively high levels of acetaldehyde which are typically significantly higher than those produced by S. cerevisiae [11]. In the present study, acetaldehyde-associated defects were not detected in the sensory analysis. Nevertheless, acetaldehyde production should be taken into consideration if H. opuntiae is employed in winemaking.
Yeasts release free fatty acids during fermentation that may add to the overall complexity of wine at low levels, but at increased concentrations they tend to enhance rancid notes [29]. In the present study, six fatty acids were found in wines. The presence of H. opuntiae in the inoculum decreased the levels of most fatty acids including those of the toxic octanoic and decanoic acids. The reduction of the latter by the use of other strains of H. opuntiae has been also reported recently [13,14]. Therefore, the use of H. opuntiae in wine fermentation may be considered as a positive contributor towards the decrease in toxicity and unpleasant notes associated with fatty acids.
Higher alcohols form a large group of volatiles in wines, which at high levels contribute negatively to their aroma, except for 2-phenylethanol that produces “flowery” and “sweet” notes [30]. Different Hanseniaspora species may enhance the levels of higher alcohols in wines and have been shown to produce higher levels of 3-methyl-1-butanol and 2-methyl-1-butanol compared to S. cerevisiae [31]. H. opuntiae in particular was previously shown to increase 3-methyl-1-butanol and 2-phenylethanol [14]. On the contrary, in the present study, H. opuntiae decreased the levels of the latter alcohols, causing a drop in the total net content of higher alcohols, while it increased the levels of 1-propanol, 1-butanol, 2-methyl-1-propanol and the herbaceous C6 alcohols 1-hexanol, among others.
The formation of esters by yeasts plays a key role in the aroma profile of wines. Therefore, the ability of Hanseniaspora yeasts to produce high levels of esters is of particular interest in winemaking. Hanseniaspora species have been shown to increase the fruity esters in wines [31]. H. uvarum, for instance, increased acetate ester levels when used in fermentations, especially those of ethyl acetate, isoamyl acetate and 2-phenylethyl acetate [32]. H. osmophila and H. vineae were also associated with elevated acetate esters in wine, especially 2-phenylethyl acetate [33,34]. However, little is known about ester production of must fermented with H. opuntiae. Recently, del Fresno et al. [31] determined six esters in wines produced with a H. opuntiae strain as a co-inoculum and found that it produced higher levels of isobutyl acetate, isoamyl acetate and up to 2.8 times higher 2-phenylethyl acetate compared to S. cerevisiae alone. Importantly, this strain produced relatively low levels of ethyl acetate, which at high concentration may spoil wine. On the contrary, other strains of H. opuntiae formed elevated amounts of ethyl acetate, which may be regarded a spoilage factor when present in high amounts, along with high levels of isoamyl acetate [13]. H. opuntiae in SQ wine increased all acetate esters compared to IS wine, except for hexyl acetate and 3-hexenyl acetate. Prominent differences were reported for ethyl acetate and 2-phenylethyl acetate as also previously observed for other Hanseniaspora species. Particularly, the concentration of 2-phenylethyl acetate, a compound responsible for imparting flowery, rosy, and honey-like fragrances with fruity undertones, exhibited a 2.6-fold increase in SQ as compared to IS. Important increments were also observed for other significant acetate esters of wine, i.e., propyl acetate and isobutyl acetate, which highly confer to the fruity character. Consistent with these observations, the level of fruity and flowery scent in the SQ wine showed a notable increase in comparison to the other wines. The elevated levels of ethyl acetate caused a slight ‘pungent’ note in the SQ as compared to other ferments. Yet, the SQ wine was the most preferred among the accessors. Still, ethyl acetate levels should be considered if H. opuntiae is to be used in wine fermentations.
Although Hanseniaspora species commonly increase the acetate ester content, certain H. uvarum strains have been also shown to raise fatty acid esters in wine including ethyl hexanoate, ethyl octanoate and ethyl decanoate [32,35]. It was shown previously that H. opuntiae produced low levels of ethyl octanoate, ethyl decanoate and ethyl dodecanoate but high levels of ethyl butyrate [13]. In the present study, the total ethyl esters content was lower in SQ compared to IS. This difference in the net content was mainly ascribed to the significant decrease in ethyl decanoate, ethyl 9-decenoate, and ethyl octanoate in SQ compared to IS. Despite the decline in total ethyl ester content, significant increases in several compounds were noted, including ethyl butyrate, ethyl heptanoate and ethyl 7-octenoate, which are associated with fruity notes.
Terpenes have a significant role in wine aroma. They predominantly originate from grapes of muscat varieties and confer a floral and fruit character to the wine. Hanseniaspora species may increase the terpenic content of the wine by de novo synthesis of monoterpenes. Specifically, b-citronellol and a-terpineol were increased by 2-fold in Hanseniaspora-fermented wines compared to Saccharomyces-fermented wines [31]. H. uvarum may produce significantly higher levels of citronellol compared to S. cerevisiae in wine fermentation [23]. H. opuntiae was also shown to produce citronellol [13,15]. Here, H. opuntiae produced a significant amount of citronellol, which confers citronella, rose and green notes to wines. This is of particular benefit for improving the terpene content and the aroma profile of ‘Sideritis’, a non-aromatic grape variety.
The use of Hanseniaspora yeasts in wine production has been associated with several positive sensory attributes. For instance, wines produced with the addition of H. uvarum or H. opuntiae were characterized by ‘hazelnut’, ‘coffee’, ‘caramel’ and ‘cherry’ notes [13]. H. opuntiae was also shown to confer increased floral and sweet notes in wine [14]. In the present study, the panel of accessors favoured wines produced with H. opuntiae over others, due to the presence of increased floral and tree-fruits aroma, body, and after-taste, and a more favourable overall acceptance of the wine. A pungent character was slightly perceived possibly due to the higher levels of acetic acid and ethyl acetate in the SQ wines. It seems that H. opuntiae has a strong impact on the sensory profile of the wine like other Hanseniaspora spp., which can be largely explained by their increased enzymatic activity compared to S. cerevisiae and also other non-Saccharomyces species [4,27]. The present findings indicate that the inclusion of H. opuntiae in wine fermentation yeast inocula, in combination with S. cerevisiae, may increase the aroma intensity and intensify the floral and fruity aromas of Sideritis wine and potentially other non-aromatic grapes/wines. Additional studies considering different H. opuntiae strains and its further application at industrial-scale wine production will provide greater insights for its potential in winemaking.
Author Contributions
Conceptualization, methodology, supervision, resources, A.N., A.M. and G.B.; investigation, data curation, formal analysis, M.-E.F., I.C. and I.M.; writing—original draft preparation, A.N.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Regional Operational Programme “Western Greece” (project: Westwine, MIS 5040235).
Institutional Review Board Statement
Started before the establishment of the Institutional Research Ethics Committee in September 2019, this research did not require ethical review. In the course of the implementation of this study, no human body, animal violation of law, morality, or “Declaration of Helsinki” was involved. Moreover, informed consent was obtained from all participants involved in the sensory analysis study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Bekris, F.; Vasileiadis, S.; Krokida, A.; Rouvali, T.; Veskoukis, A.S.; Liadaki, K.; Kouretas, D.; Karpouzas, D.G. Vineyard-Mediated Factors Are Still Operative in Spontaneous and Commercial Fermentations Shaping the Vinification Microbial Community and Affecting the Antioxidant and Anticancer Properties of Wines. Food Res. Int. 2023, 173, 113359. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces Commercial Starter Cultures: Scientific Trends, Recent Patents and Innovation in the Wine Sector. Recent Pat. Food Nutr. Agric. 2019, 11, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Mallouchos, A.; Filippousi, M.-E.; Banilas, G.; Nisiotou, A. Molecular Characterization and Enological Potential of A High Lactic Acid-Producing Lachancea thermotolerans Vineyard Strain. Foods 2020, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Velenosi, M.; Crupi, P.; Perniola, R.; Marsico, A.D.; Salerno, A.; Alexandre, H.; Archidiacono, N.; Ventura, M.; Cardone, M.F. Color Stabilization of Apulian Red Wines through the Sequential Inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Molecules 2021, 26, 907. [Google Scholar] [CrossRef] [PubMed]
- Cadez, N.; Poot, G.A.; Raspor, P.; Smith, M.T. Hanseniaspora meyeri sp. nov. Hanseniaspora clermontiae sp. nov., Hanseniaspora lachancei sp. nov. and Hanseniaspora opuntiae sp. nov., Novel Apiculate Yeast Species. Int. J. Syst. Evol. Microbiol. 2003; 53, 1671–1680. [Google Scholar] [CrossRef]
- Chalvantzi, I.; Banilas, G.; Tassou, C.; Nisiotou, A. Biogeographical Regionalization of Wine Yeast Communities in Greece and Environmental Drivers of Species Distribution at a Local Scale. Front. Microbiol. 2021, 12, 705001. [Google Scholar] [CrossRef] [PubMed]
- Nisiotou, A.A.; Nychas, G.J.E. Yeast Populations Residing on Healthy or Botrytis-Infected Grapes from a Vineyard in Attica, Greece. Appl. Environ. Microbiol. 2007, 73, 2765–2768. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, N.; Badura, J.; von Wallbrunn, C.; Pretorius, I.S. Exploring Future Applications of the Apiculate Yeast Hanseniaspora. Crit. Rev. Biotechnol. 2023, 44, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Mančić, S.; Stamenković Stojanović, S.; Danilović, B.; Djordjević, N.; Malićanin, M.; Lazić, M.; Karabegović, I. Oenological Characterization of Native Hanseniaspora uvarum Strains. Fermentation 2022, 8, 92. [Google Scholar] [CrossRef]
- Rossouw, D.; Bauer, F.F. Exploring the Phenotypic Space of Non-Saccharomyces Wine Yeast Biodiversity. Food Microbiol. 2016, 55, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Zhang, B.Q.; Duan, C.Q.; Yan, G.L. Effects of Different Pre-Fermentation Cold Maceration Time on Aroma Compounds of Saccharomyces cerevisiae Co-Fermentation with Hanseniaspora opuntiae or Pichia kudriavzevii. LWT 2018, 92, 177–186. [Google Scholar] [CrossRef]
- Badura, J.; Kiene, F.; Brezina, S.; Fritsch, S.; Semmler, H.; Rauhut, D.; Pretorius, I.S.; von Wallbrunn, C.; van Wyk, N. Aroma Profiles of Vitis vinifera L. Cv. Gewürztraminer Must Fermented with Co-Cultures of Saccharomyces cerevisiae and Seven Hanseniaspora spp. Fermentation 2023, 9, 109. [Google Scholar] [CrossRef]
- Legras, J.; Karst, F. Optimisation of Interdelta Analysis for Saccharomyces cerevisiae Strain Characterisation. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Barquet, M.; Martín, V.; Medina, K.; Pérez, G.; Carrau, F.; Gaggero, C. Tandem Repeat-TRNA (TRtRNA) PCR Method for the Molecular Typing of Non-Saccharomyces Subspecies. Appl. Microbiol. Biotechnol. 2012, 93, 807–814. [Google Scholar] [CrossRef] [PubMed]
- International Organization of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis, Vol. 1; OIV: Paris, France, 2015; Available online: https://www.oiv.int/public/medias/2270/compendium-2015-en-vol1.pdf (accessed on 5 March 2024).
- Gump, B.H.; Zoecklein, B.W.; Fugelsang, K.C. Food Microbiology Protocols. In Methods in Biotechnology; Spencer, J.F.T., Ragout de Spencer, A.L., Eds.; Humana Press: Totowa, NJ, USA, 2001; pp. 283–296. [Google Scholar]
- Plioni, I.; Bekatorou, A.; Mallouchos, A.; Kandylis, P.; Chiou, A.; Panagopoulou, E.A.; Dede, V.; Styliara, P. Corinthian Currants Finishing Side-Stream: Chemical Characterization, Volatilome, and Valorisation through Wine and Baker’s Yeast Production-Technoeconomic Evaluation. Food Chem. 2021, 342, 128161. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D.; Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Koufos, G.C.; Mavromatis, T.; Koundouras, S.; Jones, G.V. Adaptive Capacity of Winegrape Varieties Cultivated in Greece to Climate Change: Current Trends and Future Projections. Oeno One 2020, 54, 1201–1219. [Google Scholar] [CrossRef]
- Martin, V.; Jose Valera, M.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Growth of Non-Saccharomyces Yeasts Affects Nutrient Availability for Saccharomyces cerevisiae during Wine Fermentation. Int. J. Food Microbiol. 2012, 157, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Nisiotou, A.; Mallouchos, A.; Tassou, C.; Banilas, G. Indigenous Yeast Interactions in Dual-Starter Fermentations May Improve the Varietal Expression of Moschofilero Wine. Front. Microbiol. 2019, 10, 1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. Interaction between Hanseniaspora uvarum and Saccharomyces cerevisiae during Alcoholic Fermentation. Int. J. Food Microbiol. 2015, 206, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Pilone, G.J. An Overview of Formation and Roles of Acetaldehyde in Winemaking with Emphasis on Microbiological Implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [CrossRef] [PubMed]
- De-La-Fuente-Blanco, A.; Sáenz-Navajas, M.P.; Ferreira, V. On the Effects of Higher Alcohols on Red Wine Aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef] [PubMed]
- del Fresno, J.M.; Escott, C.; Carrau, F.; Herbert-Pucheta, J.E.; Vaquero, C.; González, C.; Morata, A. Improving Aroma Complexity with Hanseniaspora spp.: Terpenes, Acetate Esters, and Safranal. Fermentation 2022, 8, 654. [Google Scholar] [CrossRef]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The Oenological Potential of Hanseniaspora Uvarum in Simultaneous and Sequential Co-Fermentation with Saccharomyces Cerevisiae for Industrial Wine Production. Front. Microbiol. 2016, 7, 670. [Google Scholar] [CrossRef] [PubMed]
- Lleixà, J.; Martín, V.; Portillo, M.d.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the Levels of 2-Phenylethyl Acetate in Wine through the Use of a Mixed Culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.J.; Mei, W.C.; Li, T.; Tao, Y.S. Increase of Medium-Chain Fatty Acid Ethyl Ester Content in Mixed H. uvarum/S. cerevisiae Fermentation Leads to Wine Fruity Aroma Enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).