Associations between Milk Coagulation Properties and Microbiological Quality in Sheep Bulk Tank Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Determination
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scintu, M.F.; Piredda, G. Typicity and biodiversity of goat and sheep milk products. Small Rum. Res. 2007, 68, 221–231. [Google Scholar] [CrossRef]
- Regulation (EU) No 1151/2012 of the European Parliament and of the Council of 21 November 2012 on quality schemes for agricultural products and foodstuffs. Off. J. Eur. Union 2012, 343, 1–29.
- Pirisi, A.; Lauret, A.; Dubeuf, J.P. Basic and incentive payments for goat and sheep milk in relation to quality. Small Rum. Res. 2007, 68, 167–178. [Google Scholar] [CrossRef]
- Jayarao, B.M.; Pillai, S.R.; Sawant, A.A.; Wolfgang, D.R.; Hegde, N.V. Guidelines for monitoring bulk tank milk somatic cell and bacterial counts. J. Dairy Sci. 2004, 8, 3561–3573. [Google Scholar] [CrossRef]
- Jiménez, L.; Caballero-Villalobos, J.; Garzón, A.; Oliete, B.; Pérez-Guzmán, M.D.; Arias, R. Exploring the relationships between coagulation, composition, and hygienic quality of bulk tank milk from Manchega sheep. Small Rum. Res. 2023, 228, 107106. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef]
- Toquet, M.; Gómez-Martín, A.; Bataller, E. Review of the bacterial composition of healthy milk, mastitis milk and colostrum in small ruminants. Res. Vet. Sci. 2023, 140, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Monsallier, F.; Verdier-Metz, I.; Agabriel, C.; Martin, B.; Montel, M.-C. Variability of microbial teat skin flora in relation to farming practices and individual dairy cow characteristics. Dairy Sci. Technol. 2012, 92, 265–278. [Google Scholar] [CrossRef]
- De Garnica, M.L.; Linage, B.; Carriedo, J.A.; de La Fuente, L.F.; García-Jimeno, M.C.; Santos, J.A.; Gonzalo, C. Relationship among specific bacterial counts and total bacterial and somatic cell counts and factors influencing their variation in ovine bulk tank milk. J. Dairy Sci. 2013, 96, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Blanco, C.; Gutierrez-Gil, B.; Marina, H.; Pelayo, R.; Suarez-Vega, A.; Acedo, A.; Arranz, J. The milk microbiota of the Spanish Churra sheep breed: New insights into the complexity of the Milk microbiome of dairy species. Animals 2020, 10, 1463. [Google Scholar] [CrossRef]
- Pazzola, M.; Dettori, M.L.; Cipolat-Gotet, C.; Cecchinato, A.; Bittante, G.; Vacca, G.M. Phenotypic factors affecting coagulation properties of milk from Sarda ewes. J. Dairy Sci. 2014, 97, 7247–7257. [Google Scholar] [CrossRef] [PubMed]
- Paschino, P.; Vacca, G.M.; Dettori, M.L.; Pazzola, M. An approach for the estimation of somatic cells’ effect in Sarda sheep milk based on the analysis of milk traits and coagulation properties. Small Rum. Res. 2019, 171, 77–81. [Google Scholar] [CrossRef]
- Nilsson, K.; Stålhammar, H.; Stenholdt Hansen, M.; Lindmark-Månsson, H.; Duchemin, S.; Fikse, F.; de Koning, D.J.; Paulsson, M.; Glantz, M. Characterisation of non-coagulating milk and effects of milk composition and physical properties on rennet-induced coagulation in Swedish Red Dairy Cattle. Int. Dairy J. 2019, 95, 50–57. [Google Scholar] [CrossRef]
- Franzoi, M.; Costa, A.; Vigolo, V.; Penasa, M.; De Marchi, M. Effect of pasteurization on coagulation properties of bovine milk and the role of major composition traits and protein fractions. J. Food Compos. Anal. 2022, 114, 104808. [Google Scholar] [CrossRef]
- Pazzola, M. Coagulation Traits of Sheep and Goat Milk. Animals 2019, 9, 540. [Google Scholar] [CrossRef]
- Vacca, G.M.; Stocco, G.; Dettori, M.L.; Bittante, G.; Pazzola, M. Goat cheese yield and recovery of fat, protein, and total solids in curd are affected by milk coagulation properties. J. Dairy Sci. 2020, 103, 1352–1365. [Google Scholar] [CrossRef]
- PDO Queso Manchego. Protected Designation of Origin “Manchego Cheese”: Activities Report of the PDO “Manchego Cheese 2023”; Unpublished Data; Fundación Consejo Regulador de la D.O. Queso Manchego: Valdepeñas, Spain, 2023. [Google Scholar]
- ISO 4833–1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 4833–2:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Surface Plating Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 17410:2019; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. International Organization for Standardization: Geneva, Switzerland, 2019.
- Dore, S.; Fadda, A.; Fresi, S.; Denti, G.; Puggioni, G.; Cannas, E.A. Pseudomonas aeruginosa: Persistence in the milking machine: Healing experiences. In Special Issue of the International Dairy Federation, Proceedings of the IDF 5th International Symposium on the Challenge to Sheep and Goats Milk Sectors, Alghero, Italy, 18–20 April 2007; International Dairy Federation: Schaerbeek, Belgium, 2008; pp. 79–80. [Google Scholar]
- ISO 16649–2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-bromo-4-chloro-3-indolyl Beta-Dglucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. International Organization for Standardization: Geneva, Switzerland, 1998.
- United States Department of Agriculture. Most Probable Number Tables, MLG Appendix 2.02; United States Department of Agriculture: Washington, DC, USA, 2003; pp. 1–8. [Google Scholar]
- Ali, A.K.A.; Shook, G.E. An optimum transformation for somatic cells concentration in milk. J. Dairy Sci. 1980, 63, 487–490. [Google Scholar] [CrossRef]
- McMahon, D.J.; Brown, R.J. Evaluation of formagraph for comparing rennet solutions. J. Dairy Sci. 1982, 65, 1639–1642. [Google Scholar] [CrossRef]
- Mariani, E.; Cipolat-Gotet, C.; Stefanon, B.; Zecconi, A.; Stocco, G.; Sandri, M.; Ablondi, M.; Mountricha, M.; Summer, A. Effect of total and differential somatic cell count on yield, composition and predicted coagulation properties from individual dairy cows. Int. Dairy J. 2022, 75, 298–307. [Google Scholar] [CrossRef]
- Quintana, A.R.; Perea, J.M.; García-Béjar, B.; Jiménez, L.; Garzón, A.; Arias, R. Dominant Yeast Community in Raw Sheep’s Milk and Potential Transfers of Yeast Species in Relation to Farming Practices. Animals 2020, 10, 906. [Google Scholar] [CrossRef]
- Alin, A. Wiley Interdisciplinary Reviews: Computational Statistics. Multicollinearity 2010, 2, 370–374. [Google Scholar]
- Manca, M.G.; Serdino, J.; Gaspa, G.; Urgeghe, P.; Ibba, I.; Contu, M.; Fresi, P.; Macciotta, N.P.P. Derivation of multivariate indices of milk composition, coagulation properties, and individual cheese yield in dairy sheep. J. Dairy Sci. 2016, 99, 4547–4557. [Google Scholar] [CrossRef]
- Abilleira, E.; Virto, M.; Nájera, A.I.; Salmerón, J.; Albisu, M.; Pérez-Elortondo, F.J.; Ruiz de Gordoa, J.C.; de Renobales, M.; Barron, L.J.R. Effects of seasonal changes in feeding management under part-time grazing on the evolution of the composition and coagulation properties of raw milk from ewes. J. Dairy Sci. 2010, 93, 3902–3909. [Google Scholar] [CrossRef]
- Cecchinato, A.; Cipolat-Gotet, C.; Casellas, J.; Penasa, M.; Rossoni, A.; Bittante, G. Genetic analysis of rennet coagulation time, curd-firming rate, and curd firmness assessed over an extended testing period using mechanical and near-infrared instruments. J. Dairy Sci. 2013, 96, 50–62. [Google Scholar] [CrossRef]
- Pazzola, M.; Cipolat-Gotet, C.; Bittante, G.; Cecchinato, A.; Dettori, M.L.; Vacca, G.M. Phenotypic and genetic relationships between indicators of the mammary gland health status and milk composition, coagulation, and curd firming in dairy sheep. J. Dairy Sci. 2018, 101, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Villalobos, J.; Arias, R.; McSweeney, P.L.H.; Garzón, A. The effect of plasmin activity over rennet coagulation in ewe milk. In Proceedings of the IDF International Symposium on Cheese Science and Technology & the IDF Symposium on Concentration and Drying Technologies of Dairy Products, Dublin, Ireland, 11–13 April 2016; p. 120. [Google Scholar]
- Pinto, G.; Caira, S.; Nicolai, M.A.; Mauriello, R.; Cuollo, M.; Pirisi, A.; Piredda, G.; Chianese, L.; Addeo, F. Proteolysis and partial dephosphorylation of casein are affected by high somatic cell counts in sheep milk. Food Res. Int. 2013, 53, 510–521. [Google Scholar] [CrossRef]
- Milan, M.J.; Caja, G.; Gonzalez-Gonzalez, R.; Fernandez-Perez, A.M.; Such, X. Structure and performance of Awassi and Assaf dairy sheep farms in northwestern Spain. J. Dairy Sci. 2011, 94, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Othmane, M.H.; Carriedo, J.A.; De La Fuente, L.F.; San Primitivo, F. Factors affecting test-day milk composition in dairy ewes, and relationships amongst various milk components. J. Dairy Res. 2022, 69, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Sevi, A.; Albenzio, M.; Taibi, L.; Dantone, D.; Massa, S.; Annicchiarico, G. Changes of somatic cell count through lactation and their effects on nutritional, renneting and bacteriological characteristics of ewe’s milk. Adv. Food Sci. 1999, 21, 122–127. [Google Scholar]
- Banks, J.M. Cheese yield. In Cheese Problems Solved; McSweeney, P.L.H., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2007; pp. 100–114. [Google Scholar]
- Jaramillo, D.P.; Zamora, A.; Guamis, B.; Rodriguez, M.; Trujillo, A.J. Cheesemaking aptitude of two Spanish dairy ewe breeds: Changes during lactation and relationship between physicochemical and technological properties. Small Rum. Res. 2008, 78, 48–55. [Google Scholar] [CrossRef]
- Todaro, M.; Scatassa, M.L.; Bonanno, A. The quality assessment of Valle del Belice sheep milk and cheeses produced in the hot summer season in Sicily. Dairy Sci. Technol. 2014, 94, 225–239. [Google Scholar] [CrossRef][Green Version]
- De Marchi, M.; Dal Zotto, R.; Cassandro, M.; Bittante, G. Milk Coagulation Ability of Five Dairy Cattle Breeds. J. Dairy Sci. 2007, 90, 3986–3992. [Google Scholar] [CrossRef]
- Pellegrini, O.; Remeuf, F.; Rivemale, M. Evolution des caracteristiques physico-chimiques et des parametres de coagulation du lait de brebis collecte dans la region de Roquefort. Lait 1994, 74, 425–442. [Google Scholar] [CrossRef]
- Nájera, A.I.; Barron, L.J.R.; Ribeiro, P.; Pelissier, F.; Abilleira, E.; Perez-Elortondo, F.J.; Albisu, M.; Salmeron, J.; Ruiz de Gordoa, J.C.; Virto, M.; et al. Seasonal changes in the technological and compositional quality of ewe’s raw miles from commercial flocks under part-time grazing. J. Dairy Res. 2009, 76, 301–307. [Google Scholar] [CrossRef] [PubMed]
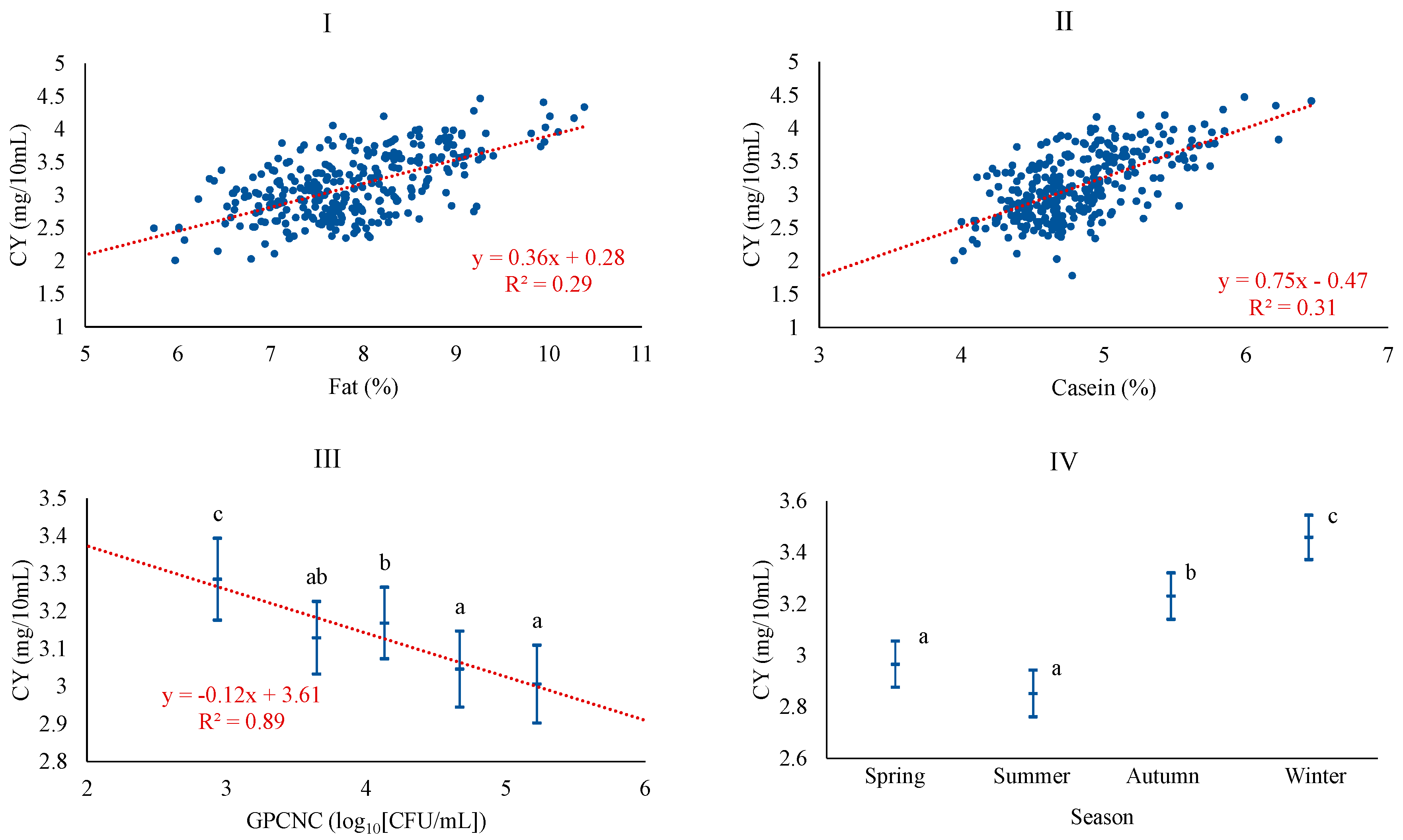
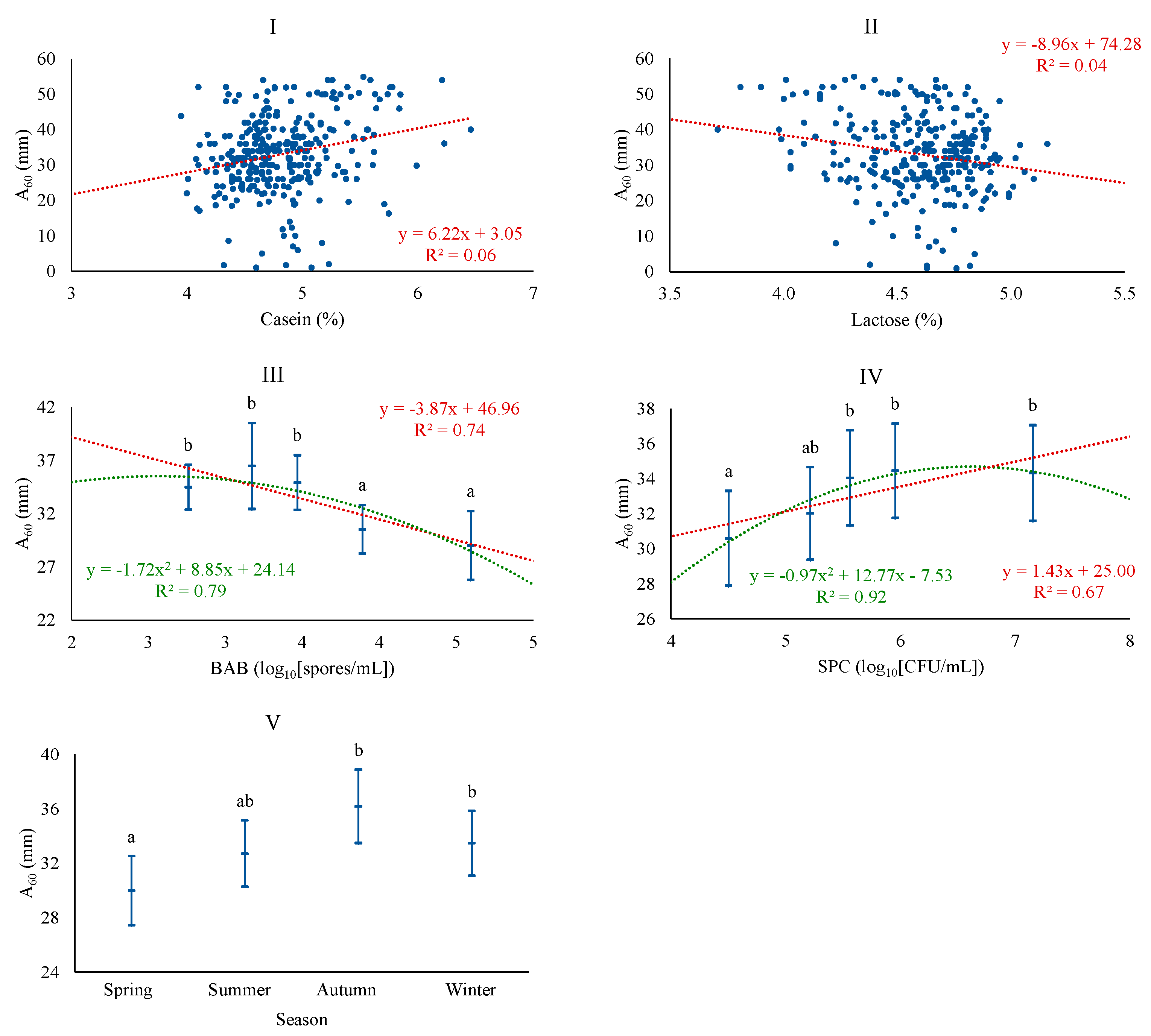
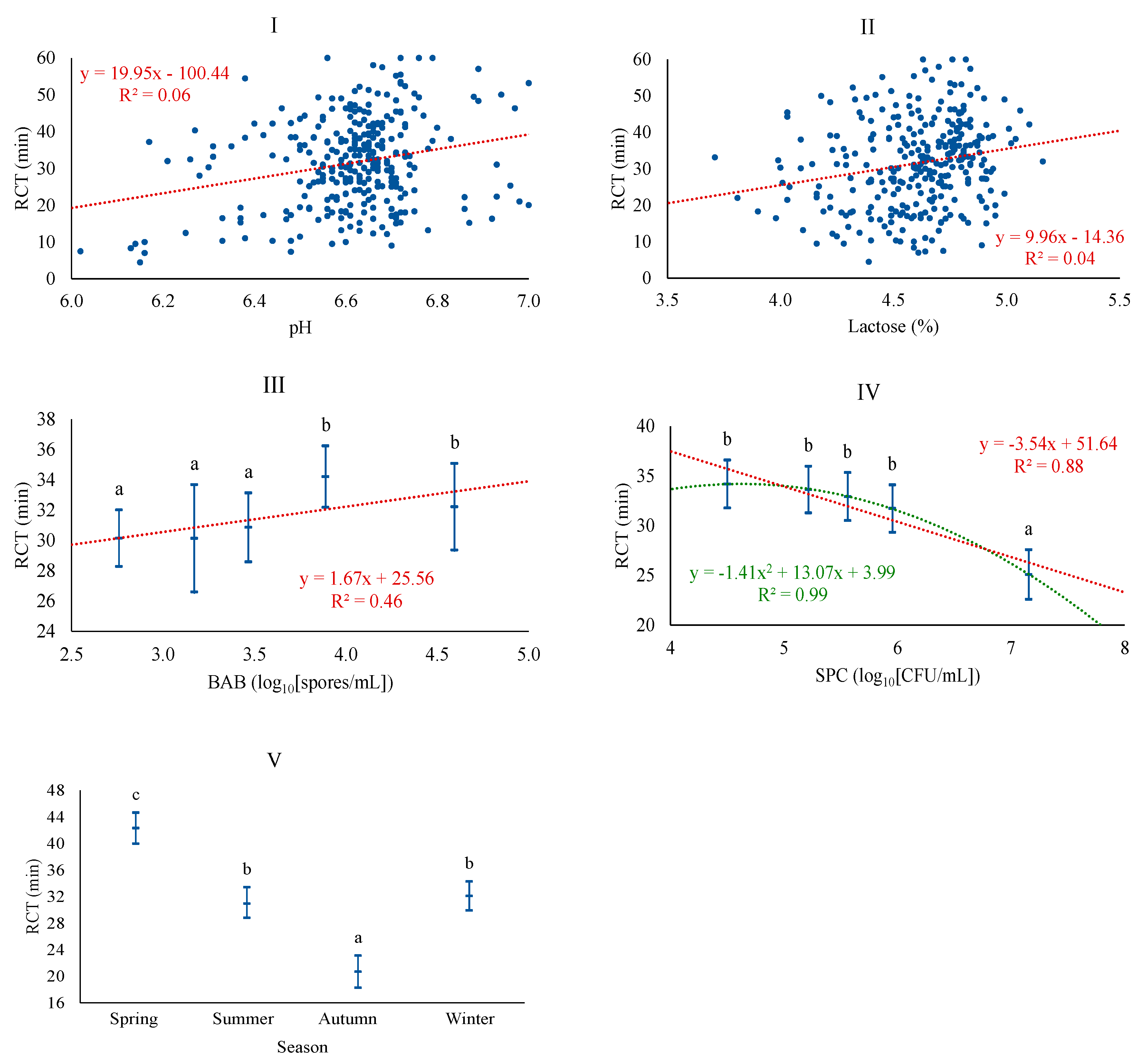
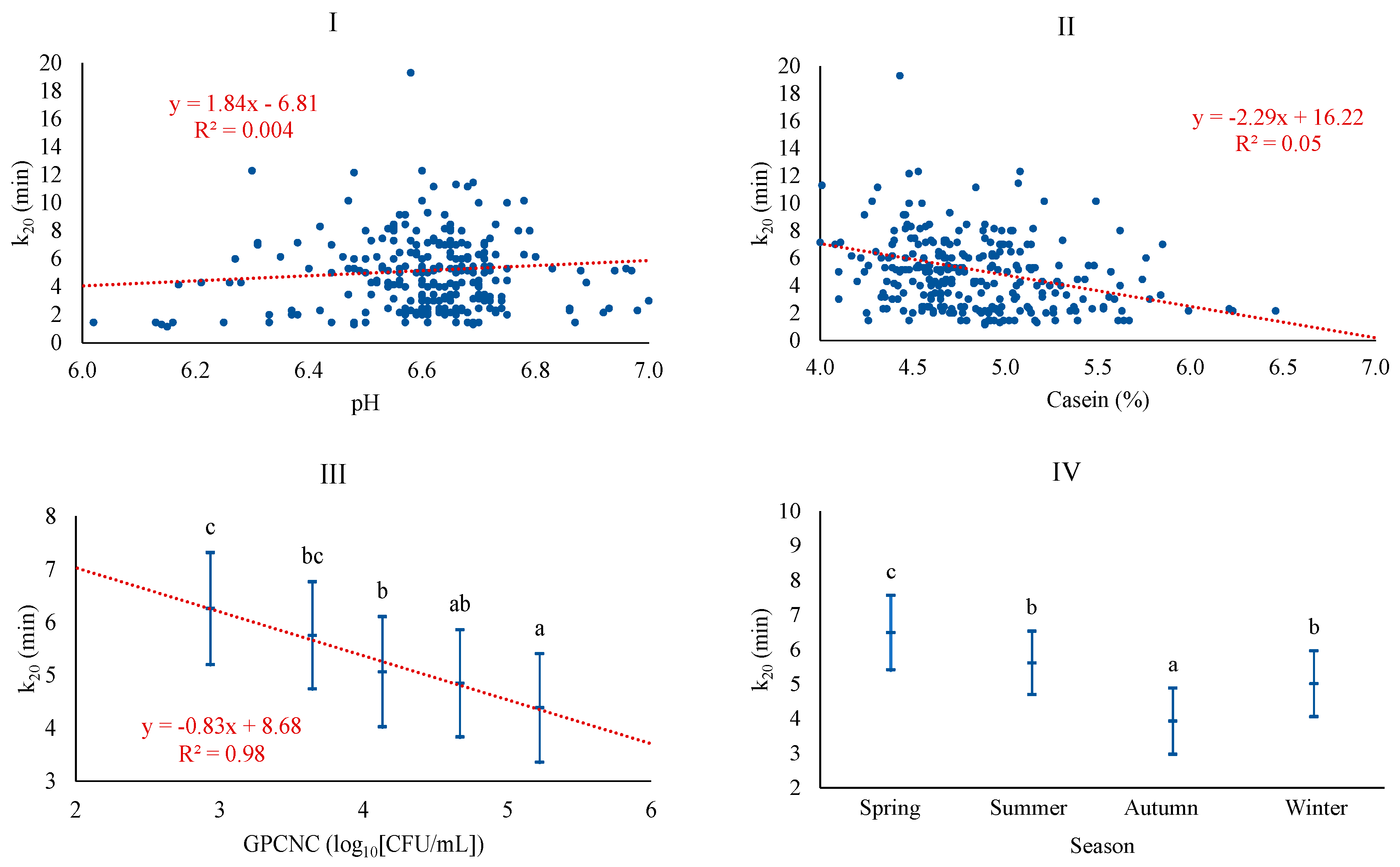
| Variable | Description | Mean | SD 1 | Q1 | Q3 |
|---|---|---|---|---|---|
| CY (g/100 mL) 2 | Curd yield | 3.13 | 0.55 | 2.76 | 3.74 |
| A60 (mm) 3 | Curd firmness at 60 min. | 33.04 | 10.76 | 26.30 | 39.98 |
| RCT (min) 4 | Rennet clotting time | 31.54 | 11.97 | 22.30 | 39.45 |
| k20 (min) 5 | Curd firming time | 8.79 | 14.17 | 3.0 | 7.0 |
| SCS 6 | Somatic cell score | 6.40 | 0.78 | 5.91 | 6.90 |
| FAT (%) | Fat content | 7.87 | 0.83 | 7.32 | 8.35 |
| CAS (%) | Casein content | 4.82 | 0.41 | 4.55 | 5.04 |
| LAC (%) | Lactose content | 4.60 | 0.24 | 4.48 | 4.79 |
| pH (−log [H+]) | pH | 6.61 | 0.15 | 6.56 | 6.69 |
| BAB 7 | Lactate-fermenting Clostridium spores | 3.42 | 0.63 | 2.96 | 3.80 |
| SPC 8 | Total mesophilic bacteria (standard plate count) | 5.65 | 0.77 | 5.11 | 6.13 |
| THERMO 8 | Thermodurics | 3.25 | 0.84 | 2.69 | 3.73 |
| PSYCHRO 8 | Psychrotrophs | 5.23 | 1.08 | 4.57 | 5.90 |
| PSEUDO 8 | Pseudomonas spp. | 3.15 | 0.84 | 2.65 | 3.72 |
| LAB 8 | Lactic acid bacteria | 4.89 | 0.73 | 4.30 | 5.40 |
| GPCNC 8 | Gram-positive catalase-negative cocci | 4.16 | 1.63 | 3.55 | 4.78 |
| ECOLI 8 | Escherichia coli | 1.63 | 1.16 | 0.13 | 2.31 |
| COLI 8 | Coliforms other than Escherichia coli | 2.91 | 1.19 | 2.35 | 3.67 |
| CPS 8 | Coagulase-positive staphylococci | 2.52 | 1.35 | 1.90 | 3.46 |
| CNS 8 | Coagulase-negative staphylococci | 4.30 | 0.54 | 4.06 | 4.50 |
| Variable | Classes | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| BAB 1 | 4.04–4.85 | 4.85–5.26 | 5.26–5.38 | 5.38–5.89 | 5.89–7.00 |
| SPC 2 | 2.48–3.04 | 3.04–3.30 | 3.30–3.63 | 3.63–4.15 | 4.15–5.04 |
| THERMO 2 | 3.99–5.01 | 5.01–5.41 | 5.41–5.70 | 5.70–6.20 | 6.20–8.10 |
| PSYCHRO 2 | 0.00–2.60 | 2.60–3.03 | 3.03–3.30 | 3.30–3.89 | 3.89–5.48 |
| PSEUDO 2 | 2.00–4.43 | 4.43–4.98 | 4.98–5.36 | 5.36–6.14 | 6.14–8.00 |
| LAB 2 | 0.00–2.55 | 2.55–2.95 | 2.95–3.34 | 3.34–3.80 | 3.80–4.53 |
| GPCNC 2 | 2.91–4.22 | 4.22–4.66 | 4.66–5.11 | 5.11–5.46 | 5.46–6.48 |
| ECOLI 2 | 0.00–2.48 | 2.48–3.39 | 3.39–4.37 | 4.37–4.97 | 4.97–5.48 |
| COLI 2 | 0.00–1.00 | 1.00–1.65 | 1.65–1.96 | 1.96–2.48 | 2.48–5.48 |
| CPS 2 | 0.00–2.45 | 2.45–2.89 | 2.89–3.31 | 3.31–3.98 | 3.98–5.48 |
| CNS 2 | 0.00–1.65 | 1.65–2.50 | 2.50–3.06 | 3.06–3.59 | 3.59–5.48 |
| Variable 1 | CY (g/100 mL) | A60 (mm) | RCT (min) | k20 (min) | ||||
|---|---|---|---|---|---|---|---|---|
| BM | WSM | BM | WSM | BM | WSM | BM | WSM | |
| Flock | ns | ns | ns | ns | ns | ns | ns | ns |
| Season | 31.27 *** | 26.47 *** | 4.35 *** | 2.55 * | 51.99 *** | 35.56 *** | 5.87 *** | 4.72 ** |
| SCS 2 | ns | ns | ns | ns | 2.27 * | ns | ns | ns |
| FAT (%) | 21.71 *** | 13.30 *** | ns | ns | ns | ns | ns | ns |
| CAS (%) | 26.07 *** | 14.71 *** | 7.60 *** | 6.98 *** | ns | ns | 5.77 ** | 5.10 * |
| LAC (%) | ns | ns | ns | 3.75 * | 70.75 *** | 12.81 *** | ns | ns |
| pH (−log[H+]) | 2.76 * | ns | ns | ns | ns | 55.69 *** | 8.51 *** | 5.68 * |
| BAB 3 | - | ns | - | 6.20 *** | - | 4.44 *** | - | ns |
| SPC 4 | - | ns | - | 3.04 ** | - | 6.25 *** | - | ns |
| THERMO 4 | - | ns | - | ns | - | ns | - | ns |
| PSYCHRO 4 | - | ns | - | ns | - | ns | - | ns |
| PSEUDO 4 | - | ns | - | ns | - | ns | - | ns |
| LAB 4 | - | ns | - | ns | - | ns | - | ns |
| GPCNC 4 | - | 3.28 ** | - | ns | - | ns | - | 2.97 * |
| ECOLI 4 | - | ns | - | ns | - | ns | - | ns |
| COLI 4 | - | ns | - | ns | - | ns | - | ns |
| CPS 4 | - | ns | - | ns | - | ns | - | ns |
| CNS 4 | - | ns | - | ns | - | ns | - | ns |
| AIC 5 | −592.06 | −445.62 | 1409.53 | 1116.48 | 1356.69 | 1067.44 | 754.21 | 634.08 |
| RMSE 6 | 0.37 | 0.33 | 10.31 | 9.76 | 9.38 | 8.82 | 3.77 | 3.03 |
| R2 7 | 54.80 | 57.9 | 8.50 | 13.80 | 39.1 | 44.44 | 10.40 | 13.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, R.; Jiménez, L.; Garzón, A.; Caballero-Villalobos, J.; Oliete, B.; Amalfitano, N.; Cecchinato, A.; Perea, J.M. Associations between Milk Coagulation Properties and Microbiological Quality in Sheep Bulk Tank Milk. Foods 2024, 13, 886. https://doi.org/10.3390/foods13060886
Arias R, Jiménez L, Garzón A, Caballero-Villalobos J, Oliete B, Amalfitano N, Cecchinato A, Perea JM. Associations between Milk Coagulation Properties and Microbiological Quality in Sheep Bulk Tank Milk. Foods. 2024; 13(6):886. https://doi.org/10.3390/foods13060886
Chicago/Turabian StyleArias, Ramón, Lorena Jiménez, Ana Garzón, Javier Caballero-Villalobos, Bonastre Oliete, Nicolò Amalfitano, Alessio Cecchinato, and José M. Perea. 2024. "Associations between Milk Coagulation Properties and Microbiological Quality in Sheep Bulk Tank Milk" Foods 13, no. 6: 886. https://doi.org/10.3390/foods13060886
APA StyleArias, R., Jiménez, L., Garzón, A., Caballero-Villalobos, J., Oliete, B., Amalfitano, N., Cecchinato, A., & Perea, J. M. (2024). Associations between Milk Coagulation Properties and Microbiological Quality in Sheep Bulk Tank Milk. Foods, 13(6), 886. https://doi.org/10.3390/foods13060886







