The Impact of Oil Type on the Performance of β-Amyrin-Based Oleogels: Formation, Physicochemical Properties, and Potential Correlation Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oleogel Preparation
2.3. Oil Binding Capacity
2.4. Texture Analysis
2.5. Polarized Light Microscopy
2.6. X-ray Diffraction Analysis
2.7. Differential Scanning Calorimetry
2.8. The Crystallization Kinetics of Oleogel
2.9. Rheological Measurement
2.10. Statistical Analysis
3. Results and Discussion
3.1. Concentration Phase Diagrams
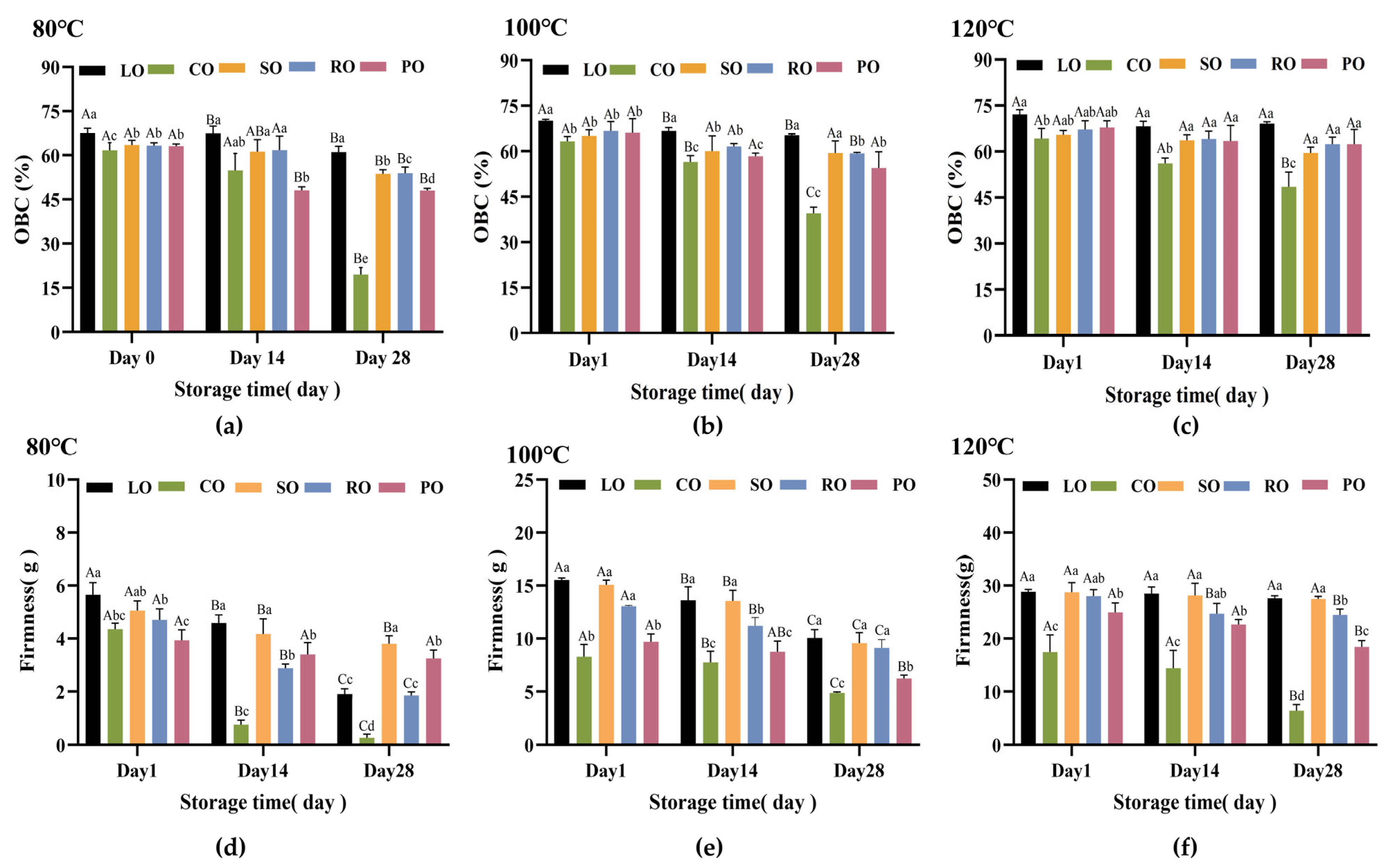
3.2. Oil Binding Capacity and Hardness Analysis
3.3. Polymorphism of Different Oleogels
3.4. Thermal Behaviour of Oleogels
3.5. The Rheological Properties of Oloegels
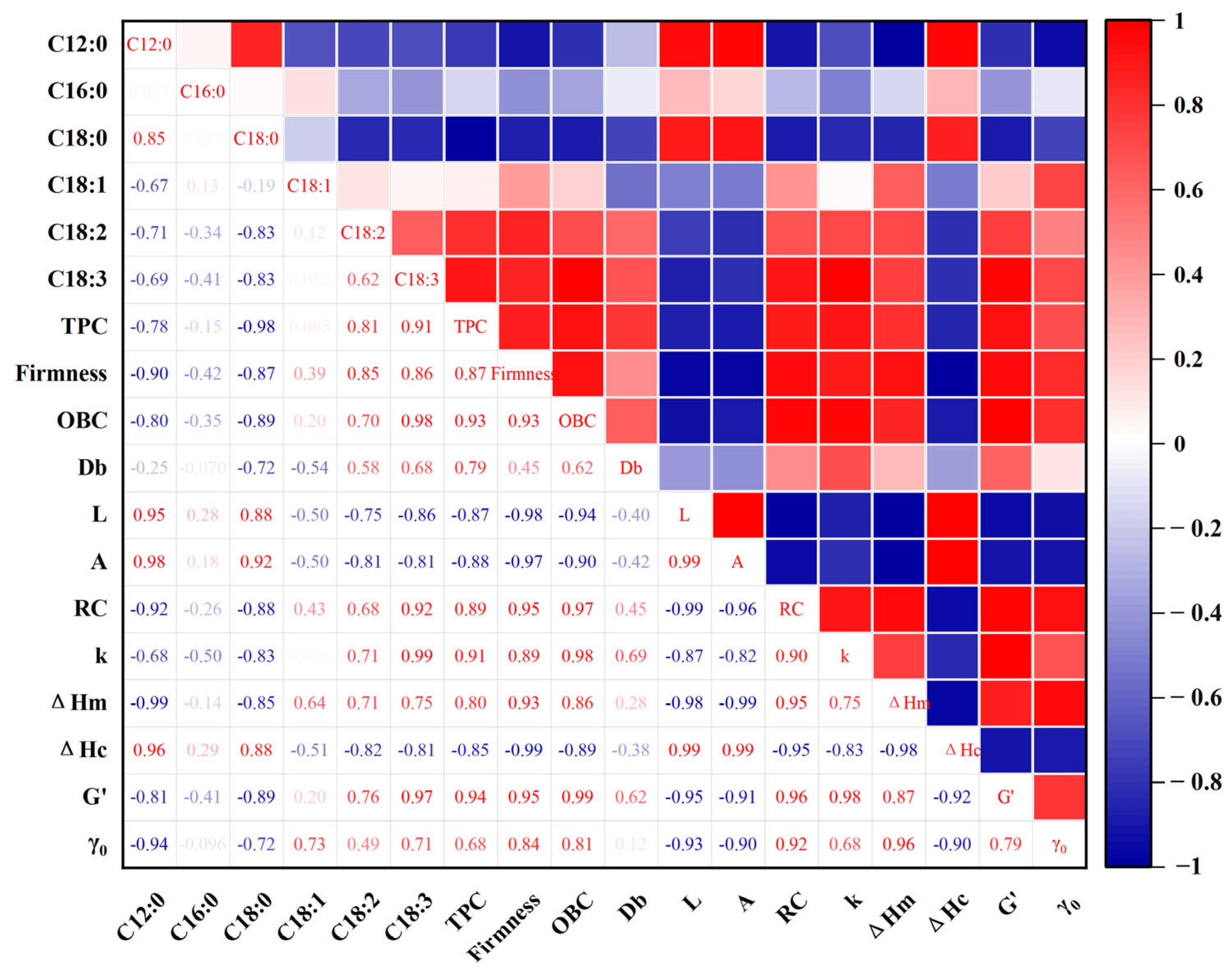
3.6. Correlation Analysis of Oleogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, W.; Chai, X.; Liu, Y.; Xu, Y.; Tan, C.-P. Crystal network structure and stability of beeswax-based oleogels with different polyunsaturated fatty acid oils. Food Chem. 2022, 381, 131745. [Google Scholar] [CrossRef]
- Li, L.; Liu, G.; Bogojevic, O.; Pedersen, J.N.; Guo, Z. Edible oleogels as solid fat alternatives: Composition and oleogelation mechanism implications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2077–2104. [Google Scholar] [CrossRef]
- McClements, D.J. Future foods: A manifesto for research priorities in structural design of foods. Food Funct. 2020, 11, 1933–1945. [Google Scholar] [CrossRef] [PubMed]
- Millao, S.; Iturra, N.; Contardo, I.; Morales, E.; Quilaqueo, M.; Rubilar, M. Structuring of oils with high PUFA content: Evaluation of the formulation conditions on the oxidative stability and structural properties of ethylcellulose oleogels. Food Chem. 2023, 405, 134772. [Google Scholar] [CrossRef]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, H. Fabrication of oleogels via a facile method by oil absorption in the aerogel templates of protein–polysaccharide conjugates. ACS Appl. Mater. Interfaces 2020, 12, 7795–7804. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of oleogelation on physical properties and oxidative stability of camellia oil-based oleogels and oleogel emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, S. Development and evaluation of a novel oleogel system based on starch–water–wax–oil. Food Funct. 2020, 11, 7727–7735. [Google Scholar] [CrossRef] [PubMed]
- Palla, C.A.; Dominguez, M.; Carrín, M.E. An overview of structure engineering to tailor the functionality of monoglyceride oleogels. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2587–2614. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Zetzl, A.K.; Barbut, S.; Marangoni, A.G. Influence of solvent quality on the mechanical strength of ethylcellulose oleogels. Carbohydr. Polym. 2016, 135, 169–179. [Google Scholar] [CrossRef]
- Oh, I.; Lee, J.; Lee, H.G.; Lee, S. Feasibility of hydroxypropyl methylcellulose oleogel as an animal fat replacer for meat patties. Food Res. Int. 2019, 122, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Strober, T.; Bot, A.; Toro-Vazquez, J.F.; Stortz, T.; Marangoni, A.G. Edible oleogels in molecular gastronomy. Int. J. Gastron. Food Sci. 2014, 2, 22–31. [Google Scholar] [CrossRef]
- Pang, M.; Wang, X.; Cao, L.; Shi, Z.; Lei, Z.; Jiang, S. Structure and thermal properties of β-sitosterol-beeswax-sunflower oleogels. Int. J. Food Sci. Technol. 2020, 55, 1900–1908. [Google Scholar] [CrossRef]
- Sun, P.; Xia, B.; Ni, Z.-J.; Wang, Y.; Elam, E.; Thakur, K.; Ma, Y.-L.; Wei, Z.-J. Characterization of functional chocolate formulated using oleogels derived from β-sitosterol with γ-oryzanol/lecithin/stearic acid. Food Chem. 2021, 360, 130017. [Google Scholar] [CrossRef]
- Silva-Avellaneda, E.; Bauer-Estrada, K.; Prieto-Correa, R.; Quintanilla-Carvajal, M. The effect of composition, microfluidization and process parameters on formation of oleogels for ice cream applications. Sci. Rep. 2021, 11, 7161. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.R.; Ghosh, S. Canola protein thermal denaturation improved emulsion-templated oleogelation and its cake-baking application. RSC Adv. 2021, 11, 25141–25157. [Google Scholar] [CrossRef]
- Yu, X.; Chen, H.; Shi, X.; Albouy, P.-A.; Guo, J.; Hu, J.; Li, M.-H. Liquid crystal gelators with photo-responsive and AIE properties. Mater. Chem. Front. 2018, 2, 2245–2253. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, H.; Guo, S.; Lu, X.; Zeng, C. Development and characterization of a novel naturally occurring pentacyclic triterpene self-stabilized pickering emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127908. [Google Scholar] [CrossRef]
- Li, Z.; Liu, A.; Du, Q.; Zhu, W.; Liu, H.; Naeem, A.; Guan, Y.; Chen, L.; Ming, L. Bioactive substances and therapeutic potential of camellia oil: An overview. Food Biosci. 2022, 49, 101855. [Google Scholar] [CrossRef]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.-R.; Machaj, F.; Ghamsari, M.; Rosik, J. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Rárová, L.; Šaman, D.; Wimmer, Z. Picolyl amides of betulinic acid as antitumor agents causing tumor cell apoptosis. Eur. J. Med. Chem. 2018, 145, 41–50. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, L.; Kang, S.; Jiang, S.; Pang, M. Influence of wax type on characteristics of oleogels from camellia oil and medium chain triglycerides. Int. J. Food Sci. Technol. 2021, 57, 2003–2014. [Google Scholar] [CrossRef]
- Bascuas, S.; Hernando, I.; Moraga, G.; Quiles, A. Structure and stability of edible oleogels prepared with different unsaturated oils and hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1458–1467. [Google Scholar] [CrossRef]
- de Vries, A.; Gomez, Y.L.; van der Linden, E.; Scholten, E. The effect of oil type on network formation by protein aggregates into oleogels. RSC Adv. 2017, 7, 11803–11812. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Y.; Zhang, Y.; Gao, Y.; Mao, L. Surfactant addition to modify the structures of ethylcellulose oleogels for higher solubility and stability of curcumin. Int. J. Biol. Macromol. 2020, 165, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Meng, Z.; Liu, Y. Comparation of micro-viscosity of liquid oil in different colloidal fat crystal networks using molecular rotors. Food Chem. 2020, 317, 126382. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A. Development, characterization, and utilization of food-grade polymer oleogels. Annu. Rev. Food Sci. Technol. 2016, 7, 65–91. [Google Scholar] [CrossRef]
- Lu, Y.C.; Li, J.L.; Ding, J.; Nie, X.H.; Yu, N.X.; Meng, X.H. Comparison of diosgenin-vegetable oils oleogels with various unsaturated fatty acids: Physicochemical properties, in-vitro digestion, and potential mechanism. Food Chem. 2023, 413, 135663. [Google Scholar] [CrossRef]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and physical properties of plant wax crystal networks and their relationship to oil binding capacity. J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Okuro, P.K.; Tavernier, I.; Bin Sintang, M.D.; Skirtach, A.G.; Vicente, A.A.; Dewettinck, K.; Cunha, R.L. Synergistic interactions between lecithin and fruit wax in oleogel formation. Food Funct. 2018, 9, 1755–1767. [Google Scholar] [CrossRef]
- Wang, L.; Wen, Y.; Su, C.; Gao, Y.; Li, Q.; Du, S.; Yu, X. Effect of water content on the physical properties and structure of walnut oleogels. RSC Adv. 2022, 12, 8987–8995. [Google Scholar] [CrossRef] [PubMed]
- Frolova, Y.; Sarkisyan, V.; Sobolev, R.; Makarenko, M.; Semin, M.; Kochetkova, A. The influence of edible oils’ composition on the properties of beeswax-based oleogels. Gels 2022, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G.; Yang, D.; Zhang, H.; Qi, X.; Jin, Q.; Wang, X. Effect of multistage process on the quality, water and oil distribution and microstructure of French fries. Food Res. Int. 2020, 137, 109229. [Google Scholar] [CrossRef]
- Borriello, A.; Antonella Miele, N.; Masi, P.; Aiello, A.; Cavella, S. Effect of fatty acid composition of vegetable oils on crystallization and gelation kinetics of oleogels based on natural wax. Food Chem. 2022, 375, 131805. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Martins, A.J.; López-Pedrouso, M.; Purriños, L.; Cerqueira, M.A.; Vicente, A.A.; Pastrana, L.M.; Zapata, C.; Lorenzo, J.M. Strategy towards replacing pork backfat with a linseed oleogel in frankfurter sausages and its evaluation on physicochemical, nutritional, and sensory characteristics. Foods 2019, 8, 366. [Google Scholar] [CrossRef]
- Gilbert, E.P. Building blocks of β-sitosterol-γ-oryzanol gels revealed by small-angle neutron scattering and real space modelling. Food Funct. 2022, 13, 7123–7131. [Google Scholar] [CrossRef] [PubMed]
- Flöter, E.; Wettlaufer, T.; Conty, V.; Scharfe, M. Oleogels—Their applicability and methods of characterization. Molecules 2021, 26, 1673. [Google Scholar] [CrossRef]
- Laredo, T.; Barbut, S.; Marangoni, A.G. Molecular interactions of polymer oleogelation. Soft Matter 2011, 7, 2734–2743. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, Y.; Cao, P.; Liu, Y. Effects of frying oils’ fatty acids profile on the formation of polar lipids components and their retention in French fries over deep-frying process. Food Chem. 2017, 237, 98–105. [Google Scholar] [CrossRef]
- Fayaz, G.; Goli, S.A.H.; Kadivar, M. A novel propolis wax-based organogel: Effect of oil type on its formation, crystal structure and thermal properties. J. Am. Oil Chem. Soc. 2017, 94, 47–55. [Google Scholar] [CrossRef]
- Scharfe, M.; Flöter, E. Oleogelation: From Scientific Feasibility to Applicability in Food Products. Eur. J. Lipid Sci. Technol. 2020, 122, 24. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fasolin, L.H.; Picone, C.S.; Pastrana, L.M.; Cunha, R.L.; Vicente, A.A. Structural and mechanical properties of organogels: Role of oil and gelator molecular structure. Food Res. Int. 2017, 96, 161–170. [Google Scholar] [CrossRef]
- Palla, C.; de Vicente, J.; Carrin, M.E.; Ruiz, M.J.G. Effects of cooling temperature profiles on the monoglycerides oleogel properties: A rheo-microscopy study. Food Res. Int. 2019, 125, 108613. [Google Scholar] [CrossRef]
- Perta-Crisan, S.; Ursachi, C.S.; Chereji, B.D.; Tolan, I.; Munteanu, F.D. Food-Grade Oleogels: Trends in Analysis, Characterization, and Applicability. Gels 2023, 9, 386. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, Y.; Zhang, W.; Yang, Q.; Yang, S.; Su, C.; Lin, Z.; Gu, J.; Hu, C. Structural Diversity of Diosgenin Hydrates: Effect of Initial Concentration, Water Volume Fraction, and Solvent on Crystallization. Cryst. Growth Des. 2016, 16, 1492–1501. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, Z.; Xie, Y.; Ma, A.; Zhang, H.; Rao, P.; Wang, Q. Synthesis, physicochemical properties, and health aspects of structured lipids: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 759–800. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. The effect of addition of high-melting monoacylglycerol and candelilla wax on pea and faba bean protein foam-templated oleogelation. J. Am. Oil Chem. Soc. 2020, 97, 1319–1333. [Google Scholar] [CrossRef]
- Joyner, H.S. Rheology of Semisolid Foods; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Holey, S.A.; Sekhar, K.P.; Mishra, S.S.; Kanjilal, S.; Nayak, R.R. Effect of oil unsaturation and wax composition on stability, properties and food applicability of oleogels. J. Am. Oil Chem. Soc. 2021, 98, 1189–1203. [Google Scholar] [CrossRef]
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef] [PubMed]

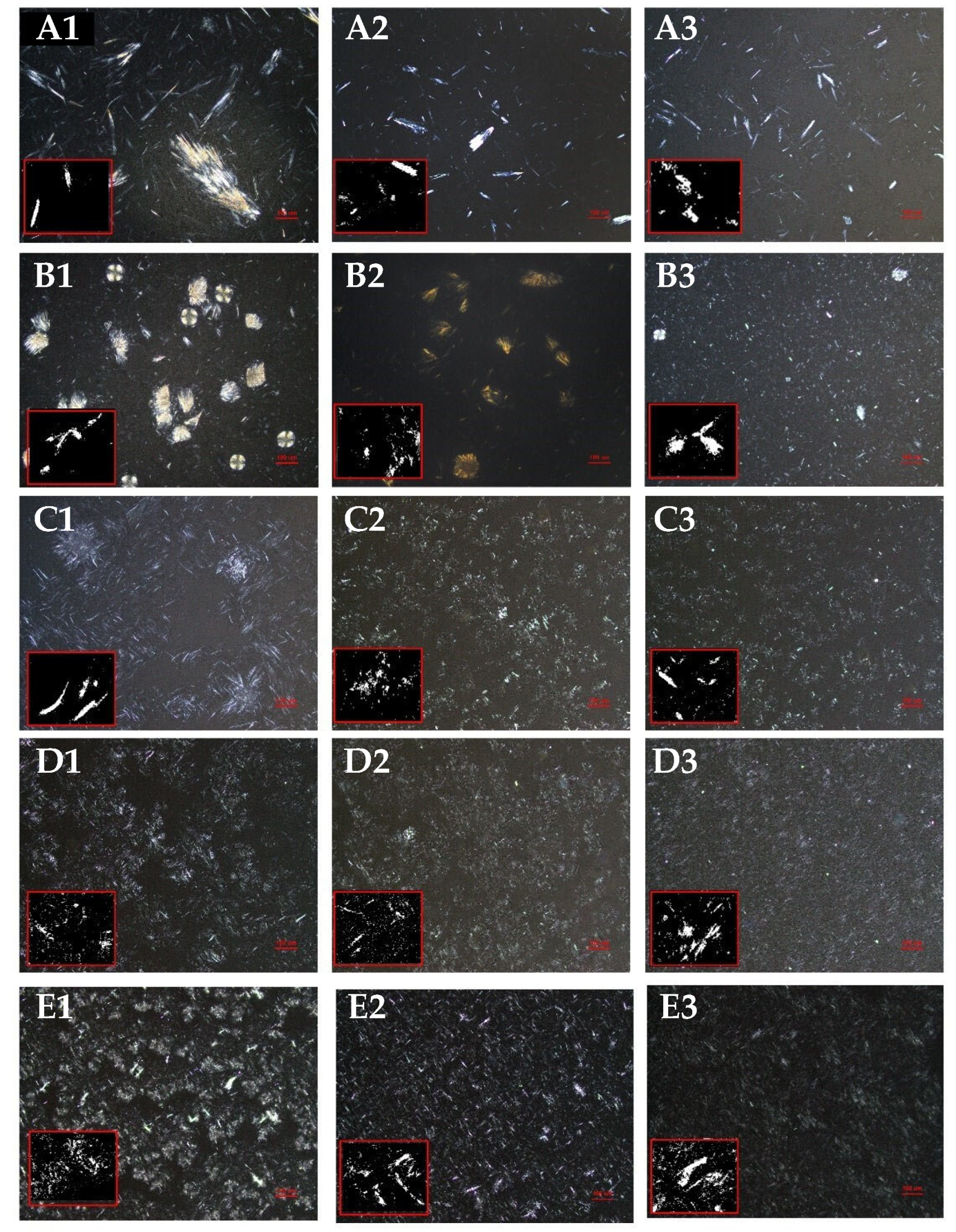


| Types | Temperature (°C) | Db | L (μm) | A |
|---|---|---|---|---|
| LO | 80 | 1.27 ± 0.03 Ca | 35.08 ± 1.15 Ad | 0.57 ± 0.00 Ab |
| 100 | 1.38 ± 0.05 Ba | 30.96 ± 1.70 Ad | 0.25 ± 0.02 Bd | |
| 120 | 1.46 ± 0.02 Aa | 21.09 ± 6.56 Bc | 0.08 ± 0.00 Cd | |
| CO | 80 | 0.82 ± 0.03 Cc | 61.10 ± 2.82 Aa | 0.86 ± 0.02 Aa |
| 100 | 0.97 ± 0.04 Bd | 52.10 ± 6.52 Aa | 0.85 ± 0.01 Aa | |
| 120 | 1.07 ± 0.02 Ad | 50.54 ± 6.14 Aa | 0.78 ± 0.03 Ba | |
| SO | 80 | 1.02 ± 0.01 Ca | 42.54 ± 1.21 Ac | 0.59 ± 0.04 Ab |
| 100 | 1.27 ± 0.05 Ab | 35.05 ± 0.72 Bcd | 0.36 ± 0.02 Bcd | |
| 120 | 1.23 ± 0.12 Bbc | 31.23 ± 1.28 Bbc | 0.13 ± 0.04 Cc | |
| RO | 80 | 1.07 ± 0.04 Bb | 45.59 ± 6.96 Abc | 0.84 ± 0.02 Aa |
| 100 | 1.11 ± 0.02 Ac | 41.08 ± 1.55 ABbc | 0.45 ± 0.02 Bc | |
| 120 | 1.29 ± 0.06 Ab | 30.35 ± 5.83 Bbc | 0.29 ± 0.03 Cbc | |
| PO | 80 | 0.85 ± 0.04 Bc | 50.74 ± 2.67 Ab | 0.86 ± 0.02 Aa |
| 100 | 1.06 ± 0.07 Acd | 46.89 ± 2.34 ABab | 0.68 ± 0.08 Bb | |
| 120 | 1.14 ± 0.23 Ac | 36.43 ± 8.57 Bb | 0.28 ± 0.02 Cb |
| 5 °C min−1 | 10 °C min−1 | |||||
|---|---|---|---|---|---|---|
| Type | n | K (min−1) | R2 | n | K (min−1) | R2 |
| LO | 2.96 ± 0.13 | 0.03 ± 0.02 | 0.95 ± 0.02 | 3.03 ± 0.47 | 0.07 ± 0.03 | 0.98 ± 0.01 |
| CO | 2.75 ± 0.19 | ND | 0.85 ± 0.02 | 2.80 ± 0.02 | 0.02 ± 0.01 | 0.87 ± 0.00 |
| SO | 2.59 ± 0.18 | 0.01 ± 0.00 | 0.96 ± 0.01 | 2.66 ± 0.32 | 0.03 ± 0.02 | 0.98 ± 0.01 |
| RO | 2.79 ± 0.07 | 0.02 ± 0.00 | 0.98 ± 0.02 | 2.97 ± 0.30 | 0.02 ± 0.01 | 0.97 ± 0.02 |
| PO | 2.75 ± 0.12 | ND | 0.97 ± 0.01 | 2.31 ± 0.07 | 0.01 ± 0.01 | 0.90 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.; Qin, S.; Xia, H.; Li, P.; Li, H.; Li, C.; Guo, S.; Zeng, C. The Impact of Oil Type on the Performance of β-Amyrin-Based Oleogels: Formation, Physicochemical Properties, and Potential Correlation Analysis. Foods 2024, 13, 876. https://doi.org/10.3390/foods13060876
Su S, Qin S, Xia H, Li P, Li H, Li C, Guo S, Zeng C. The Impact of Oil Type on the Performance of β-Amyrin-Based Oleogels: Formation, Physicochemical Properties, and Potential Correlation Analysis. Foods. 2024; 13(6):876. https://doi.org/10.3390/foods13060876
Chicago/Turabian StyleSu, Shuxian, Si Qin, Huiping Xia, Peiwang Li, Haiyan Li, Chenjia Li, Shiyin Guo, and Chaoxi Zeng. 2024. "The Impact of Oil Type on the Performance of β-Amyrin-Based Oleogels: Formation, Physicochemical Properties, and Potential Correlation Analysis" Foods 13, no. 6: 876. https://doi.org/10.3390/foods13060876
APA StyleSu, S., Qin, S., Xia, H., Li, P., Li, H., Li, C., Guo, S., & Zeng, C. (2024). The Impact of Oil Type on the Performance of β-Amyrin-Based Oleogels: Formation, Physicochemical Properties, and Potential Correlation Analysis. Foods, 13(6), 876. https://doi.org/10.3390/foods13060876







