Leveraging Plasma-Activated Seawater for the Control of Human Norovirus and Bacterial Pathogens in Shellfish Depuration
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Characterization of PASWs
2.2. Phage Inactivation by PASWs
2.3. Surrogate Inactivation by PASWs
2.4. Human Norovirus Inactivation by PASWs
2.5. Bacterial Inactivation by PASWs
2.6. HIE Cytotoxicity
2.7. Mussel Viability
2.8. Statistical Analyses
3. Results
3.1. Characterization of PASWs
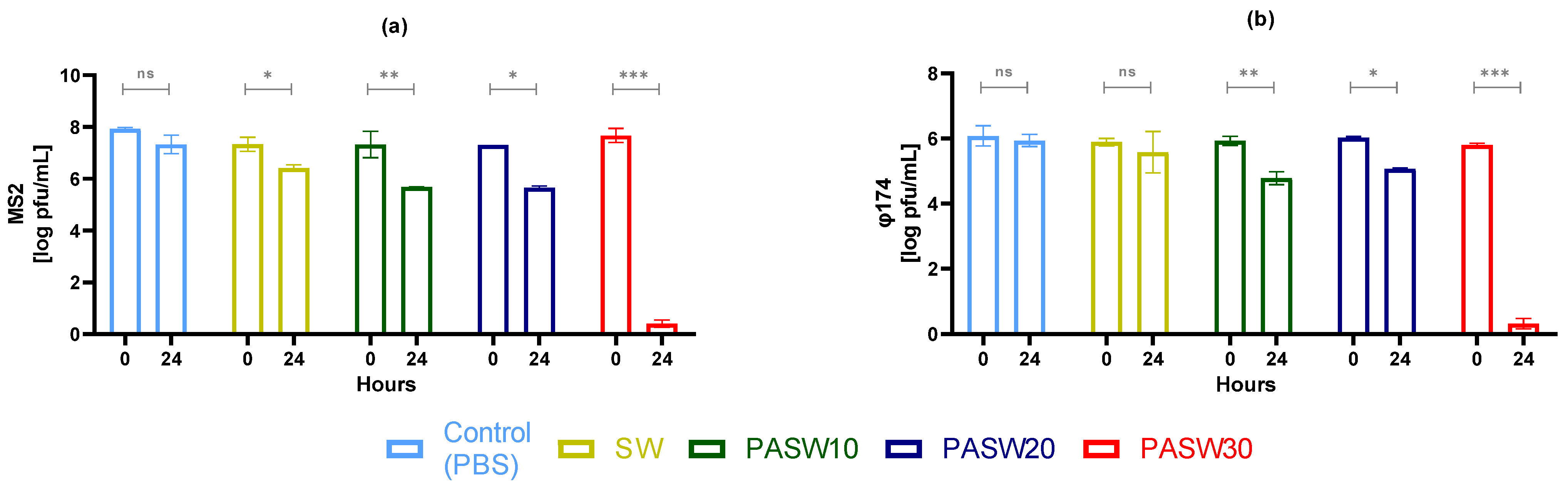
3.2. Phage Inactivation by PASWs
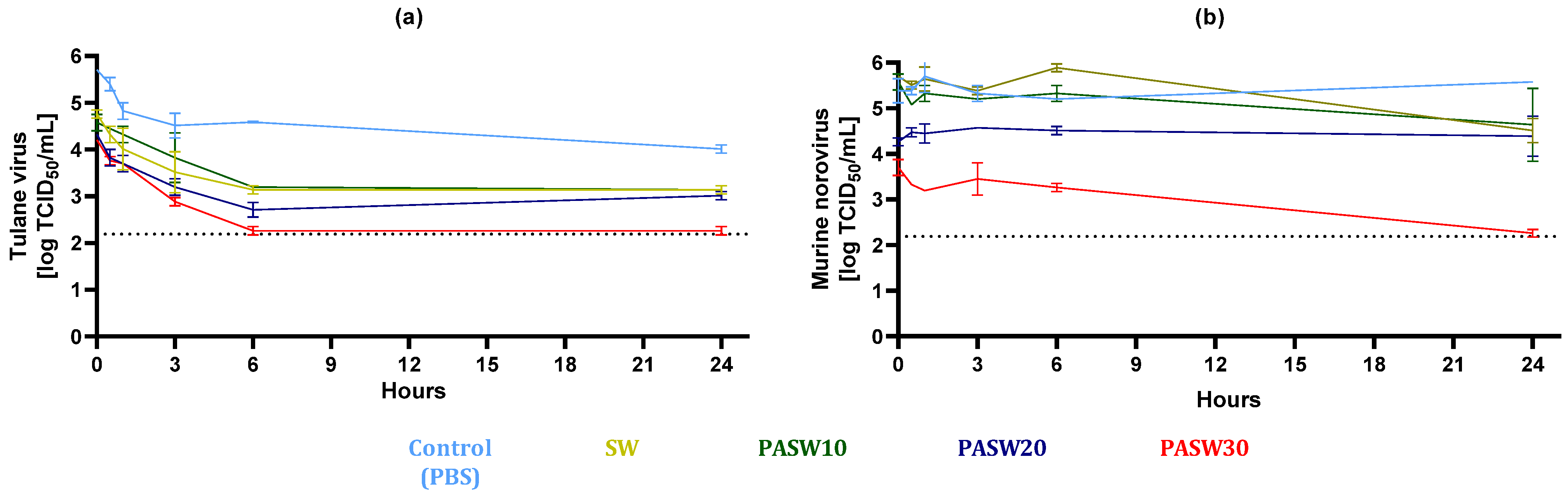
3.3. Kinetic Inactivation of HuNoV Surrogates
3.4. Inactivation of HuNoV
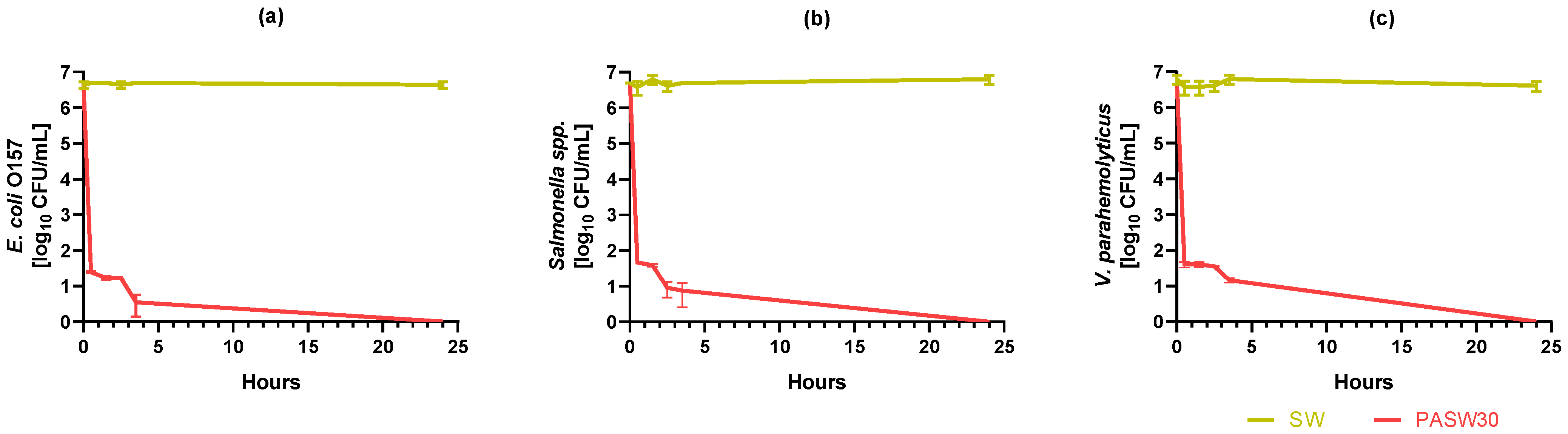
3.5. Kinetic Bacterial Inactivation
3.6. HIE Cytotoxicity
3.7. Mussel Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical Applications of Plasma Treatments: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef] [PubMed]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cai, D.; Xiao, S.; Ning, M.; Zhou, R.; Zhang, S.; Chen, X.; Ostrikov, K.; Dai, X. In Vivo Pen: A Novel Plasma Source for In Vivo Cancer Treatment. J. Cancer 2020, 11, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Salvi, D. Recent Progress in the Application of Plasma-Activated Water (PAW) for Food Decontamination. Curr. Opin. Food Sci. 2021, 42, 51–60. [Google Scholar] [CrossRef]
- Oh, Y.J.; Song, A.Y.; Min, S.C. Inhibition of Salmonella Typhimurium on Radish Sprouts Using Nitrogen-Cold Plasma. Int. J. Food Microbiol. 2017, 249, 66–71. [Google Scholar] [CrossRef]
- Fernández, A.; Noriega, E.; Thompson, A. Inactivation of Salmonella Enterica Serovar Typhimurium on Fresh Produce by Cold Atmospheric Gas Plasma Technology. Food Microbiol. 2013, 33, 24–29. [Google Scholar] [CrossRef]
- Choi, S.; Puligundla, P.; Mok, C. Corona Discharge Plasma Jet for Inactivation of Escherichia coli O157:H7 and Listeria Monocytogenes on Inoculated Pork and Its Impact on Meat Quality Attributes. Ann. Microbiol. 2016, 66, 685–694. [Google Scholar] [CrossRef]
- Baier, M.; Görgen, M.; Ehlbeck, J.; Knorr, D.; Herppich, W.B.; Schlüter, O. Non-Thermal Atmospheric Pressure Plasma: Screening for Gentle Process Conditions and Antibacterial Efficiency on Perishable Fresh Produce. Innov. Food Sci. Emerg. Technol. 2014, 22, 147–157. [Google Scholar] [CrossRef]
- Hertwig, C.; Reineke, K.; Ehlbeck, J.; Knorr, D.; Schlüter, O. Decontamination of Whole Black Pepper Using Different Cold Atmospheric Pressure Plasma Applications. Food Control 2015, 55, 221–229. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H.; Kong, M.G. Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. Appl. Environ. Microbiol. 2018, 84, e00726-18. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H.A.; Williams, P.; Gangal, U.; Youssef, M.M.; El-Sohaimy, S.A.A.; Bruggeman, P.J.; Goyal, S.M. Virucidal Effect of Cold Atmospheric Gaseous Plasma on Feline Calicivirus, a Surrogate for Human Norovirus. Appl. Environ. Microbiol. 2015, 81, 3612–3622. [Google Scholar] [CrossRef] [PubMed]
- Baert, L.; Debevere, J.; Uyttendaele, M. The Efficacy of Preservation Methods to Inactivate Foodborne Viruses. Int. J. Food Microbiol. 2009, 131, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, B.; Li, Y.; Boulaaba, A.; Binder, A.; Schotte, U.; Zimmermann, J.L.; Morfill, G.; Klein, G. Inactivation of a Foodborne Norovirus Outbreak Strain with Nonthermal Atmospheric Pressure Plasma. mBio 2015, 6, e02300-14. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-C.; Park, S.Y.; Choe, W.; Ha, S.-D. Inactivation of Murine Norovirus-1 and Hepatitis a Virus on Fresh Meats by Atmospheric Pressure Plasma Jets. Food Res. Int. 2015, 76, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, A.; Niemira, B.A.; Gurtler, J.B.; Sites, J.; Boyd, G.; Kingsley, D.H.; Li, X.; Chen, H. Nonthermal Inactivation of Norovirus Surrogates on Blueberries Using Atmospheric Cold Plasma. Food Microbiol. 2017, 63, 1–5. [Google Scholar] [CrossRef]
- Nayak, G.; Aboubakr, H.A.; Goyal, S.M.; Bruggeman, P.J. Reactive Species Responsible for the Inactivation of Feline Calicivirus by a Two-dimensional Array of Integrated Coaxial Microhollow Dielectric Barrier Discharges in Air. Plasma Process. Polym. 2018, 15, 1700119. [Google Scholar] [CrossRef]
- Herianto, S.; Shih, M.-K.; Lin, C.-M.; Hung, Y.-C.; Hsieh, C.-W.; Wu, J.-S.; Chen, M.-H.; Chen, H.-L.; Hou, C.-Y. The Effects of Glazing with Plasma-Activated Water Generated by a Piezoelectric Direct Discharge Plasma System on Whiteleg Shrimp (Litopenaeus vannamei). LWT 2022, 154, 112547. [Google Scholar] [CrossRef]
- Patange, A.; Lu, P.; Boehm, D.; Cullen, P.J.; Bourke, P. Efficacy of Cold Plasma Functionalised Water for Improving Microbiological Safety of Fresh Produce and Wash Water Recycling. Food Microbiol. 2019, 84, 103226. [Google Scholar] [CrossRef]
- Andrasch, M.; Stachowiak, J.; Schlüter, O.; Schnabel, U.; Ehlbeck, J. Scale-up to Pilot Plant Dimensions of Plasma Processed Water Generation for Fresh-Cut Lettuce Treatment. Food Packag. Shelf Life 2017, 14, 40–45. [Google Scholar] [CrossRef]
- Wong, K.S.; Lim, W.T.H.; Ooi, C.W.; Yeo, L.Y.; Tan, M.K. In Situ Generation of Plasma-Activated Aerosols via Surface Acoustic Wave Nebulization for Portable Spray-Based Surface Bacterial Inactivation. Lab Chip 2020, 20, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- de Castro Medeiros, L.; de Alencar, F.L.S.; Navoni, J.A.; de Araujo, A.L.C.; do Amaral, V.S. Toxicological Aspects of Trihalomethanes: A Systematic Review. Environ. Sci. Pollut. Res. 2019, 26, 5316–5332. [Google Scholar] [CrossRef] [PubMed]
- Jenns, K.; Sassi, H.P.; Zhou, R.; Cullen, P.J.; Carter, D.; Mai-Prochnow, A. Inactivation of Foodborne Viruses: Opportunities for Cold Atmospheric Plasma. Trends Food Sci. Technol. 2022, 124, 323–333. [Google Scholar] [CrossRef]
- Rahman, M.; Hasan, M.S.; Islam, R.; Rana, R.; Sayem, A.S.M.; Sad, M.A.A.; Matin, A.; Raposo, A.; Zandonadi, R.P.; Han, H.; et al. Plasma-Activated Water for Food Safety and Quality: A Review of Recent Developments. Int. J. Environ. Res. Public Health 2022, 19, 6630. [Google Scholar] [CrossRef]
- Oliveira, J.; Cunha, A.; Castilho, F.; Romalde, J.L.; Pereira, M.J. Microbial Contamination and Purification of Bivalve Shellfish: Crucial Aspects in Monitoring and Future Perspectives—A Mini-Review. Food Control 2011, 22, 805–816. [Google Scholar] [CrossRef]
- RASFF Window. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search?searchQueries=eyJkYXRlIjp7InN0YXJ0UmFuZ2UiOiIyMDIyLTEyLTMxVDIzOjAwOjAwLjAwMFoiLCJlbmRSYW5nZSI6IjIwMjMtMTItMzBUMjM6MDA6MDAuMDAwWiJ9LCJub3RpZmljYXRpb25TdGF0dXMiOnsibm90aWZpY2F0aW9uU3RhdHVzIjpbWzFdXX0sInByb2R1Y3QiOnsicHJvZHVjdENhdGVnb3J5IjpbWzE4NDMyXV19LCJyaXNrIjp7ImhhemFyZENhdGVnb3J5IjpbWzIxODAxXV19fQ%3D%3D (accessed on 17 January 2024).
- European Food Safety Authority (EFSA). Analysis of the European Baseline Survey of Norovirus in Oysters. EFSA J. 2019, 17, e05762. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. Infections. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Mancini, M.E.; Alessiani, A.; Donatiello, A.; Didonna, A.; D’Attoli, L.; Faleo, S.; Occhiochiuso, G.; Carella, F.; Di Taranto, P.; Pace, L.; et al. Systematic Survey of Vibrio spp. and Salmonella spp. in Bivalve Shellfish in Apulia Region (Italy): Prevalence and Antimicrobial Resistance. Microorganisms 2023, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Mok, J.S.; Ryu, A.; Kwon, J.Y.; Kim, B.; Park, K. Distribution of Vibrio Species Isolated from Bivalves and Bivalve Culture Environments along the Gyeongnam Coast in Korea: Virulence and Antimicrobial Resistance of Vibrio parahaemolyticus Isolates. Food Control 2019, 106, 106697. [Google Scholar] [CrossRef]
- Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the Monitoring of Zoonoses and Zoonotic Agents, Amending Council Decision 90/424/EEC and Repealing Council Directive 92/117/EEC. Available online: http://data.europa.eu/eli/dir/2003/99/oj (accessed on 3 March 2024).
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1441/2007 of 5 December 2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs (Text with EEA Relevance). Available online: http://data.europa.eu/eli/reg/2007/1441/oj (accessed on 3 March 2024).
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Pavoni, E.; Bertasi, B.; Galuppini, E.; Mangeri, L.; Meletti, F.; Tilola, M.; Carta, V.; Todeschi, S.; Losio, M.-N. Detection of Hepatitis a Virus and Norovirus in Different Food Categories: A 6-Year Survey in Italy. Food Environ. Virol. 2022, 14, 69–76. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 Laying Down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and Amending Commission Regulation (EC) No 2074/2005 as Regards Official Controls (Text with EEA Relevance). Available online: http://data.europa.eu/eli/reg_impl/2019/627/oj (accessed on 3 March 2024).
- Le Mennec, C.; Parnaudeau, S.; Rumebe, M.; Le Saux, J.C.; Piquet, J.C.; Le Guyader, S.F. Follow-Up of Norovirus Contamination in an Oyster Production Area Linked to Repeated Outbreaks. Food Environ. Virol. 2017, 9, 54–61. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.; Polo, D.; Le Saux, J.; Le Guyader, F.S. Depuration and Relaying: A Review on Potential Removal of Norovirus from Oysters. Compr. Rev. Food Sci. Food Saf. 2017, 16, 692–706. [Google Scholar] [CrossRef] [PubMed]
- UNI EN ISO 15216-1:2017; Microbiology of the Food Chain—Horizontal Method for Determination of Hepatitis a Virus and Norovirus Using Real-Time RT-PCR—Part 1: Method for Quantification. International Organization for Standardization, Vernier: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/65681.html (accessed on 3 March 2024).
- Estes, M.K.; Ettayebi, K.; Tenge, V.R.; Murakami, K.; Karandikar, U.; Lin, S.C.; Ayyar, B.V.; Cortes-Penfield, N.W.; Haga, K.; Neill, F.H.; et al. Human Norovirus Cultivation in Nontransformed Stem Cell-Derived Human Intestinal Enteroid Cultures: Success and Challenges. Viruses 2019, 11, 638. [Google Scholar] [CrossRef] [PubMed]
- Kamarasu, P.; Hsu, H.-Y.; Moore, M.D. Research Progress in Viral Inactivation Utilizing Human Norovirus Surrogates. Front. Sustain. Food Syst. 2018, 2, 89. [Google Scholar] [CrossRef]
- Cromeans, T.; Park, G.W.; Costantini, V.; Lee, D.; Wang, Q.; Farkas, T.; Lee, A.; Vinjé, J. Comprehensive Comparison of Cultivable Norovirus Surrogates in Response to Different Inactivation and Disinfection Treatments. Appl. Environ. Microbiol. 2014, 80, 5743–5751. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.P. Critical Review of Norovirus Surrogates in Food Safety Research: Rationale for Considering Volunteer Studies. Food Environ. Virol. 2012, 4, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Blondin-Brosseau, M.; Harlow, J.; Doctor, T.; Nasheri, N. Examining the Persistence of Human Coronavirus 229E on Fresh Produce. Food Microbiol. 2021, 98, 103780. [Google Scholar] [CrossRef] [PubMed]
- Danso, D.; Chow, J.; Streita, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef] [PubMed]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of Human Noroviruses in Stem Cell-Derived Human Enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef]
- Costantini, V.; Morantz, E.K.; Browne, H.; Ettayebi, K.; Zeng, X.L.; Atmar, R.L.; Estes, M.K.; Vinjé, J. Human Norovirus Replication in Human Intestinal Enteroids as Model to Evaluate Virus Inactivation. Emerg. Infect. Dis. 2018, 24, 1453–1464. [Google Scholar] [CrossRef]
- Escudero-Abarca, B.I.; Goulter, R.M.; Arbogast, J.W.; Leslie, R.A.; Green, K.; Jaykus, L.A. Efficacy of Alcohol-Based Hand Sanitizers against Human Norovirus Using RNase-RT-QPCR with Validation by Human Intestinal Enteroid Replication. Lett. Appl. Microbiol. 2020, 71, 605–610. [Google Scholar] [CrossRef]
- Desdouits, M.; Polo, D.; Le Mennec, C.; Strubbia, S.; Zeng, X.L.; Ettayebi, K.; Atmar, R.L.; Estes, M.K.; Le Guyader, F.S. Use of Human Intestinal Enteroids to Evaluate Persistence of Infectious Human Norovirus in Seawater. Emerg. Infect. Dis. 2022, 28, 1475–1479. [Google Scholar] [CrossRef]
- Randazzo, W.; Costantini, V.; Morantz, E.K.; Vinjé, J. Human Intestinal Enteroids to Evaluate Human Norovirus GII.4 Inactivation by Aged-Green Tea. Front. Microbiol. 2020, 11, 1917. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Pérez, A.; Martínez, A.; Rodrigo, D.; Sánchez, G. High Pressure Treatment and Green Tea Extract Synergistically Control Enteric Virus Contamination in Beverages. Food Control 2023, 144, 109384. [Google Scholar] [CrossRef]
- Kulawik, P.; Kumar Tiwari, B. Recent Advancements in the Application of Non-Thermal Plasma Technology for the Seafood Industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 3199–3210. [Google Scholar] [CrossRef]
- Abedon, S.T.; Yin, J. Bacteriophage Plaques: Theory and Analysis. In Bacteriophages; Springer: Berlin/Heidelberg, Germany, 2009; pp. 161–174. [Google Scholar]
- Falcó, I.; Randazzo, W.; Rodríguez-Díaz, J.; Gozalbo-Rovira, R.; Luque, D.; Aznar, R.; Sánchez, G. Antiviral Activity of Aged Green Tea Extract in Model Food Systems and under Gastric Conditions. Int. J. Food Microbiol. 2019, 292, 101–106. [Google Scholar] [CrossRef]
- Carmona, N.; Randazzo, W. Passaging human intestinal organoids and monolayers set up. Protoc. Exch. 2023. [Google Scholar] [CrossRef]
- Wales, S.Q.; Pandiscia, A.; Kulka, M.; Sánchez, G.; Randazzo, W. Challenges for Estimating Human Norovirus Infectivity by Viability RT-QPCR as Compared to Replication in Human Intestinal Enteroids. Int. J. Food Microbiol. 2024, 411, 110507. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H.A.; Sampedro Parra, F.; Collins, J.; Bruggeman, P.; Goyal, S.M. Ìn Situ Inactivation of Human Norovirus GII.4 by Cold Plasma: Ethidium Monoazide (EMA)-Coupled RT-QPCR Underestimates Virus Reduction and Fecal Material Suppresses Inactivation. Food Microbiol. 2020, 85, 103307. [Google Scholar] [CrossRef]
- Baek, K.H.; Yong, H.I.; Yoo, J.H.; Kim, J.W.; Byeon, Y.S.; Lim, J.; Yoon, S.Y.; Ryu, S.; Jo, C. Antimicrobial Effects and Mechanism of Plasma Activated Fine Droplets Produced from Arc Discharge Plasma on Planktonic Listeria monocytogenes and Escherichia coli O157:H7. J. Phys. D Appl. Phys. 2020, 53, 124002. [Google Scholar] [CrossRef]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.; von Woedtke, T. Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process. Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Su, X.; Tian, Y.; Zhou, H.; Li, Y.; Zhang, Z.; Jiang, B.; Yang, B.; Zhang, J.; Fang, J. Inactivation Efficacy of Nonthermal Plasma-Activated Solutions against Newcastle Disease Virus. Appl. Environ. Microbiol. 2018, 84, e02836-17. [Google Scholar] [CrossRef]
- Wang, Q.; Salvi, D. Evaluation of Plasma-Activated Water (PAW) as a Novel Disinfectant: Effectiveness on Escherichia Coli and Listeria Innocua, Physicochemical Properties, and Storage Stability. LWT 2021, 149, 111847. [Google Scholar] [CrossRef]
- Campbell, V.M.; Hall, S.; Salvi, D. Antimicrobial Effects of Plasma-Activated Simulated Seawater (PASW) on Total Coliform and Escherichia coli in Live Oysters during Static Depuration. Fishes 2023, 8, 396. [Google Scholar] [CrossRef]
- Campbell, V.M.; Wang, Q.; Hall, S.G.; Salvi, D. Physicochemical Properties and Antimicrobial Impacts of Plasma-activated Simulated Seawater on Escherichia coli. JSFA Rep. 2022, 2, 228–235. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Dielectric Barrier Discharge Cold Atmospheric Plasma: Bacterial Inactivation Mechanism. J. Food Saf. 2019, 39, e12705. [Google Scholar] [CrossRef]
- Epifanio, C.E.; Srna, R.F. Toxicity of Ammonia, Nitrite Ion, Nitrate Ion, and Orthophosphate to Mercenaria Mercenaria and Crassostrea Virginica. Mar. Biol. 1975, 33, 241–246. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.h.; Weltmann, K.-D.; von Woedtke, T.h. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Herianto, S.; Hou, C.; Lin, C.; Chen, H. Nonthermal Plasma-activated Water: A Comprehensive Review of This New Tool for Enhanced Food Safety and Quality. Compr. Rev. Food Sci. Food Saf. 2021, 20, 583–626. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Bhartiya, P.; Kaushik, N.; Shin, Y.; Nguyen, L.N.; Park, J.S.; Kim, D.; Choi, E.H. Nitric-Oxide Enriched Plasma-Activated Water Inactivates 229E Coronavirus and Alters Antiviral Response Genes in Human Lung Host Cells. Bioact. Mater. 2023, 19, 569–580. [Google Scholar] [CrossRef]
- Wolff, A.; Günther, T.; Albert, T.; Johne, R. Effect of Sodium Chloride, Sodium Nitrite and Sodium Nitrate on the Infectivity of Hepatitis E Virus. Food Environ. Virol. 2020, 12, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Lee, J.E.; Lim, M.Y.; Ko, G. Effect of Temperature, PH, and NaCl on the Inactivation Kinetics of Murine Norovirus. J. Food Prot. 2012, 75, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Mitra, S.; Baek, E.J.; Nguyen, L.N.; Bhartiya, P.; Kim, J.H.; Choi, E.H.; Kaushik, N.K. The Inactivation and Destruction of Viruses by Reactive Oxygen Species Generated through Physical and Cold Atmospheric Plasma Techniques: Current Status and Perspectives. J. Adv. Res. 2023, 43, 59–71. [Google Scholar] [CrossRef]
- Boulais, M.; Chenevert, K.J.; Demey, A.T.; Darrow, E.S.; Robison, M.R.; Roberts, J.P.; Volety, A. Oyster Reproduction Is Compromised by Acidification Experienced Seasonally in Coastal Regions. Sci. Rep. 2017, 7, 13276. [Google Scholar] [CrossRef]
- Russell, S. The Effect of Electrolyzed Oxidative Water Applied Using Electrostatic Spraying on Pathogenic and Indicator Bacteria on the Surface of Eggs. Poult. Sci. 2003, 82, 158–162. [Google Scholar] [CrossRef]
- Zver, M.; Dobnik, D.; Zaplotnik, R.; Mozetič, M.; Filipić, A.; Primc, G. Non-Thermal Plasma Inactivation of Viruses in Water Solutions. J. Water Process. Eng. 2023, 53, 103839. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, Y.; Wei, K.; Li, W.; Yao, M.; Zhang, J.; Grinshpun, S.A. MS2 Virus Inactivation by Atmospheric-Pressure Cold Plasma Using Different Gas Carriers and Power Levels. Appl. Environ. Microbiol. 2015, 81, 996–1002. [Google Scholar] [CrossRef]
- Filipić, A.; Dobnik, D.; Tušek Žnidarič, M.; Žegura, B.; Štern, A.; Primc, G.; Mozetič, M.; Ravnikar, M.; Žel, J.; Gutierrez Aguirre, I. Inactivation of Pepper Mild Mottle Virus in Water by Cold Atmospheric Plasma. Front. Microbiol. 2021, 12, 618209. [Google Scholar] [CrossRef]
- Hanbal, S.E.; Takashima, K.; Miyashita, S.; Ando, S.; Ito, K.; Elsharkawy, M.M.; Kaneko, T.; Takahashi, H. Atmospheric-Pressure Plasma Irradiation Can Disrupt Tobacco Mosaic Virus Particles and RNAs to Inactivate Their Infectivity. Arch. Virol. 2018, 163, 2835–2840. [Google Scholar] [CrossRef]
- Shaffer, M.; Huynh, K.; Costantini, V.; Bibby, K.; Vinjé, J. Viable Norovirus Persistence in Water Microcosms. Environ. Sci. Technol. Lett. 2022, 9, 851–855. [Google Scholar] [CrossRef]
- Rexin, D.; Rachmadi, A.T.; Hewitt, J. Persistence of Infectious Human Norovirus in Estuarine Water. Food Environ. Virol. 2024. [Google Scholar] [CrossRef]
- Mohamed, H.; Nayak, G.; Rendine, N.; Wigdahl, B.; Krebs, F.C.; Bruggeman, P.J.; Miller, V. Non-Thermal Plasma as a Novel Strategy for Treating or Preventing Viral Infection and Associated Disease. Front. Phys. 2021, 9, 683118. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Gangal, U.; Youssef, M.M.; Goyal, S.M.; Bruggeman, P.J. Inactivation of Virus in Solution by Cold Atmospheric Pressure Plasma: Identification of Chemical Inactivation Pathways. J. Phys. D Appl. Phys. 2016, 49, 204001. [Google Scholar] [CrossRef]
- Yamashiro, R.; Misawa, T.; Sakudo, A. Key Role of Singlet Oxygen and Peroxynitrite in Viral RNA Damage during Virucidal Effect of Plasma Torch on Feline Calicivirus. Sci. Rep. 2018, 8, 17947. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Cui, Q.; Xu, Y.; Wang, B.; Tian, M.; Li, Q.; Liu, Z.; Liu, D.; Chen, H.; Kong, M.G. Systemic Study on the Safety of Immuno-Deficient Nude Mice Treated by Atmospheric Plasma-Activated Water. Plasma Sci. Technol. 2018, 20, 044003. [Google Scholar] [CrossRef]

| PASW | NO2− (mg/L) | NO3− (mg/L) | H2O2 (mg/L) | pH |
|---|---|---|---|---|
| SW | 0 | 0 | 0 | 8.13 ± 0.31 b |
| PASW10 | 5 ± 0.5 b | 25 ± 1 a | 1 ± 0.2 b | 7.95 ± 0.11 b |
| PASW20 | 0.5 ± 0.5 a | 298 ± 3 b | 5 ± 0.3 c | 7.73 ± 0.17 b |
| PASW30 | 100 ± 0.5 c | 310 ± 10 b | 0.5 ± 0.1 a | 5.00 ± 0.22 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandiscia, A.; Lorusso, P.; Manfredi, A.; Sánchez, G.; Terio, V.; Randazzo, W. Leveraging Plasma-Activated Seawater for the Control of Human Norovirus and Bacterial Pathogens in Shellfish Depuration. Foods 2024, 13, 850. https://doi.org/10.3390/foods13060850
Pandiscia A, Lorusso P, Manfredi A, Sánchez G, Terio V, Randazzo W. Leveraging Plasma-Activated Seawater for the Control of Human Norovirus and Bacterial Pathogens in Shellfish Depuration. Foods. 2024; 13(6):850. https://doi.org/10.3390/foods13060850
Chicago/Turabian StylePandiscia, Annamaria, Patrizio Lorusso, Alessio Manfredi, Gloria Sánchez, Valentina Terio, and Walter Randazzo. 2024. "Leveraging Plasma-Activated Seawater for the Control of Human Norovirus and Bacterial Pathogens in Shellfish Depuration" Foods 13, no. 6: 850. https://doi.org/10.3390/foods13060850
APA StylePandiscia, A., Lorusso, P., Manfredi, A., Sánchez, G., Terio, V., & Randazzo, W. (2024). Leveraging Plasma-Activated Seawater for the Control of Human Norovirus and Bacterial Pathogens in Shellfish Depuration. Foods, 13(6), 850. https://doi.org/10.3390/foods13060850






