Wheat Protein Hydrolysates Improving the Stability of Purple Sweet Potato Anthocyanins under Neutral pH after Commercial Sterilization at 121 °C
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Measurement of Degradation
2.3.1. Color
2.3.2. Anthocyanin Content
2.3.3. Brown Indexes (BI) and Polymeric Color Indexes (PCI)
2.4. Degradation Kinetics of Anthocyanins
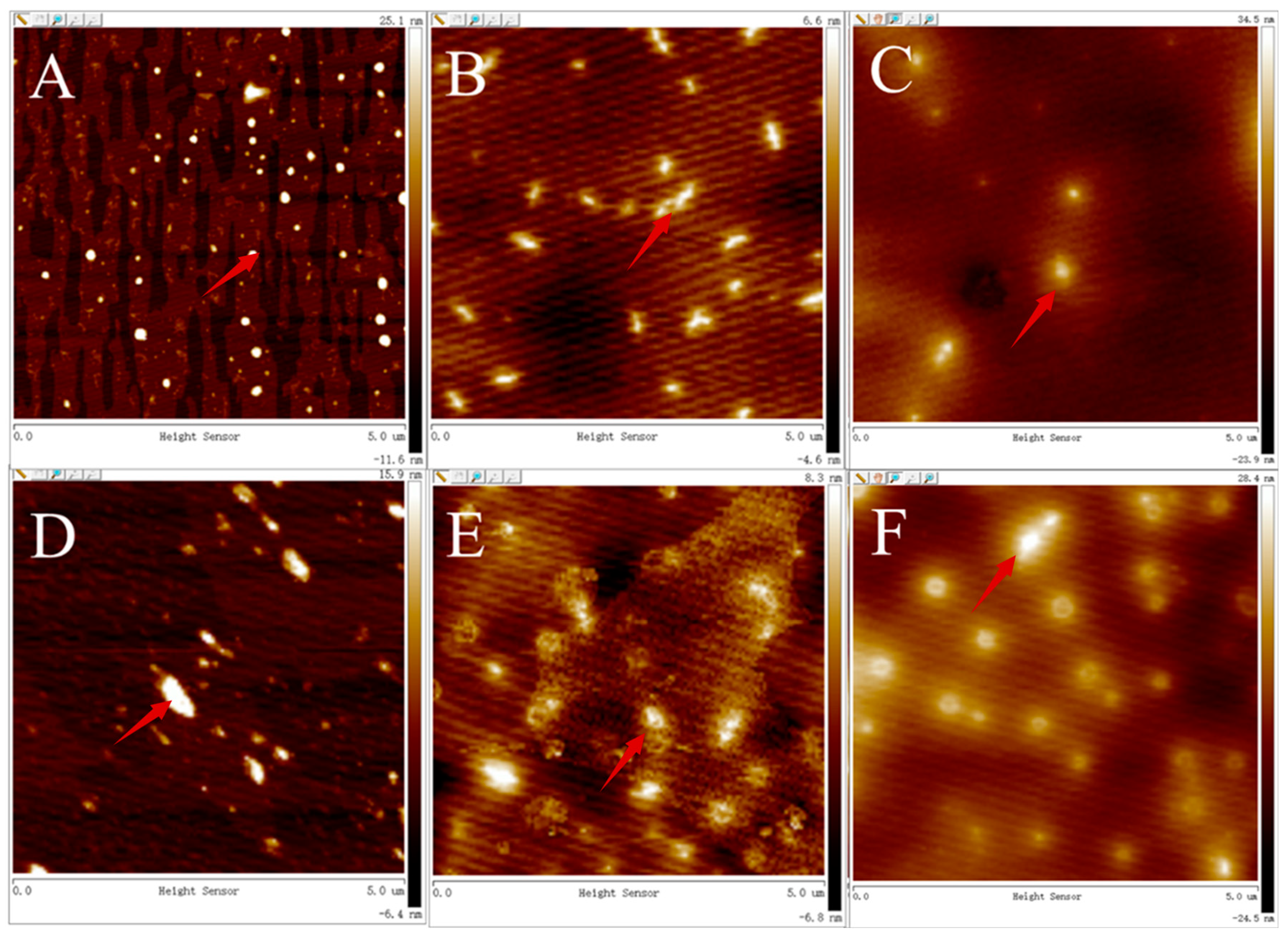
2.5. Atomic force Microscope (AFM)
2.6. Zeta Potential and Particle Size
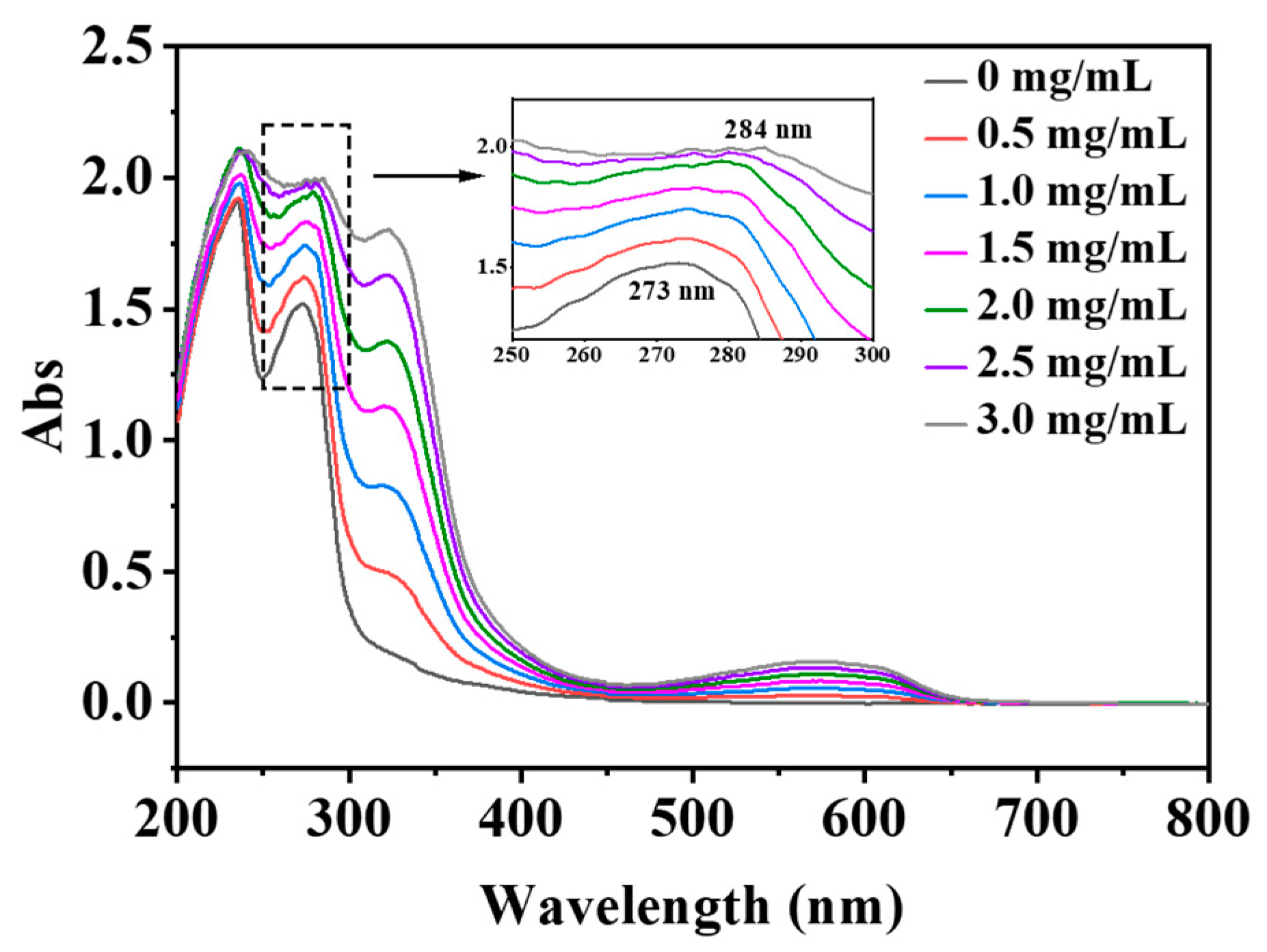
2.7. Spectral Characteristics
2.7.1. UV–Vis Spectroscopy
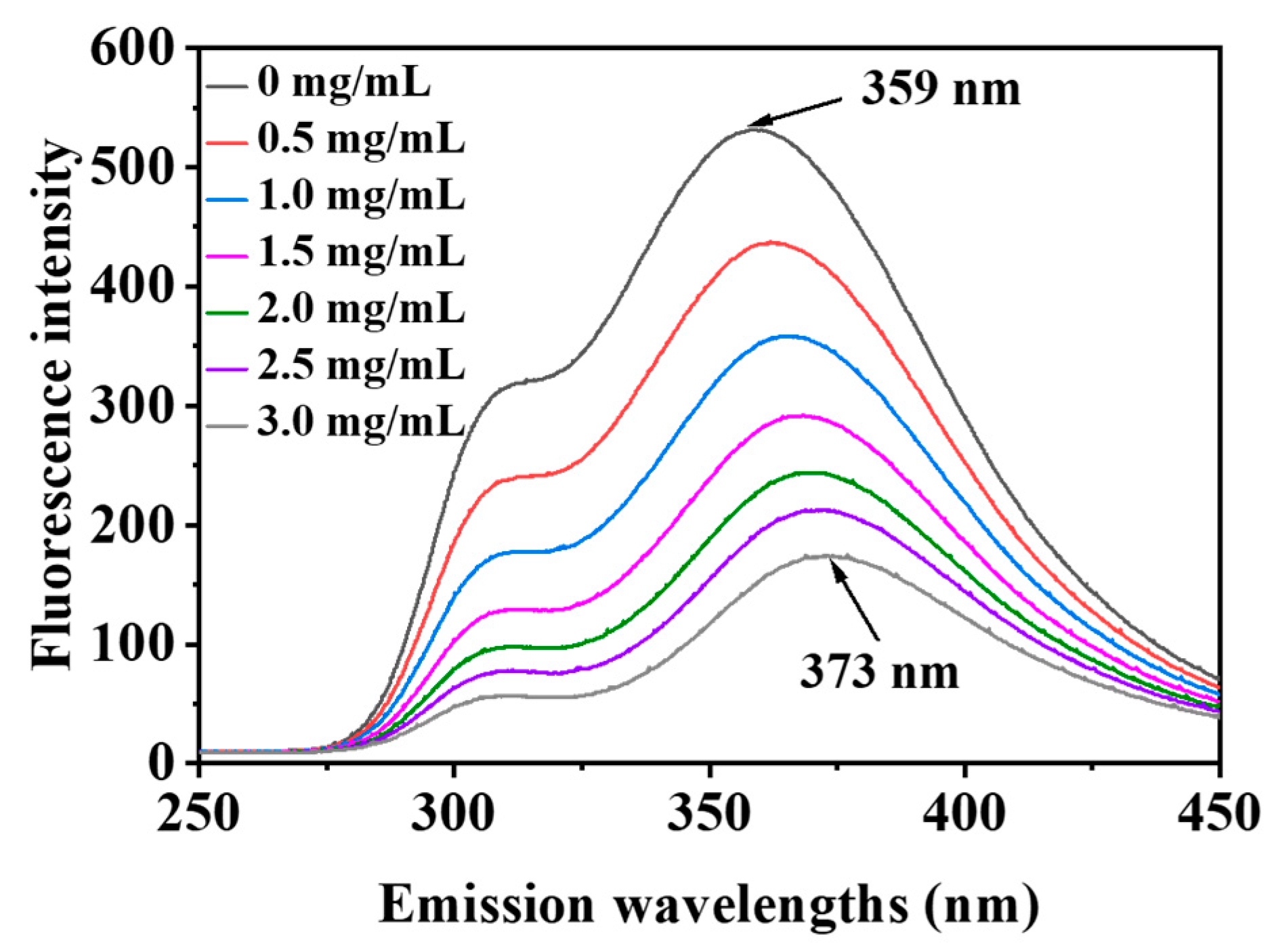
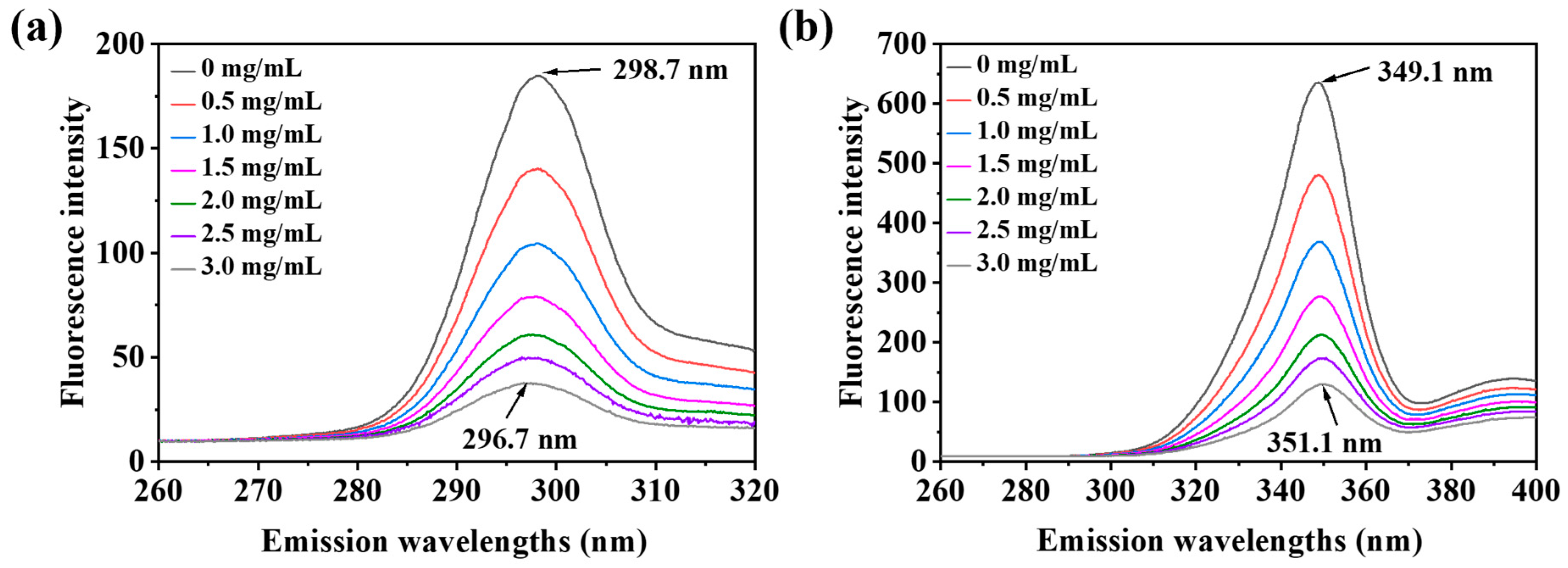
2.7.2. Fluorescence Spectroscopy
2.8. Antioxidant Capacity
2.8.1. DPPH Free Radical Scavenging Activity
2.8.2. ABTS Free Radical Scavenging Activity
2.8.3. Ferric Reducing Antioxidant Power (FRAP)
2.9. In Vitro Digestion
2.10. Statistical Analysis
3. Results and Discussion
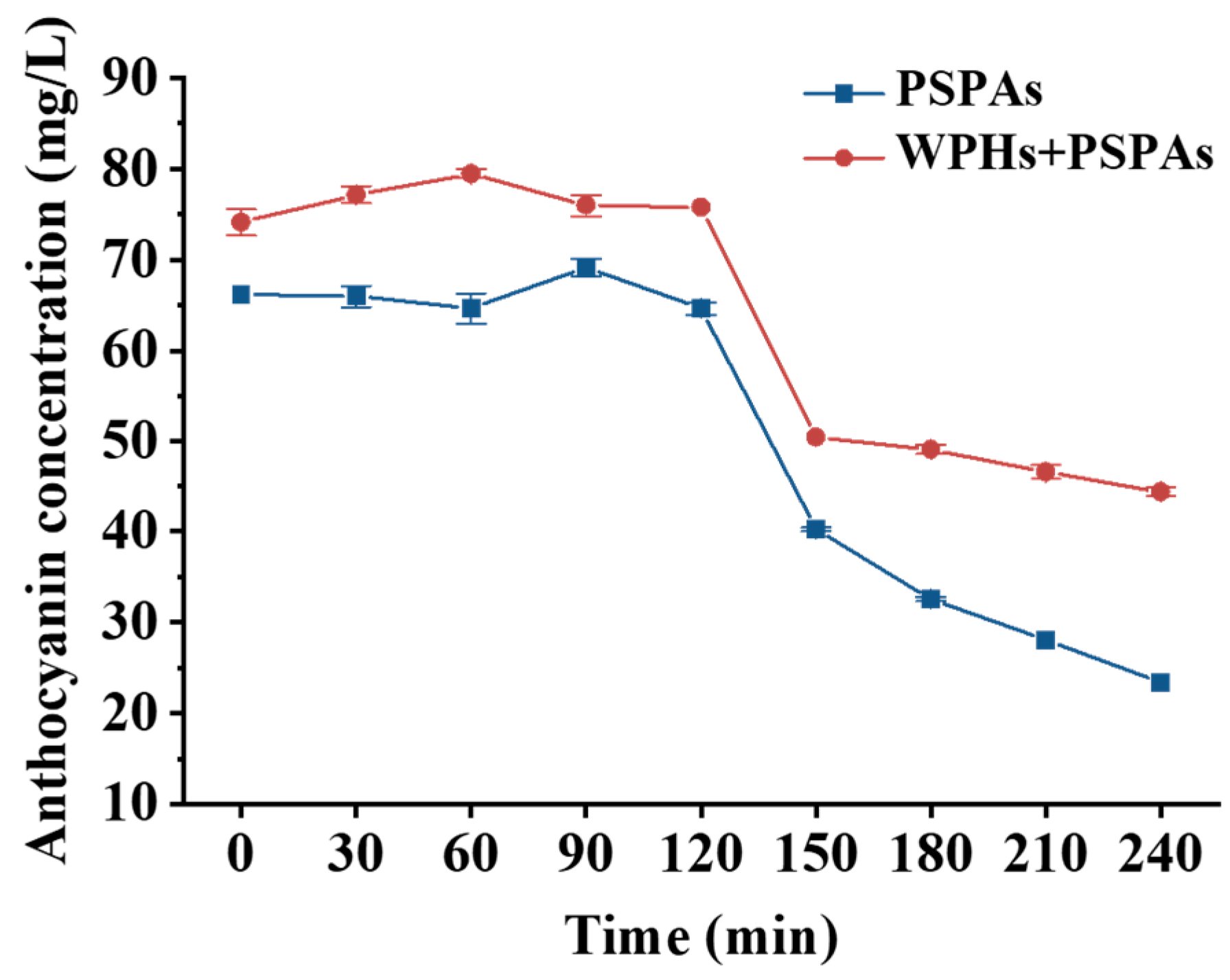
3.1. Color Stability and Anthocyanins Concentration
3.2. BI and PCI
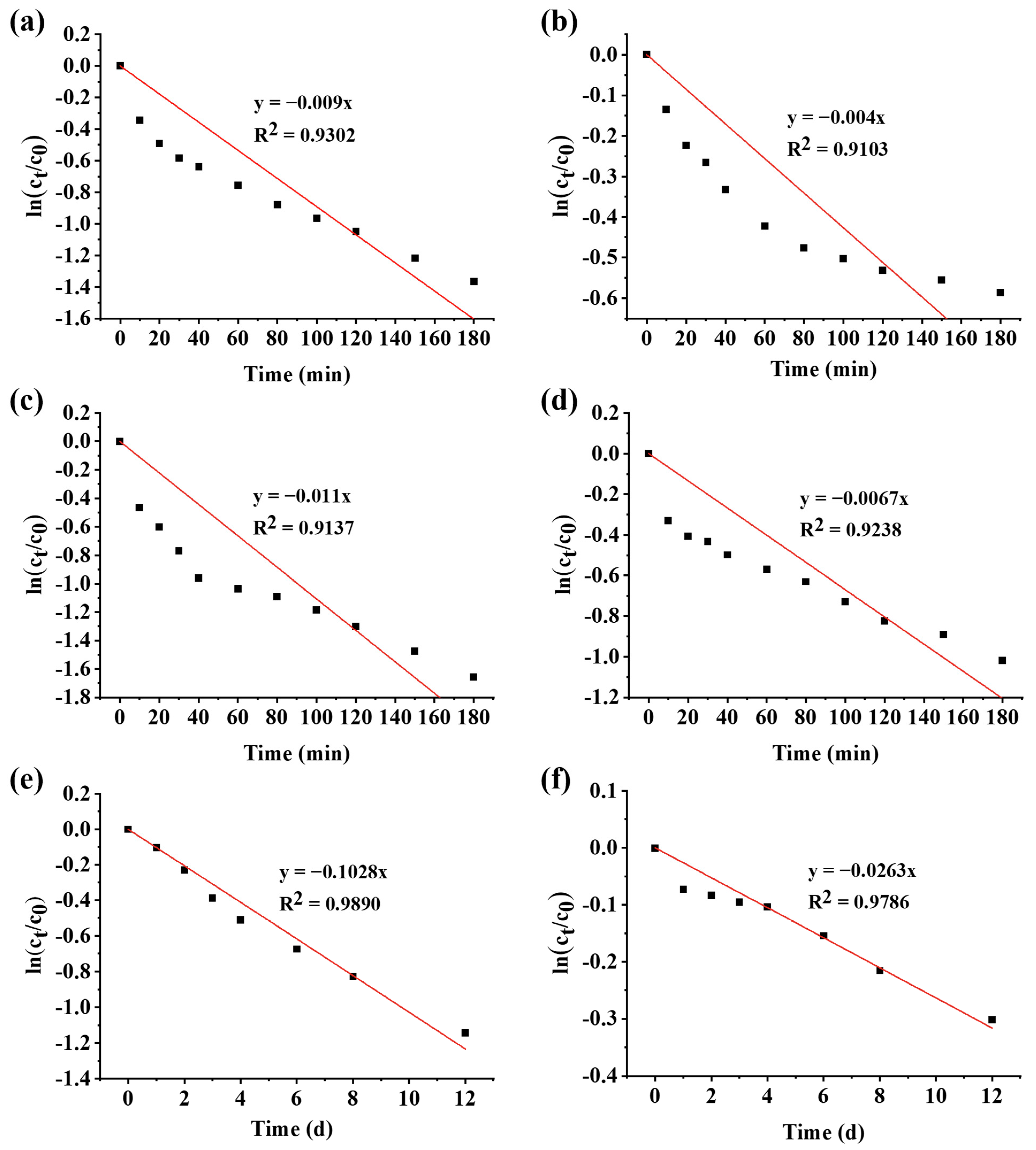
3.3. Degradation Kinetics
3.4. Changes of Particle Size and Morphology
3.5. Zeta Potential
3.6. UV–Vis Spectroscopy
3.7. Fluorescence Spectroscopy
3.8. Synchronous Fluorescence Spectroscopy
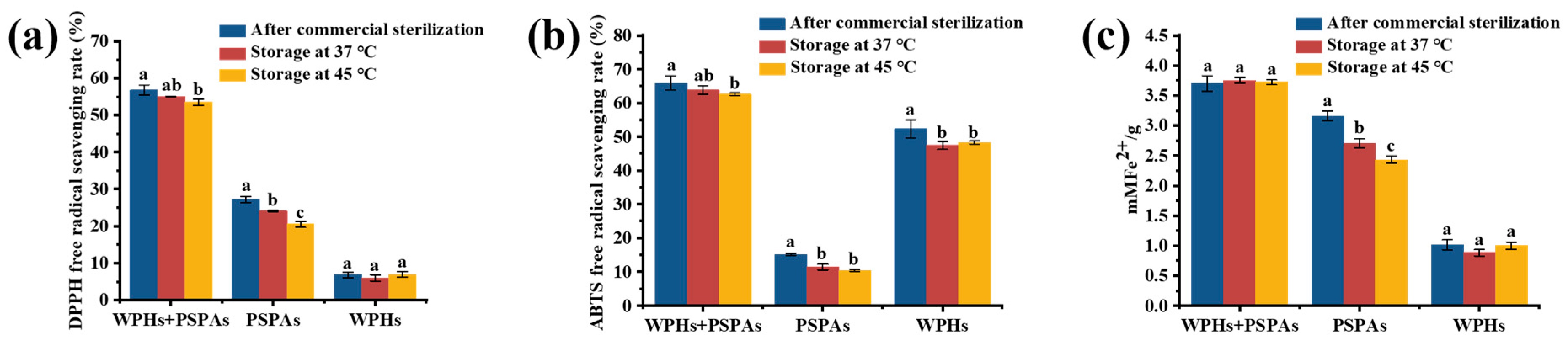
3.9. Antioxidant Ability
3.10. In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WPHs | Wheat protein hydrolysates |
| PSPAs | Purple sweet potato anthocyanins |
| WPHs+PSPAs | Purple sweet potato anthocyanins solution with wheat protein hydrolysates added |
| BI | Brown indexes |
| PCI | Polymeric color indexes |
| AFM | Atomic force microscope |
References
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2018, 274, 460–470. [Google Scholar] [CrossRef]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ichiyanagi, T.; Komiyama, T.; Sato, S.; Konishi, T. Effects of anthocyanins on psychological stress-induced oxidative stress and neurotransmitter status. J. Agric. Food Chem. 2008, 56, 7545–7550. [Google Scholar] [CrossRef]
- Nizamutdinova, I.T.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Jeong, Y.-K.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean seed coats preferentially inhibit TNF-α-mediated induction of VCAM-1 over ICAM-1 through the regulation of GATAs and IRF-1. J. Agric. Food Chem. 2009, 57, 7324–7330. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research advances of purple sweet potato anthocyanins: Extraction, identification, stability, bioactivity, application, and biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhu, H.; Xu, M.; Qin, F.; Chen, J. Complexation of bovine β-lactoglobulin with malvidin-3-O-glucoside and its effect on the stability of grape skin anthocyanin extracts. Food Chem. 2016, 209, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Berrios Jde, J.; Powers, J.R.; Tang, J. Thermal degradation of anthocyanins from purple potato (cv. Purple Majesty) and impact on antioxidant capacity. J. Agric. Food Chem. 2011, 59, 11040–11049. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Interactions of milk α- and β-casein with malvidin-3-O-glucoside and their effects on the stability of grape skin anthocyanin extracts. Food Chem. 2016, 199, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.; Chou, S.; Tian, J.; Lang, Y.; Shen, Y.; Ran, X.; Gao, N.; Li, B. Effect of whey protein isolate on the stability and antioxidant capacity of blueberry anthocyanins: A mechanistic and in vitro simulation study. Food Chem. 2021, 336, 127700. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Y.; Li, L.; Qi, B.; Ju, M.; Xu, Y.; Zhang, Y.; Sui, X. Covalent conjugates of anthocyanins to soy protein: Unravelling their structure features and in vitro gastrointestinal digestion fate. Food Res. Int. 2019, 120, 603–609. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, S.; Chen, Q.; Liu, Q.; Kong, B. Antioxidant activities and emulsifying properties of porcine plasma protein hydrolysates modified by oxidized tannic acid and oxidized chlorogenic acid. Process Biochem. 2019, 79, 105–113. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Liu, Y.; Fang, L.; Wei, Y.; Gu, R.; Lin, M.; Chen, L.; Zhou, Z. The effect of fermented wheat protein hydrolysate on the exercise performance in mice. J. Funct. Foods 2022, 97, 105217. [Google Scholar] [CrossRef]
- Lu, X.; Ma, R.; Zhan, J.; Liu, C.; Tian, Y. Starch digestion retarded by wheat protein hydrolysates with different degrees of hydrolysis. Food Chem. 2023, 408, 135153. [Google Scholar] [CrossRef] [PubMed]
- Agyare, K.; Xiong, Y.; Addo, K. Influence of salt and pH on the solubility and structural characteristics of transglutaminase-treated wheat gluten hydrolysate. Food Chem. 2007, 107, 1131–1137. [Google Scholar] [CrossRef]
- Horiguchi, N.; Horiguchi, H.; Suzuki, Y. Effect of wheat gluten hydrolysate on the immune system in healthy human subjects. Biosci. Biotechnol. Biochem. 2014, 69, 2445–2449. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Nakanishi, T.; Tani, T.; Tsuda, T. Low-molecular fraction of wheat protein hydrolysate stimulates glucagon-like peptide-1 secretion in an enteroendocrine L cell line and improves glucose tolerance in rats. Nutr. Res. 2017, 37, 37–45. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Peng, X.; Xing, Y.-F.; Wang, Y.; Zeng, W.; Su, N.; Zhang, C.; Lu, D.-N.; Xing, X.-H. Increased stability and intracellular antioxidant activity of chlorogenic acid depend on its molecular interaction with wheat gluten hydrolysate. Food Chem. 2020, 325, 126873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pan, F.; Mehmood, A.; Zhang, Y.; Hao, S.; Rehman, A.U.; Li, J.; Wang, C.; Wang, Y. Protective effect and mechanism of action of xanthan gum on the color stability of black rice anthocyanins in model beverage systems. Int. J. Biol. Macromol. 2020, 164, 3800–3807. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Salah, M.; Xu, X. Effect of microencapsulation using soy protein isolate and gum arabic as wall material on red raspberry anthocyanin stability, characterization, and simulated gastrointestinal conditions. Ultrason. Sonochem. 2020, 63, 104927. [Google Scholar] [CrossRef]
- Lang, Y.; Li, B.; Gong, E.; Shu, C.; Si, X.; Gao, N.; Zhang, W.; Cui, H.; Meng, X. Effects of α-casein and β-casein on the stability, antioxidant activity and bioaccessibility of blueberry anthocyanins with an in vitro simulated digestion. Food Chem. 2021, 334, 127526. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Xu, J.; Zhang, L.; Liu, L.; Zhang, L. Nanoencapsulation of anthocyanin by an amphiphilic peptide for stability enhancement. Food Hydrocoll. 2021, 118, 106741. [Google Scholar] [CrossRef]
- Guan, Y.; Zhong, Q. The improved thermal stability of anthocyanins at pH 5.0 by gum arabic. LWT Food Sci. Technol. 2015, 64, 706–712. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Wang, X.; Tang, H.; Wu, N.; Wu, F.; Yu, D.; Elfalleh, W. Effects of (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2019, 98, 105306. [Google Scholar] [CrossRef]
- Safari, R.; Yaghoubzadeh, Z. Antioxidant activity of bioactive peptides extracted from sea cucumber (Holothuria leucospilata). Int. J. Pept. Res. Ther. 2020, 26, 2393–2398. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT Food Sci. Technol. 2020, 117, 108621. [Google Scholar] [CrossRef]
- Siddiq, M.; Dolan, K.D.; Perkins-Veazie, P.; Collins, J.K. Effect of pectinolytic and cellulytic enzymes on the physical, chemical, and antioxidant properties of blueberry (Vaccinium corymbosum L.) juice. LWT Food Sci. Technol. 2018, 92, 127–132. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Fenger, J.A.; Moloney, M.; Robbins, R.J.; Collins, T.M.; Dangles, O. The influence of acylation, metal binding and natural antioxidants on the thermal stability of red cabbage anthocyanins in neutral solution. Food Funct. 2019, 10, 6740–6751. [Google Scholar] [CrossRef]
- Roidoung, S.; Dolan, K.D.; Siddiq, M. Gallic acid as a protective antioxidant against anthocyanin degradation and color loss in vitamin-C fortified cranberry juice. Food Chem. 2016, 210, 422–427. [Google Scholar] [CrossRef]
- Muche, B.M.; Speers, R.A.; Rupasinghe, H.P.V. Storage temperature impacts on anthocyanins degradation, color changes and haze development in juice of “Merlot” and “Ruby” grapes (Vitis vinifera). Front. Nutr. 2018, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Peron, D.V.; Fraga, S.; Antelo, F. Thermal degradation kinetics of anthocyanins extracted from juçara (Euterpe edulis Martius) and “Italia” grapes (Vitis vinifera L.), and the effect of heating on the antioxidant capacity. Food Chem. 2017, 232, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, H.; Kido, H.; Misawa, H.; Yaguchi, J.; Otsuki, T.; Chino, M.; Takeda, M.; Yamagata, K. Stability to light, heat, and hydrogen peroxide at different pH values and DPPH radical scavenging activity of acylated anthocyanins from red radish extract. J. Agric. Food Chem. 2007, 55, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Bucheli, P.; Jing, H. Effects of casein- and whey protein–dextran conjugates on the stability of bog bilberry anthocyanin extract. Int. J. Food Sci. Technol. 2009, 44, 1452–1458. [Google Scholar] [CrossRef]
- Cabrita, L.; Fossen, T.; Andersen, Ø.M. Colour and stability of the six common anthocyanidin 3-glucosides in aqueous solutions. Food Chem. 2000, 68, 101–107. [Google Scholar] [CrossRef]
- Rawel, H.M.; Kroll, J.; Hohl, U.C. Model studies on reactions of plant phenols with whey proteins. Nahr. Food 2001, 45, 72–81. [Google Scholar] [CrossRef]
- Wang, W.; Nema, S.; Teagarden, D. Protein aggregation—Pathways and influencing factors. Int. J. Pharm. 2010, 390, 89–99. [Google Scholar] [CrossRef]
- Zang, Z.; Chou, S.; Geng, L.; Si, X.; Ding, Y.; Lang, Y.; Cui, H.; Gao, N.; Chen, Y.; Wang, M.; et al. Interactions of blueberry anthocyanins with whey protein isolate and bovine serum protein: Color stability, antioxidant activity, in vitro simulation, and protein functionality. LWT Food Sci. Technol. 2021, 152, 112269. [Google Scholar] [CrossRef]
- Siebert, K.J. Effects of protein-polyphenol interactions on beverage haze, stabilization, and analysis. J. Agric. Food Chem. 1999, 47, 353–362. [Google Scholar] [CrossRef]
- Millet, M.; Poupard, P.; Guilois-Dubois, S.; Poiraud, A.; Fanuel, M.; Rogniaux, H.; Guyot, S. Heat-unstable apple pathogenesis-related proteins alone or interacting with polyphenols contribute to haze formation in clear apple juice. Food Chem. 2020, 309, 125636. [Google Scholar] [CrossRef]
- Antosiewicz, J.M.; Shugar, D. UV–Vis spectroscopy of tyrosine side-groups in studies of protein structure. Part 2: Selected applications. Biophys. Rev. 2016, 8, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Ariga, G.G.; Naik, P.N.; Chimatadar, S.A.; Nandibewoor, S.T. Interactions between epinastine and human serum albumin: Investigation by fluorescence, UV–vis, FT–IR, CD, lifetime measurement and molecular docking. J. Mol. Struct. 2017, 1137, 485–494. [Google Scholar] [CrossRef]
- Salah, M.; Xu, X. Anthocyanin-β-lactoglobulin nanoparticles in acidic media: Synthesis, characterization and interaction study. J. Mol. Struct. 2021, 1232, 129995. [Google Scholar] [CrossRef]
- Yu, X.; Cai, X.; Luo, L.; Wang, J.; Ma, M.; Wang, M.; Zeng, L. Influence of tea polyphenol and bovine serum albumin on tea cream formation by multiple spectroscopy methods and molecular docking. Food Chem. 2020, 333, 127432. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yin, Z.; Qie, X.; Chen, Y.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Interaction of soy protein isolate hydrolysates with cyanidin-3-O-glucoside and its effect on the in vitro antioxidant capacity of the complexes under neutral condition. Molecules 2021, 26, 1721. [Google Scholar] [CrossRef] [PubMed]
- Hossein Razavizadegan Jahromi, S.; Farhoosh, R.; Hemmateenejad, B.; Varidi, M. Characterization of the binding of cyanidin-3-glucoside to bovine serum albumin and its stability in a beverage model system: A multispectroscopic and chemometrics study. Food Chem. 2020, 311, 126015. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, B.; Liao, Z.; Jiao, Y.; Yi, P. Study on the interaction between Besifloxacin and bovine serum albumin by spectroscopic techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 116–121. [Google Scholar] [CrossRef]
- Lang, Y.; Li, E.; Meng, X.; Tian, J.; Ran, X.; Zhang, Y.; Zang, Z.; Wang, W.; Li, B. Protective effects of bovine serum albumin on blueberry anthocyanins under illumination conditions and their mechanism analysis. Food Res. Int. 2019, 122, 487–495. [Google Scholar] [CrossRef]
- Karami, Z.; Peighambardoust, S.H.; Hesari, J.; Akbari-Adergani, B.; Andreu, D. Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates. Food Biosci. 2019, 32, 100450. [Google Scholar] [CrossRef]
- Torskangerpoll, K.; Andersen, Ø.M. Colour stability of anthocyanins in aqueous solutions at various pH values. Food Chem. 2005, 89, 427–440. [Google Scholar] [CrossRef]

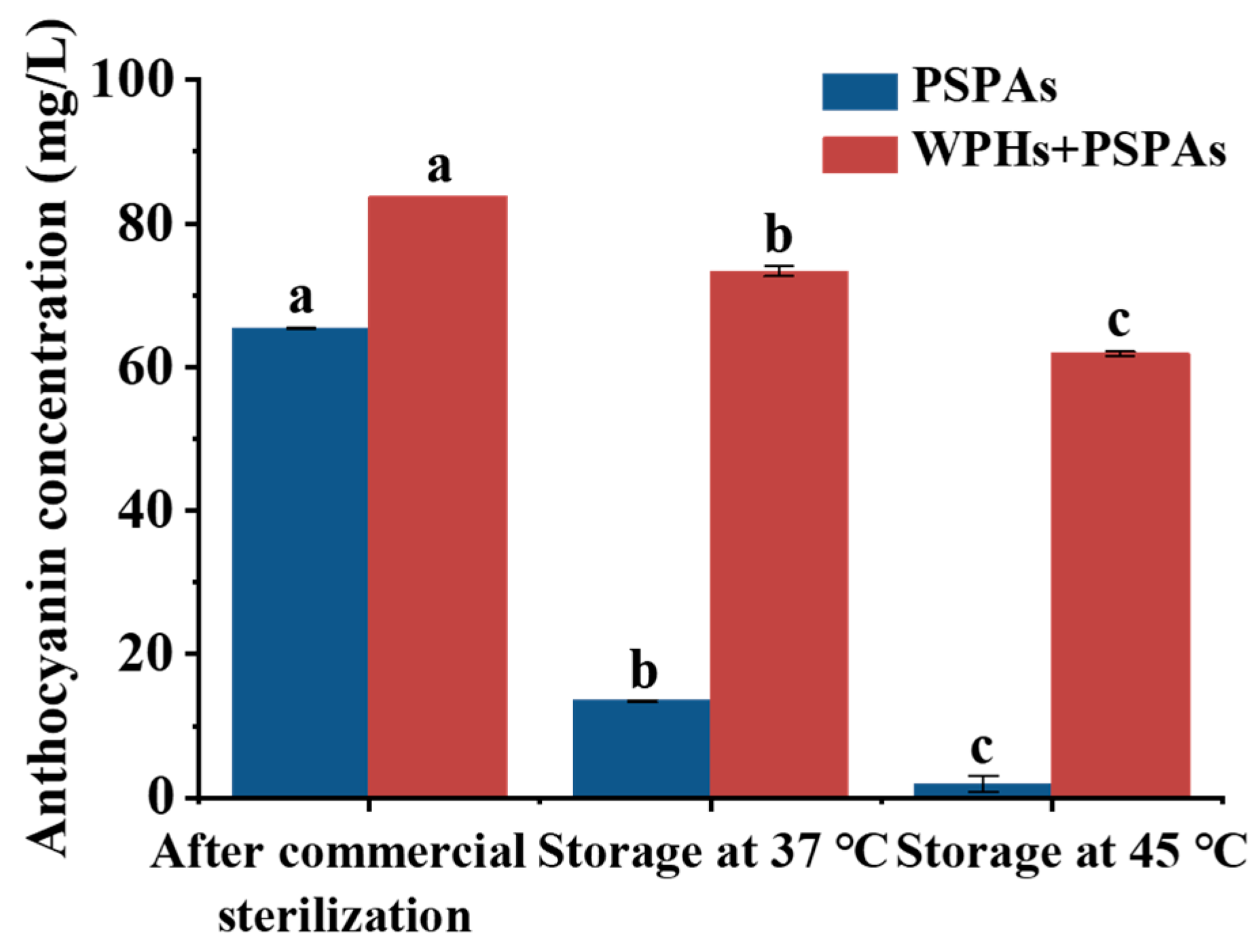
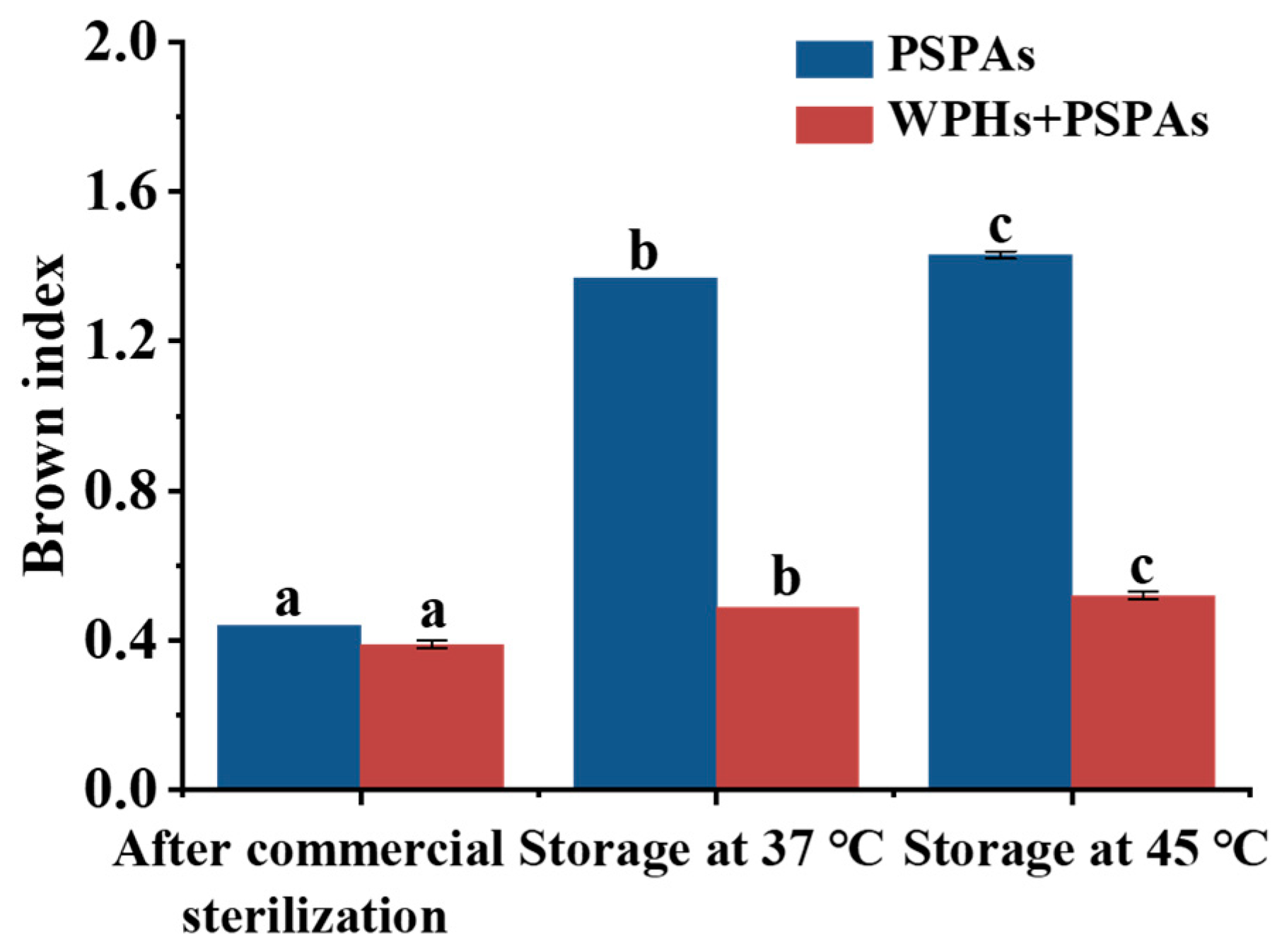
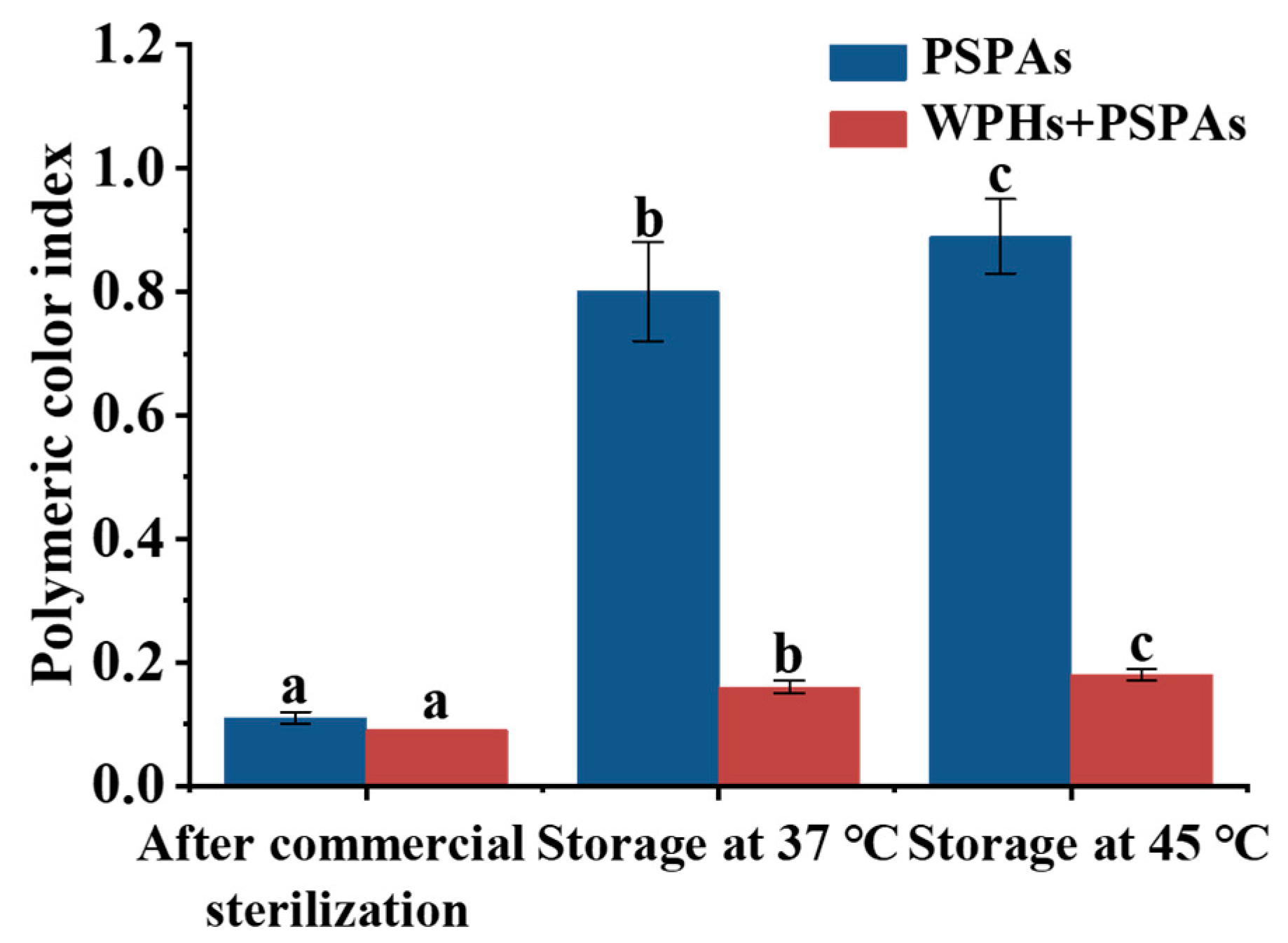
| PSPAs | WPHs+PSPAs | |||||
|---|---|---|---|---|---|---|
| After Commercial Sterilization | Storage at 37 °C | Storage at 45 °C | After Commercial Sterilization | Storage at 37 °C | Storage at 45 °C | |
| L* | 26.16 ± 0.06 c | 28.8 ± 0.04 b | 30.83 ± 0.17 a | 25.29 ± 0.05 e | 25.47 ± 0.03 de | 25.57 ± 0.35 d |
| a* | 9.78 ± 0.45 c | 12.52 ± 0.13 b | 12.95 ± 0.25 a | 5.23 ± 0.12 d | 5.07 ± 0.17 d | 5.15 ± 0.25 d |
| b* | 0.59 ± 0.02 c | 5.46 ± 0.11 b | 9.38 ± 0.40 a | −0.24 ± 0.05 d | −0.22 ± 0.07 d | 0.31 ± 0.22 c |
| ΔE | -- | 6.19 ± 0.29 b | 10.46 ± 0.54 a | -- | 0.29 ± 0.09 c | 0.77 ± 0.15 c |
| C* | 9.80 ± 0.45 c | 13.66 ± 0.17 b | 15.70 ± 0.47 a | 5.24 ± 0.13 d | 5.07 ± 0.18 d | 5.16 ± 0.33 d |
| h | 3.45 ± 0.04 c | 23.56 ± 0.21 b | 36.69 ± 0.63 a | −2.63 ± 0.61 d | −2.48 ± 0.87 d | 3.44 ± 2.28 c |
| Sample | k (10−2 min−1) | Half-Life (min) |
|---|---|---|
| WPHs+PSPAs, sterilization at 80 °C | 0.456 | 152.00 |
| PSPAs, sterilization at 80 °C | 0.892 | 77.70 |
| WPHs+PSPAs, sterilization at 121 °C | 0.671 | 103.30 |
| PSPAs, sterilization at 121 °C | 1.107 | 62.61 |
| k (10−2d−1) | Half-life (d) | |
| WPHs+PSPAs, illumination | 2.634 | 26.35 |
| PSPAs, illumination | 10.285 | 6.73 |
| PSPAs | WPHs+PSPAs | |||||
|---|---|---|---|---|---|---|
| After Commercial Sterilization | Storage at 37 °C | Storage at 45 °C | After Commercial Sterilization | Storage at 37 °C | Storage at 45 °C | |
| Particle size (nm) | 167.58 ± 27.39 c | 293.68 ± 19.56 b | 351.21 ± 5.86 a | 343.13 ± 34.03 b | 460.91 ± 58.95 a | 511.89 ± 40.25 a |
| Zeta potential | −51.01 ± 2.75 a | −49.55 ± 2.34 a | −48.89 ± 1.68 a | −35.01 ± 2.94 a | −33.49 ± 2.85 a | −35.43 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Qiao, B.; Lu, X.; Xiao, J.; Yu, L.; Niu, L. Wheat Protein Hydrolysates Improving the Stability of Purple Sweet Potato Anthocyanins under Neutral pH after Commercial Sterilization at 121 °C. Foods 2024, 13, 843. https://doi.org/10.3390/foods13060843
Feng Y, Qiao B, Lu X, Xiao J, Yu L, Niu L. Wheat Protein Hydrolysates Improving the Stability of Purple Sweet Potato Anthocyanins under Neutral pH after Commercial Sterilization at 121 °C. Foods. 2024; 13(6):843. https://doi.org/10.3390/foods13060843
Chicago/Turabian StyleFeng, Yaping, Bingqian Qiao, Xue Lu, Jianhui Xiao, Lili Yu, and Liya Niu. 2024. "Wheat Protein Hydrolysates Improving the Stability of Purple Sweet Potato Anthocyanins under Neutral pH after Commercial Sterilization at 121 °C" Foods 13, no. 6: 843. https://doi.org/10.3390/foods13060843
APA StyleFeng, Y., Qiao, B., Lu, X., Xiao, J., Yu, L., & Niu, L. (2024). Wheat Protein Hydrolysates Improving the Stability of Purple Sweet Potato Anthocyanins under Neutral pH after Commercial Sterilization at 121 °C. Foods, 13(6), 843. https://doi.org/10.3390/foods13060843






