Shelf Life and Functional Quality of Almond Bagasse Powders as Influenced by Dehydration and Storing Conditions
Abstract
1. Introduction
2. Materials and Method
2.1. Process for Obtaining Almond Bagasse and Almond Bagasse Powder
2.2. Evaluation of Storage Stability of the Almond Bagasse Powder
2.3. Physico-Chemical Properties
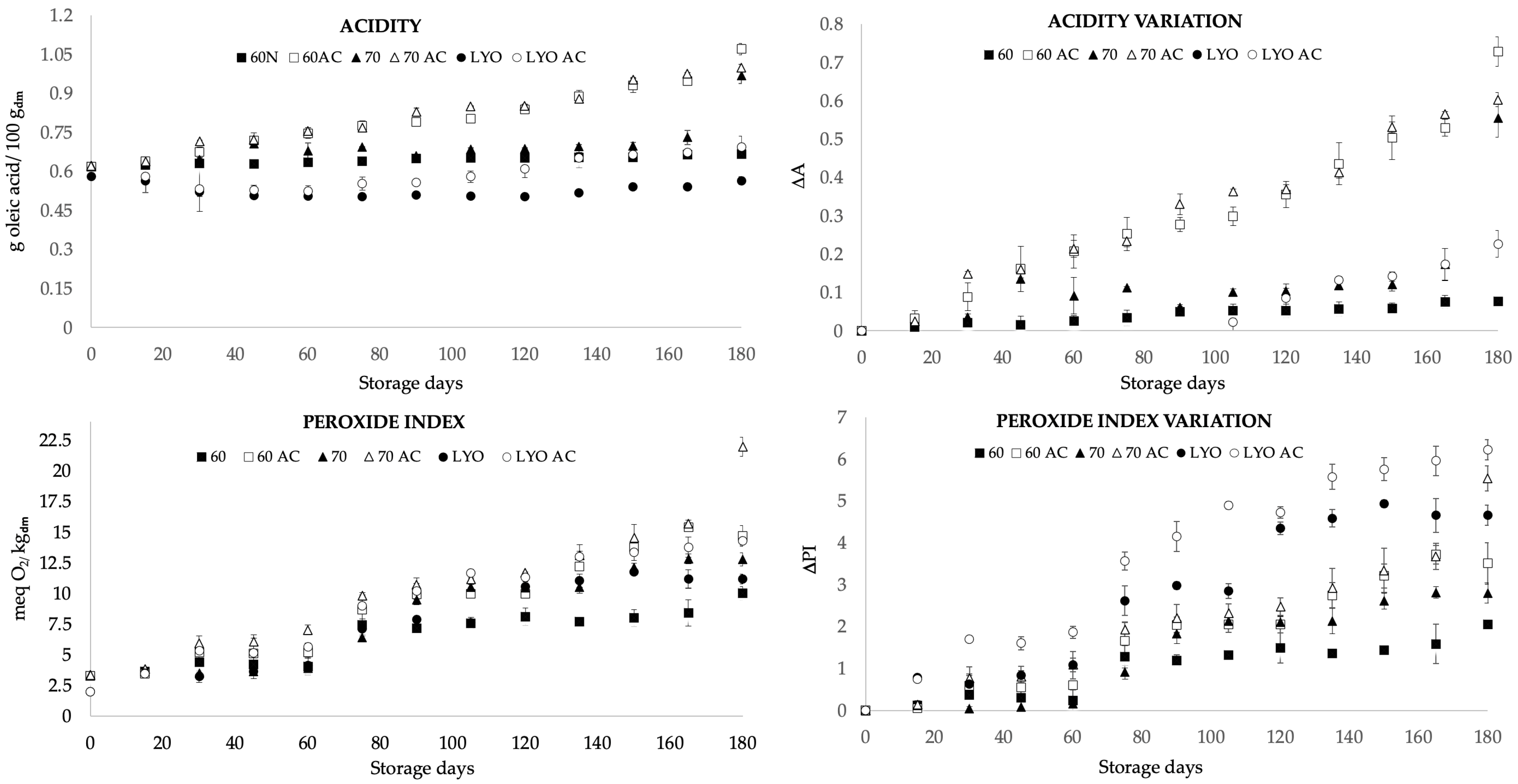
2.4. Acidity and Peroxide Value
2.5. Determination of Phenolic Compounds and Antiradical Capacity
2.5.1. Total Phenol Content
2.5.2. Antiradical Capacity by DPPH and ABTS Methods
2.5.3. Phenolic Compounds by HPLC Analysis
2.6. Microbiological Analyses
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, Z. The Uses and Properties of Almond Oil. Complement. Ther. Clin. Pract. 2010, 16, 10–12. [Google Scholar] [CrossRef]
- De Souza, R.G.M.; Schincaglia, R.M.; Pimente, G.D.; Mota, J.F. Nuts and Human Health Outcomes: A Systematic Review. Nutrients 2017, 9, 1311. [Google Scholar] [CrossRef]
- Kamil, A.; Chen, C.Y.O. Health Benefits of Almonds beyond Cholesterol Reduction. J. Agric. Food Chem. 2012, 60, 6694–6702. [Google Scholar] [CrossRef]
- Almond Production Worldwide 2024|Statista. Available online: https://www.statista.com/statistics/632829/almond-production-worldwide/ (accessed on 14 December 2023).
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 November 2023).
- Yada, S.; Lapsley, K.; Huang, G. A Review of Composition Studies of Cultivated Almonds: Macronutrients and Micronutrients. J. Food Compos. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Tomishima, H.; Luo, K.; Mitchell, A.E. The Almond (Prunus Dulcis): Chemical Properties, Utilization, and Valorization of Coproducts. Annu. Rev. Food Sci. Technol. 2022, 13, 145–166. [Google Scholar] [CrossRef]
- Fernandes, G.D.; Gómez-Coca, R.B.; Pérez-Camino, M.D.C.; Moreda, W.; Barrera-Arellano, D. Chemical Characterization of Major and Minor Compounds of Nut Oils: Almond, Hazelnut, and Pecan Nut. J. Chem. 2017, 2017, 2609549. [Google Scholar] [CrossRef]
- Martínez, M.L.; Penci, M.C.; Marin, M.A.; Ribotta, P.D.; Maestri, D.M. Screw Press Extraction of Almond (Prunus Dulcis (Miller) D.A. Webb): Oil Recovery and Oxidative Stability. J. Food Eng. 2013, 119, 40–45. [Google Scholar] [CrossRef]
- de Souza, T.S.P.; Dias, F.F.G.; Oliveira, J.P.S.; de Moura Bell, J.M.L.N.; Koblitz, M.G.B. Biological Properties of Almond Proteins Produced by Aqueous and Enzyme-Assisted Aqueous Extraction Processes from Almond Cake. Sci. Rep. 2020, 10, 10873. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic Effects of Almonds and Almond Skins on Intestinal Microbiota in Healthy Adult Humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Blumberg, J.B.; Oliver Chen, C.Y. The Influence of Roasting, Pasteurisation, and Storage on the Polyphenol Content and Antioxidant Capacity of California Almond Skins. Food Chem. 2010, 123, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef]
- Lorente, D.; Duarte Serna, S.; Betoret, E.; Betoret, N. Opportunities for the Valorization of Waste Generated by the Plant-Based Milk Substitutes Industry. In Advanced Technologies in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–66. ISBN 9780323885102. [Google Scholar]
- Bartkiene, E.; Bartkevics, V.; Pugajeva, I.; Borisova, A.; Zokaityte, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Klupsaite, D.; Zadeike, D.; et al. Challenges Associated with Byproducts Valorization—Comparison Study of Safety Parameters of Ultrasonicated and Fermented Plant-Based Byproducts. Foods 2020, 9, 614. [Google Scholar] [CrossRef]
- Duarte, S.; Betoret, E.; Barrera, C.; Seguí, L.; Betoret, N. Integral Recovery of Almond Bagasse through Dehydration: Physico-Chemical and Technological Properties and Hot Air-Drying Modelling. Sustainability 2023, 15, 10704. [Google Scholar] [CrossRef]
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguí, L. Valorization of Vegetable Fresh-Processing Residues as Functional Powdered Ingredients. A Review on the Potential Impact of Pretreatments and Drying Methods on Bioactive Compounds and Their Bioaccessibility. Front. Sustain. Food Syst. 2021, 5, 654313. [Google Scholar] [CrossRef]
- AOAC 934.06, 1934 AOAC 934.06-1934(1996); Loss on Drying (Moisture) in Dried Fruit: AOAC Official Method. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=695 (accessed on 11 February 2023).
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-Dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Rani, P.; Kumar, A.; Purohit, S.R.; Rao, P.S. Impact of Fermentation and Extrusion Processing on Physicochemical, Sensory and Bioactive Properties of Rice-Black Gram Mixed Flour. LWT 2018, 89, 155–163. [Google Scholar] [CrossRef]
- AOAC, 2000 Peroxide Value of Oils and Fats 965.33.12. Official Methods of Analysis of AOAC International. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=2886 (accessed on 25 November 2023).
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of Total Content of Phenolic Compounds and Their Antioxidant Activity in Vegetables—Evaluation of Spectrophotometric Methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de Diversos Métodos Químicos Para Determinar Actividad Antioxidante en Pulpa de Frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Caprioli, G.; Nzekoue, F.K.; Giusti, F.; Vittori, S.; Sagratini, G. Optimization of an Extraction Method for the Simultaneous Quantification of Sixteen Polyphenols in Thirty-One Pulse Samples by Using HPLC-MS/MS Dynamic-MRM Triple Quadrupole. Food Chem. 2018, 266, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A Comprehensive Investigation of the Behaviour of Phenolic Compounds in Legumes during Domestic Cooking and in Vitro Digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Tanleque-Alberto, F.; Juan-Borrás, M.; Escriche, I. Antioxidant Characteristics of Honey from Mozambique Based on Specific Flavonoids and Phenolic Acid Compounds. J. Food Compos. Anal. 2020, 86, 103377. [Google Scholar] [CrossRef]
- Inglett, G.E.; Chen, D.; Liu, S.X. Physical Properties of Gluten-Free Sugar Cookies Made from Amaranth–Oat Composites. LWT Food Sci. Technol. 2015, 63, 214–220. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Fontana, A.J.; Schmidt, S.J.; Labuza, T.P. Water Activity in Foods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–435. [Google Scholar] [CrossRef]
- Medeiros Arlindo, D.; José de Melo Queiroz, A.; Maria Feitosa de Figueiredo, R. Armazenamento de pimentão em pó em embalagem de polietileno. Rev. Bras. Prod. Agroindustriais 2007, 9, 111–118. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Breda, C.A.; Sanjinez-Argandoña, E.J.; Correia, C.D.A.C. Shelf Life of Powdered Campomanesia Adamantium Pulp in Controlled Environments. Food Chem. 2012, 135, 2960–2964. [Google Scholar] [CrossRef]
- Tan, S.L.; Sulaiman, R.; Rukayadi, Y.; Ramli, N.S. Physical, Chemical, Microbiological Properties and Shelf Life Kinetic of Spray-Dried Cantaloupe Juice Powder during Storage. LWT 2021, 140, 110597. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef]
- Morrison, W.R. The Free Fatty Acid Content of Some Wheat Flours. J. Sci. Food Agric. 1963, 14, 870–873. [Google Scholar] [CrossRef]
- Fargerson, S.I. Thermal Degradation of Carbohydrate: A Review. J. Agric. Food Chem. 1969, 17, 747–750. [Google Scholar] [CrossRef]
- Gardner, H.W. Lipid Hydroperoxide Reactivity with Proteins and Amino Acids: A Review. Biochem. Biophys. Res. Commun. 1979, 27, 198. [Google Scholar] [CrossRef]
- CEE Reglamento (CEE) n.o 2568/91 Del Consejo, de 11 de Julio de 1991, Sobre Las Características de Los Aceites de Oliva y de Los Aceites de Orujo de Oliva y Sobre Sus Métodos de Análisis. Available online: https://eur-lex.europa.eu/ES/legal-content/summary/olive-oil-analysis.html (accessed on 11 December 2023).
- Nader, J.; Afif, C.; Louka, N. Impact of a Novel Partial Defatting Technology on Oxidative Stability and Sensory Properties of Peanut Kernels. Food Chem. 2021, 334, 127581. [Google Scholar] [CrossRef]
- El Bernoussi, S.; Boujemaa, I.; Harhar, H.; Belmaghraoui, W.; Matthäus, B.; Tabyaoui, M. Evaluation of Oxidative Stability of Sweet and Bitter Almond Oils under Accelerated Storage Conditions. J. Stored Prod. Res. 2020, 88, 101662. [Google Scholar] [CrossRef]
- Turan, A.; Karaosmanoğlu, H. Effect of Drying Methods on Long Term Storage of Hazelnut. Food Sci. Technol. 2019, 39, 406–412. [Google Scholar] [CrossRef]
- Maskan, M.; Karataş, Ş. Storage Stability of Whole-Split Pistachio Nuts (Pistachia vera L.) at Various Conditions. Food Chem. 1999, 66, 227–233. [Google Scholar] [CrossRef]
- BOE Ley 17/2011, de 5 de Julio, de Seguridad Alimentaria y Nutrición. State Official Bulletin, 5 July 2011. pp. 71283–71319. Available online: https://www.boe.es/boe/dias/2011/07/06/pdfs/BOE-A-2011-11604.pdf (accessed on 25 November 2023).
- Shakoor, A.; Zhang, C.; Xie, J.; Yang, X. Maillard Reaction Chemistry in Formation of Critical Intermediates and Flavour Compounds and Their Antioxidant Properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef] [PubMed]
- Sreeramulu, N. Auxins, Inhibitors and Phenolics in Bambarranut Seeds (Voandzeia subterranea Thouars) in Relation to Loss of Viability During Storage. Ann. Bot. 1983, 51, 209–216. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard Reaction Products as Food-Born Antioxidant and Antibrowning Agents in Model and Real Food Systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Talcott, S.T.; Passeretti, S.; Duncan, C.E.; Gorbet, D.W. Polyphenolic Content and Sensory Properties of Normal and High Oleic Acid Peanuts. Food Chem. 2005, 90, 379–388. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E.; Lee, S.U.; Kozukue, N. Stability of Green Tea Catechins in Commercial Tea Leaves during Storage for 6 Months. J. Food Sci. 2009, 74, H47–H51. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to Ferric Reducing Antioxidant Power (FRAP) and 2,2′-Diphenyl-1- Picrylhydrazyl (DPPH) Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Del Caro, A.; Piga, A.; Vacca, V.; Agabbio, M. Changes of Flavonoids, Vitamin C and Antioxidant Capacity in Minimally Processed Citrus Segments and Juices during Storage. Food Chem. 2004, 84, 99–105. [Google Scholar] [CrossRef]
- Bodart, M.; de Peñaranda, R.; Deneyer, A.; Flamant, G. Photometry and Colorimetry Characterisation of Materials in Daylighting Evaluation Tools. Build. Environ. 2008, 43, 2046–2058. [Google Scholar] [CrossRef]
| HAD60 | HAD70 | LYO | |
|---|---|---|---|
| Water activity | 0.13 ± 0.03 b | 0.23 ± 0.01 c | 0.05 ± 0.01 a |
| Humidity (g/gdm) | 0.009 ± 0.009 a | 0.007 ± 0.001 a | 0.0011 ± 0.0009 a |
| Hygroscopicity (gw/g) | 0.116 ± 0.004 a | 0.20 ± 0.01 b | 0.224 ± 0.007 c |
| Peroxide (meq/kgdm) | 3.3 ± 0.2 b | 3.4 ± 0.2 b | 1.9 ± 0.1 a |
| Acidity (g/100 g dm) | 0.619 ± 0.008 b | 0.622 ± 0.001 b | 0.58 ± 0.01 a |
| Mesophiles (log CFU/gdm) | n.d | n.d | n.d |
| Mold and yeast (log CFU/gdm) | n.d | n.d | n.d |
| Phenolic compounds (mg/100 gdm) | |||
| 4-Hydroxibezoic acid | 60.0± 0.9 a | 70.7 ± 0.9 b | 84.5 ± 1.4 c |
| Epicatechin | 458 ± 17 a | 498 ± 7 b | 585.0 ± 0.9 c |
| Vanillin | 94.6 ± 0.9 a | 128.4 ± 0.9 c | 98.7 ± 1.4 b |
| Rutin | 206.4 ± 0.9 c | 195.2 ± 0.2 b | 151.6 ± 0.9 a |
| Quercitin 3-glucoside | 63.0 ± 0.9 a | 66.1 ± 0.9 b | 112.2 ± 0.9 c |
| p-Coumaric acid | 39.0 ± 0.9 a | 39.4 ± 0.9 a | 51.1 ± 0.9 b |
| Sinapic acid | 61.6 ± 0.4 a | 63.9 ± 0.4 b | 120.5 ± 0.5 c |
| Ferulic acid | 63.1 ± 0.4 a | 65.0 ± 0.4 b | 107.8 ± 0.5 c |
| Apigenin-7-glucoside | 60 ± 11 b | 65 ± 10 b | 36 ± 16 a |
| Clorogenic acid | 78.2 ± 0.9 a | 81.6 ± 0.9 b | 87.4 ± 0.9 c |
| Sum of specific phenols | 1181 ± 12 a | 1272 ± 1 b | 1435 ± 9 c |
| Color | |||
| L | 58.1 ± 0.5 b | 51.3 ± 0.5 a | 61.86 ± 0.09 c |
| a* | 7.15 ± 0.06 a | 8.1 ± 0.2 b | 8.0 ± 0.6 b |
| b* | 15.27 ± 0.05 a | 19.3 ± 0.4 c | 16.5 ± 0.4 b |
| Cab | 64.93 ± 0.02 a | 67.2 ± 0.4 c | 65.1 ± 0.5 b |
| HAD60 | HAD70 | LYO | HAD60 AC | HAD70 AC | LYO AC | |
|---|---|---|---|---|---|---|
| Water activity | 0.245 ± 0.001 b | 0.274 ± 0.002 c | 0.10 ± 0.01 a | 0.341 ± 0.001 d | 0.41 ± 0.02 e | 0.30 ± 0.02 c |
| Xw (g/gdm) | 0.013 ± 0.01 b | 0.0137 ± 0.0002 b | 0.0030 ± 0.0001 a | 0.017 ± 0.001 c | 0.026 ± 0.004 d | 0.0183 ± 0.0005 c |
| Hygroscopicity (gw/g) | 0.195 ± 0.006 a | 0.220 ± 0.004 b | 0.22 ± 0.02 b | 0.202 ± 0.009 c | 0.208 ± 0.005 d | 0.208 ± 0.006 cd |
| Peroxides (meq/gdm) | 7.14 ± 0.08 a | 9.5 ± 0.4 c | 7.9 ± 0.1 b | 9.92 ± 0.02 a | 10.7 ± 0.5 b | 10.2 ± 0.7 b |
| Acidity (g/100 gdm) | 0.65 ± 0.01 c | 0.68 ± 0.01 c | 0.52 ± 0.03 a | 0.79 ± 0.01 d | 0660 ± 0.006 e | 0.579 ± 0.004 b |
| Mesophiles (log CFU/gdm) | 1 ± 0 b | 0 a | 1 ± 0 b | 1.5 ± 0.7 b | 0 a | 2 ± 0 c |
| Mold and yeast (log CFU/gdm) | 1 ± 0 b | 0 a | 1 ± 0 b | 1.5 ± 0.7 a | 1.5 ± 0.7 a | 1.5 ± 0.7 a |
| Phenolic compounds (mg/100 gdm) | ||||||
| 4-Hydroxibezoic acid | 69.7 ± 0.5 d | 72.1 ± 0.1 e | 28.7± 0.3 a | 66.7 ± 0.6 c | 72.6 ± 0.6 e | 34.3 ± 0.5 b |
| Epicatechin | 460.6 ± 0.1 a | 773.7 ± 33 b | 701.0 ± 38 b | 441 ± 86 a | 829.9 ± 17 c | 697.6 ± 21 b |
| Vanillin | 256.8 ± 4 d | 310.9 ± 1 f | 87.3 ± 0.9 b | 229.8 ± 2 c | 294.5 ± 3 e | 79.0 ± 0.9 a |
| Rutin | 154.7 ± 0.3 b | 153.1 ± 0.8 b | 256.3 ± 3 e | 162.9 ± 0.5 c | 146.7 ± 0.03 a | 242.2 ± 1.1 d |
| Quercitin 3-glucoside | 63.0 ± 0.04 b | 51.9 ± 0.2 a | 106.9 ± 1.2 d | 61.4 ± 0.8 b | 51.5 ± 1.2 a | 82.1 ± 1 c |
| p-Coumaric acid | 36.6 ± 1.1 a | 52.9 ± 0.3 c | 39.9 ± 0.04 b | 59.3 ± 1.3 d | 53.1 ± 1.7 c | 39.6 ± 0.8 b |
| Sinapic acid | 78.0 ± 0.7 b | 87.0 ± 0.9 c | 69.9 ± 0.5 c | 80.4 ± 0.6 b | 85.2 ± 0.3 c | 72.6 ± 2 a |
| Ferulic acid | 77.3 ± 0.8 b | 83.2 ± 0.8 c | 70.9 ± 1 a | 79.0 ± 0.8 b | 82.5 ± 1 c | 72.0 ± 0.6 a |
| Apigenin-7-glucoside | 77 ± 1 c | 69.2 ± 0.6 a | 102 ± 6 d | 75.5 ± 0.1 bc | 69.6 ± 0.3 ab | 100.8 ± 0.8 d |
| Clorogenic acid | 82.2 ± 3 a | 88.7 ± 1 b | 98.6 ± 1 c | 85.8 ± 1 ab | 102.40 ± 0.01 c | 99.7 ± 2 c |
| Sum of specific phenols | 1356.4 ± 0.4 a | 1743 ± 29 c | 1562 ± 25 b | 1342 ± 4 a | 1788 ± 8 c | 1520 ± 3 b |
| Color | ||||||
| L | 58.6 ± 0.2 b | 59.3 ± 0.2 c | 61.7 ± 0.6 d | 57.22 ± 0.12 a | 58.7 ± 0.2 b | 72.94 ± 0.01 e |
| a* | 6.99 ± 0.14 c | 6.80 ± 0.04 b | 7.06 ± 0.14 c | 7.33 ± 0.02 d | 7.30 ± 0.01 d | 5.29 ± 0.01 a |
| b* | 15.2 ± 0.5 b | 15.72 ± 0.09 c | 15.78 ± 0.03 c | 15.86 ± 0.12 c | 16.55 ± 0.09 d | 14.67 ± 0.01 a |
| Cab | 16.7 ± 0.5 b | 17.13 ± 0.07 c | 17.29 ± 0.03 c | 17.10 ± 0.01 c | 18.09 ± 0.08 d | 15.60 ± 0.01 a |
| ΔE | 0.63 ± 0.44 a | 1.3 ± 0.6 a | 1.3 ± 0.7 a | 1.2 ± 0.1 a | 6.6 ± 0.5 c | 3.19 ± 0.04 d |
| HAD60 | HAD70 | LYO | HAD60 AC | HAD70 AC | LYO AC | |
|---|---|---|---|---|---|---|
| Water activity | 0.30 ± 0.03 ab | 0.34 ± 0.03 b | 0.280 ± 0.002 a | 0.431 ± 0.002 a | 0.484 ± 0.002 b | 0.432 ± 0.004 a |
| Humidity (g/gdm) | 0.021 ± 0.001 b | 0.024 ± 0.001 b | 0.0154 ± 0.0004 a | 0.0314 ± 0.0004 d | 0.032 ± 0.002 d | 0.0292 ± 0.0005 c |
| Hygroscopicity (gw/g) | 0.168 ± 0.006 a | 0.214 ± 0.005 b | 0.213 ± 0.005 b | 0.179 ± 0.01 a | 0.220 ± 0.013 b | 0.223 ± 0.006 b |
| Peroxide (meq/gdm) | 10.0 ± 0.6 a | 12.8 ± 0.5 c | 11.2 ± 0.4 b | 14.7 ± 0.9 d | 23 ± 2 e | 14.3 ± 0.3 d |
| Acidity (g/100 g dm) | 0.666 ± 0.004 b | 0.97 ± 0.03 d | 0.56 ± 0.01 a | 1.07 ± 0.02 e | 1.00 ± 0.01 e | 0.734 ± 0.009 c |
| Mesophiles (log CFU/gdm) | 2.5 ± 0.7 b | 1 ± 0 a | 2.5 ± 0.7 b | 3 ± 0 c | 1.5 ± 0.7 a | 3 ± 0 c |
| Mold and yeast (log CFU/gdm) | 2.5 ± 0.7 c | 1 ± 0 a | 2 ± 0 b | 4 ± 0 b | 3 ± 0 a | 4 ± 1 c |
| Phenolic compounds (mg/100 gdm) | ||||||
| 4-Hydroxibezoic acid | 80.8 ± 1.3 c | 75.6 ± 0.2 b | 89 ± 2 d | 64.6 ± 0.4 a | 91.0 ± 0.4 e | 96 ± 4 f |
| Epicatechin | 463 ± 7 a | 747 ± 6 c | 607 ± 4 b | 747.5 ± 6.1 c | 945.6 ± 24.7 d | 659.1 ± 0.9 a |
| Vanillin | 263 ± 6 c | 322 ± 2 e | 113 ± 1 a | 150 ± 2 b | 290.3 ± 0.5 d | 110.7 ± 1.2 a |
| Rutin | 143.6 ± 0.9 c | 145± 3 c | 75 ± 2 a | 111 ± 2.0 b | 173.4 ± 0.4 d | 226.1 ± 0.3 e |
| Quercitin 3-glucoside | 68 ± 2 c | 61.4 ± 0.4 b | 81 ± 2 c | 53.2 ± 1.1 a | 56.4 ± 0.6 a | 76.9 ± 1.4 d |
| p-Coumaric acid | 55 ± 2 d | 38 ± 1 a | 48.5 ± 0.2 c | 53.4 ± 0.4 d | 46.3 ± 0.7 b | 49.1 ± 0.2 c |
| Sinapic acid | 80 ± 2 b | 91.1 ± 1.5 c | 76.2 ± 0.4 a | 71.4 ± 1.2 a | 84 ± 2 b | 74 ± 2 a |
| Ferulic acid | 79 ± 2 b | 86.9 ± 0.8 c | 74.0 ± 1.2 a | 71.5 ± 0.1 a | 84.7 ± 1.1 c | 72.9 ± 0.3 a |
| Apigenin-7-glucoside | 80.6 ± 0.1 c | 75 ± 2 bc | 81 ± 6 d | 68.3 ± 1.1 a | 71.6 ± 1.4 b | 79 ± 8 c |
| Clorogenic acid | 75 ± 2 a | 87.7 ± 0.9 b | 88.0 ± 1.4 b | 83.2 ± 0.6 b | 95.1 ± 1.2 d | 91.0 ± 0.4 c |
| Sum of specific phenols | 1388 ± 10 b | 1923 ± 1 e | 1336 ± 1 a | 1475 ± 9 c | 1938 ± 4 f | 1541 ± 2 d |
| Color | ||||||
| L | 57.883 ± 0.004 c | 54.5 ± 0.3 a | 60.20 ± 0.01 d | 56.593 ± 0.006 b | 56.73 ± 0.01 bc | 61.587 ± 0.002 e |
| a* | 6.85 ± 0.01 a | 8.98 ± 0.13 d | 6.958 ± 0.002 a | 9.00 ± 0.01 e | 7.0 ± 0.6 b | 7.664 ± 0.002 c |
| b* | 14.84 ± 0.01 a | 18.1 ± 0.4 a | 15.25 ± 0.01 a | 19.09 ± 0.02 c | 14.93 ± 0.01 a | 16.643 ± 0.004 b |
| Cab | 65.22 ± 0.01 e | 63.6 ± 0.3 c | 65.47 ± 0.01 c | 64.74 ± 0.02 d | 65.972 ± 0.003 f | 65.274 ± 0.003 b |
| ΔE | 0.7 ± 0.3 a | 5.0 ± 0.4 e | 2.3 ± 0.4 b | 4.5 ± 0.2 d | 7.1 ± 0.6 f | 3.56 ± 0.01 c |
| Days | HAD60 | HAD70 | LYO | HAD60 AC | HAD70 AC | LYO AC | |
|---|---|---|---|---|---|---|---|
| DPPH (mgTE/g dm) | 0 | 0.20 ± 0.02 a | 0.23 ± 0.01 a | 0.24 ± 0.007 a | 0.20 ± 0.02 a | 0.23 ± 0.01 b | 0.319 ± 0.007 c |
| 60 | 0.201 ± 0.013 a | 0.218 ± 0.02 ab | 0.22 ± 0.02 c | 0.2372 ± 0.0013 b | 0.21 ± 0.01 a | 0.333 ± 0.009 d | |
| 90 | 0.391 ± 0.006 d | 0.37 ± 0.01 a | 0.36 ± 0.01 bc | 0.37 ± 0.01 bcd | 0.34 ± 0.01 b | 0.38 ± 0.01 cd | |
| 180 | 0.31 ± 0.02 a | 0.307 ± 0.014 c | 0.344 ± 0.009 d | 0.27 ± 0.01 b | 0.213 ± 0.009 a | 0.404 ± 0.007 e | |
| ABTS (mgTE/g dm) | 0 | 1.04 ± 0.02 b | 1.092 ± 0.014 b | 0.56 ± 0.05 a | 1.04 ± 0.02 b | 1.092 ± 0.014 b | 0.56 ± 0.05 a |
| 60 | 0.73 ± 0.02 b | 0.788 ± 0.012 c | 0.53 ± 0.02 a | 0.75 ± 0.08 ab | 0.84 ± 0.04 b | 0.65 ± 0.04 a | |
| 90 | 0.88 ± 0.05 c | 0.95 ± 0.04 d | 0.44 ± 0.01 a | 0.86 ± 0.03 c | 0.91 ± 0.04 cd | 0.52 ± 0.02 b | |
| 180 | 0.79 ± 0.05 c | 0.99 ± 0.03 d | 0.55 ± 0.02 a | 1.07 ± 0.01 e | 0.813 ± 0.003 c | 0.60 ± 0.02 b | |
| Phenols (mg GAE/g dms) | 0 | 0.31 ± 0.02 a | 0.355 ± 0.006 b | 0.313 ± 0.007 a | 0.31 ± 0.02 a | 0.355 ± 0.006 b | 0.313 ± 0.007 a |
| 60 | 0.39 ± 0.02 b | 0.535± 0.015 cd | 0.291 ± 0.004 a | 0.47 ± 0.02 c | 0.54 ± 0.02 e | 0.3 ± 0.4 a | |
| 90 | 0.323 ± 0.002 a | 0.37 ± 0.01 b | 0.32 ± 0.01 a | 0.44 ± 0.03 c | 0.43 ± 0.02 c | 0.34 ± 0.01 ab | |
| 180 | 0.443 ± 0.011 b | 0.51 ± 0.02 c | 0.242 ± 0.006 a | 0.63 ± 0.06 d | 0.552 ± 0.011 c | 0.248 ± 0.013 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, S.; Betoret, E.; Betoret, N. Shelf Life and Functional Quality of Almond Bagasse Powders as Influenced by Dehydration and Storing Conditions. Foods 2024, 13, 744. https://doi.org/10.3390/foods13050744
Duarte S, Betoret E, Betoret N. Shelf Life and Functional Quality of Almond Bagasse Powders as Influenced by Dehydration and Storing Conditions. Foods. 2024; 13(5):744. https://doi.org/10.3390/foods13050744
Chicago/Turabian StyleDuarte, Stevens, Ester Betoret, and Noelia Betoret. 2024. "Shelf Life and Functional Quality of Almond Bagasse Powders as Influenced by Dehydration and Storing Conditions" Foods 13, no. 5: 744. https://doi.org/10.3390/foods13050744
APA StyleDuarte, S., Betoret, E., & Betoret, N. (2024). Shelf Life and Functional Quality of Almond Bagasse Powders as Influenced by Dehydration and Storing Conditions. Foods, 13(5), 744. https://doi.org/10.3390/foods13050744








