Characterization of the Effects of Low-Sodium Salt Substitution on Sensory Quality, Protein Oxidation, and Hydrolysis of Air-Dried Chicken Meat and Its Molecular Mechanisms Based on Tandem Mass Tagging-Labeled Quantitative Proteomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing of Air-Dried Chicken
2.3. Sensory Analysis
2.4. Extraction of Myofibrillar Protein from Air-Dried Chicken
2.5. Determination of the Protein Oxidation Levels in Air-Dried Chicken
2.5.1. Determination of the Myofibrillar Protein Carbonyl Content
2.5.2. Determination of the Total Sulfhydryl Content of Myofibrillar Proteins
2.5.3. Determination of the Surface Hydrophobicity and Solubility of Myofibrillar Proteins
2.5.4. Determination of the Dimeric Tyrosine Content of Myofibrillar Proteins
2.5.5. Tryptophan Fluorescence Spectrometry of Myofibrillar Protein
2.6. Determination of Proteolysis during the Processing of Air-Dried Chicken
2.6.1. Determination of P.I. (Proteolysis Index)
2.6.2. Determination of Cathepsin B and L Activity
2.6.3. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
2.7. Texture Profile Analysis (TPA)
2.8. TMT Proteomics
2.8.1. Reversed-Phase Chromatographic Separation
2.8.2. Chromatographic and Mass Spectrometry Conditions
2.9. Statistical Analysis
3. Results and Discussion
3.1. Sensory Evaluation
3.2. Analysis of the Total Carbonyl Content in Different Treatments
3.3. Analysis of the Total Sulfhydryl Content in Different Treatments
3.4. Analysis of Surface Hydrophobicity and Protein Solubility in Different Treatments
3.5. Analysis of Dimeric Tyrosine Content with Different Salt Substitutions
3.6. Analysis of Tryptophan Fluorescence in Different Treatments
3.7. Analysis of the Proteolysis Index in Different Treatments
3.8. Analysis of Cathepsin B and L Activity, SDS-PAGE, and Texture in Different Treatments
3.9. Relationship between Protein Oxidation and Protein Proteolysis
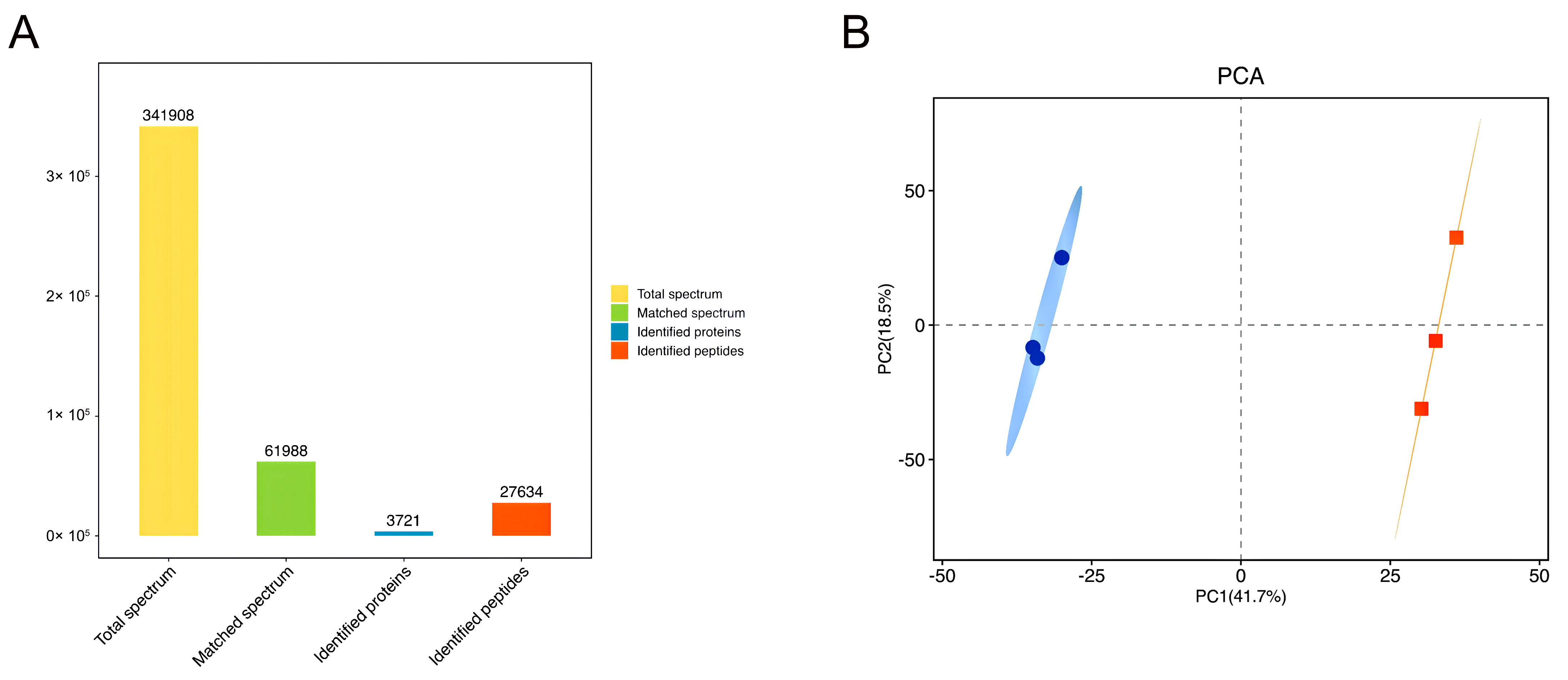
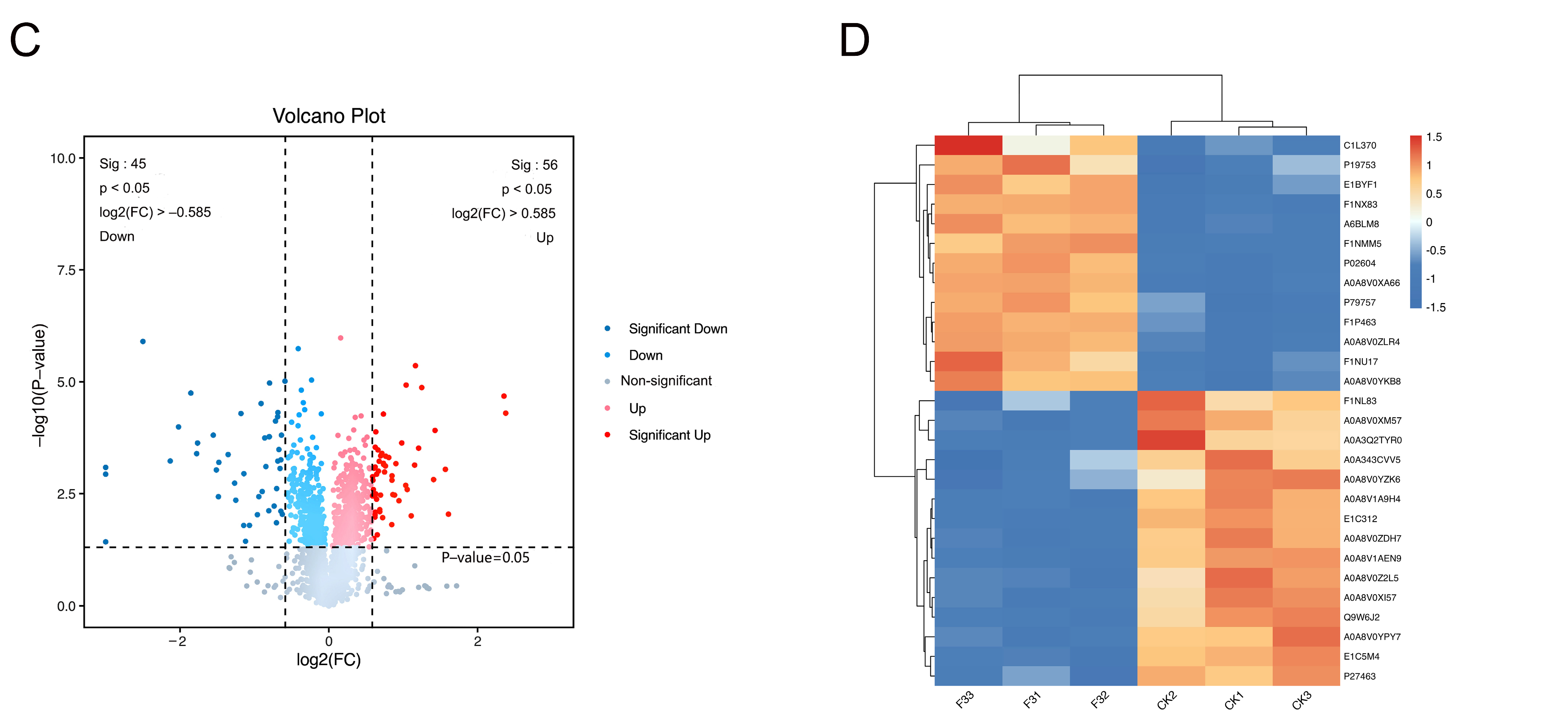
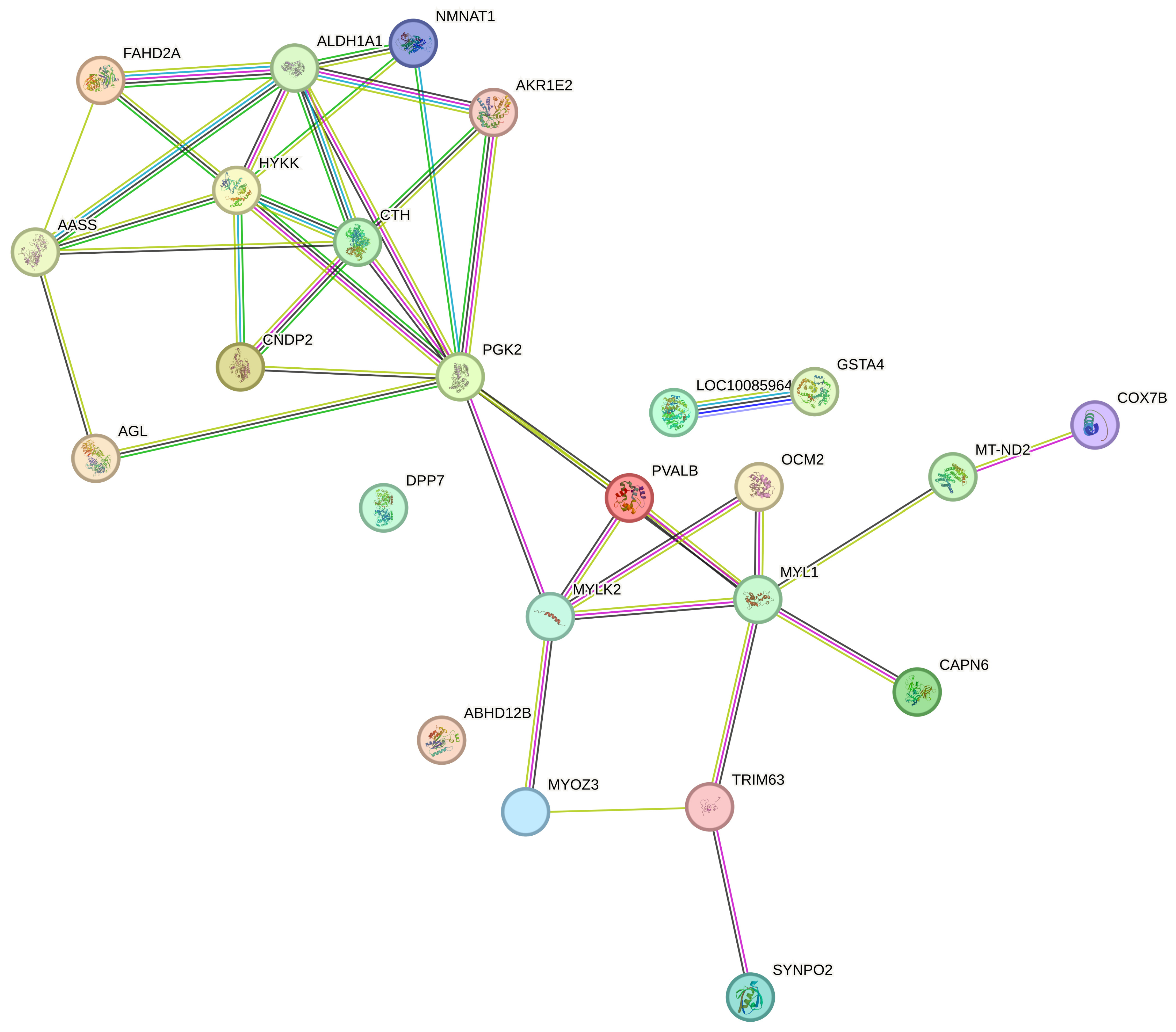
3.10. Proteomics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, N.; Xu, B.; Zhao, L.; Huang, M.; Zhou, G. Effects of high-temperature–short time (HTST) drying process on proteolysis, lipid oxidation and sensory attributes of Chinese dry-cured chicken. CyTA-J. Food 2016, 14, 440–448. [Google Scholar] [CrossRef][Green Version]
- Zhu, Y.; Chen, X.; Pan, N.; Liu, S.; Su, Y.; Xiao, M.; Shi, W.; Liu, Z. The effects of five different drying methods on the quality of semi-dried Takifugu obscurus fillets. LWT 2022, 161, 113340. [Google Scholar] [CrossRef]
- Liu, X.; Piao, C.; Ju, M.; Zhang, J.; Zhang, W.; Cui, F.; Li, G.; Cui, M. Effects of low salt on lipid oxidation and hydrolysis, fatty acids composition and volatiles flavor compounds of dry-cured ham during ripening. LWT 2023, 187, 115347. [Google Scholar] [CrossRef]
- Barretto, T.L.; Pollonio, M.A.R.; Telis-Romero, J.; da Silva Barretto, A.C. Improving sensory acceptance and physicochemical properties by ultrasound application to restructured cooked ham with salt (NaCl) reduction. Meat Sci. 2018, 145, 55–62. [Google Scholar] [CrossRef]
- Gan, X.; Li, H.; Wang, Z.; Emara, A.M.; Zhang, D.; He, Z. Does protein oxidation affect proteolysis in low sodium Chinese traditional bacon processing? Meat Sci. 2019, 150, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Lametsch, R.; Akcan, T.; Ruiz-Carrascal, J. Pork proteins oxidative modifications under the influence of varied time-temperature thermal treatments: A chemical and redox proteomics assessment. Meat Sci. 2018, 140, 134–144. [Google Scholar] [CrossRef]
- Cittadini, A.; Dominguez, R.; Gomez, B.; Pateiro, M.; Perez-Santaescolastica, C.; Lopez-Fernandez, O.; Sarries, M.V.; Lorenzo, J.M. Effect of NaCl replacement by other chloride salts on physicochemical parameters, proteolysis and lipolysis of dry-cured foal “cecina”. J. Food Sci. Technol. 2020, 57, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Heres, A.; Mora, L.; Toldra, F. Bioactive and Sensory Di- and Tripeptides Generated during Dry-Curing of Pork Meat. Int. J. Mol. Sci. 2023, 24, 1574. [Google Scholar] [CrossRef]
- Zhou, C.-Y.; Pan, D.-D.; Bai, Y.; Li, C.-B.; Xu, X.-L.; Zhou, G.-H.; Cao, J.-X. Evaluating endogenous protease of salting exudates during the salting process of Jinhua ham. LWT 2019, 101, 76–82. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Gao, H.; Zhao, Y.; Wang, J.; Wang, S. Substituting sodium by various metal ions affects the cathepsins activity and proteolysis in dry-cured pork butts. Meat Sci. 2020, 166, 108132. [Google Scholar] [CrossRef]
- Liu, C.; Wan, J.; Zhou, Y.; Hu, K.; Zhu, Q.; Tang, P.; Xu, S.; Song, L. Proteome profile of glycrol-mediated salt-reduction cured meat reveals the formation mechanism of eating quality. Food Chem. 2022, 382, 132395. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, X.; Pan, D.; Xia, Q.; Sun, Y.; Geng, F.; Cao, J. TMT-labeled quantitative proteomic reveals the mechanism of proteolysis and taste improvement of dry-cured bacon with Staphylococcus co-inoculation. Food Chem. 2024, 436, 137711. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, E.; Castillo, L.S.; Sánchez, J.C.; Siche, R.; de Almeida, M.A.; Behrens, J.H.; Selani, M.M.; Contreras-Castillo, C.J. Descriptive analysis of bacon smoked with Brazilian woods from reforestation: Methodological aspects, statistical analysis, and study of sensory characteristics. Meat Sci. 2018, 140, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, Y.; Fei, Y.; Xu, X.; Zhou, G. Effect of microbial transglutaminase on NMR relaxometry and microstructure of pork myofibrillar protein gel. Eur. Food Res. Technol. 2008, 228, 665–670. [Google Scholar] [CrossRef]
- Soglia, F.; Petracci, M.; Ertbjerg, P. Novel DNPH-based method for determination of protein carbonylation in muscle and meat. Food Chem. 2016, 197 Pt A, 670–675. [Google Scholar] [CrossRef]
- Chin, K.B.; Go, M.Y.; Xiong, Y.L. Konjac flour improved textural and water retention properties of transglutaminase-mediated, heat-induced porcine myofibrillar protein gel: Effect of salt level and transglutaminase incubation. Meat Sci. 2009, 81, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, X.; Liu, D.; Zhou, G.; Han, M.; Wang, P. Rheological behavior, conformational changes and interactions of water-soluble myofibrillar protein during heating. Food Hydrocoll. 2018, 77, 524–533. [Google Scholar] [CrossRef]
- Zhu, Z.; Mao, X.; Wu, Q.; Zhang, J.; Deng, X. Effects of oxidative modification of peroxyl radicals on the structure and foamability of chickpea protein isolates. J. Food Sci. 2021, 86, 824–833. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J.; Wójciak, K.M.; Neffe-Skocińska, K. Physico-chemical and proteolytic changes during cold storage of dry-cured pork loins with probiotic strains ofLAB. Int. J. Food Sci. Technol. 2019, 55, 1069–1079. [Google Scholar] [CrossRef]
- Zhao, G.M.; Zhou, G.H.; Wang, Y.L.; Xu, X.L.; Huan, Y.J.; Wu, J.Q. Time-related changes in cathepsin B and L activities during processing of Jinhua ham as a function of pH, salt and temperature. Meat Sci. 2005, 70, 381–388. [Google Scholar] [CrossRef]
- Feng, L.; Qiao, Y.; Zou, Y.; Huang, M.; Kang, Z.; Zhou, G. Effect of Flavourzyme on proteolysis, antioxidant capacity and sensory attributes of Chinese sausage. Meat Sci. 2014, 98, 34–40. [Google Scholar] [CrossRef]
- Lawless, H.T.; Rapacki, F.; Horne, J.; Hayes, A. The taste of calcium and magnesium salts and anionic modifications. Food Qual. Prefer. 2003, 14, 319–325. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, X.; Zhao, S.; Wei, Q.; Dou, M. Effect of the partial substitution of NaCl with blended KCl, MgCl2 and arginine on sensory profiles and storage characteristics of cooked marinated chicken. J. Food Meas. Charact. 2023, 17, 5948–5958. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Cittadini, A.; Bermúdez, R.; Munekata, P.E.; Domínguez, R. Influence of partial replacement of NaCl with KCl, CaCl2 and MgCl2 on proteolysis, lipolysis and sensory properties during the manufacture of dry-cured lacón. Food Control 2015, 55, 90–96. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Guo, A. Animal and Plant Protein Oxidation: Chemical and Functional Property Significance. Foods 2020, 10, 40. [Google Scholar] [CrossRef]
- Estevez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Han, Y.; Ge, G.; Zhao, M.; Sun, W. Partial substitution of NaCl with chloride salt mixtures: Impact on oxidative characteristics of meat myofibrillar protein and their rheological properties. Food Hydrocoll. 2019, 96, 36–42. [Google Scholar] [CrossRef]
- Mao, J.; Fu, J.; Zhu, Z.; Jin, D.; Shen, S.; Yuan, Y.; Chen, Y. Impact of KCl and ultrasound on the structural properties of myofibrillar proteins in low sodium semi-dried large yellow croaker (Pseudosciaena crocea). LWT 2023, 178, 114604. [Google Scholar] [CrossRef]
- Dominguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein Oxidation in Muscle Foods: A Comprehensive Review. Antioxidants 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.; Han, Y.; Zheng, J.; Zhao, M.; Sun, W. Physicochemical characteristics and gel-forming properties of myofibrillar protein in an oxidative system affected by partial substitution of NaCl with KCl, MgCl2 or CaCl2. Food Chem. 2020, 309, 125614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Effect of peroxyl radicals on the structure and gel properties of isolated rabbit meat myofibrillar proteins. Int. J. Food Sci. Technol. 2018, 53, 2687–2696. [Google Scholar] [CrossRef]
- Kherb, J.; Flores, S.C.; Cremer, P.S. Role of carboxylate side chains in the cation Hofmeister series. J. Phys. Chem. B 2012, 116, 7389–7397. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Tang, H.; Zhao, Z.; Song, S. Hofmeister Series: Insights of Ion Specificity from Amphiphilic Assembly and Interface Property. ACS Omega 2020, 5, 6229–6239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Huan, H.; Bu, Y.; Li, X.; Shiuan, D.; Li, J.; Sun, X. Effects of hydroxyl radical induced oxidation on water holding capacity and protein structure of jumbo squid (Dosidicus gigas) mantle. Int. J. Food Sci. Technol. 2019, 54, 2159–2168. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Li, Q.; Zhang, L.; Wang, Z.; Han, L.; Yu, Q. Oxidation of myofibrillar protein and crosslinking behavior during processing of traditional air-dried yak (Bos grunniens) meat in relation to digestibility. LWT 2021, 142, 110984. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef]
- Yang, H.H.; Zhong, C.; Sun, L.C.; Li, Y.K.; Chen, H.; Wu, G.P. Effects of partial substitution of NaCl on myofibrillar protein properties from pearl mussel Hyriopsis cumingii muscle: Structural characteristics and aggregation behaviors. Food Chem. 2021, 356, 129734. [Google Scholar] [CrossRef]
- Zhou, C.-Y.; Wu, J.-Q.; Tang, C.-B.; Li, G.; Dai, C.; Bai, Y.; Li, C.-B.; Xu, X.-L.; Zhou, G.-H.; Cao, J.-X. Comparing the proteomic profile of proteins and the sensory characteristics in Jinhua ham with different processing procedures. Food Control 2019, 106, 106694. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Pan, D.D.; Cao, J.X.; Zhou, G.H. A comprehensive review on molecular mechanism of defective dry-cured ham with excessive pastiness, adhesiveness, and bitterness by proteomics insights. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3838–3857. [Google Scholar] [CrossRef]
- Dang, D.S.; Buhler, J.F.; Davis, H.T.; Thornton, K.J.; Scheffler, T.L.; Matarneh, S.K. Inhibition of mitochondrial calcium uniporter enhances postmortem proteolysis and tenderness in beef cattle. Meat Sci. 2020, 162, 108039. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Long, M.; Tang, J.; Yu, X.; Wang, J.; Zhang, J. Proteolysis and sensory properties of dry-cured bacon as affected by the partial substitution of sodium chloride with potassium chloride. Meat Sci. 2014, 96, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, C.; Liu, X.; He, L.; Zhang, M.; Huang, S.; Huang, S.; Liu, Y.; Zhang, Y.; Jin, G. A multivariate insight into the organoleptic properties of porcine muscle by ultrasound-assisted brining: Protein oxidation, water state and microstructure. LWT 2022, 159, 113136. [Google Scholar] [CrossRef]
- Xia, M.; Chen, Y.; Guo, J.; Feng, X.; Yin, X.; Wang, L.; Wu, W.; Li, Z.; Sun, W.; Ma, J. Effects of oxidative modification on textural properties and gel structure of pork myofibrillar proteins. Food Res. Int. 2019, 121, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; He, Z.; Wang, Z.; Li, S.; Wang, Z.; Li, H. Effects of NaCl content and drying temperature on lipid oxidation, protein oxidation, and physical properties of dry-cured chicken. J. Food Sci. 2020, 85, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.M.; Dornelles, R.C.P.; Vidal, A.R.; Fontoura, A.; Kubota, E.H.; Mello, R.O.; Kempka, A.P.; Demiate, I.M. Development of cooked and smoked chicken sausage with reduced sodium and fat. J. Appl. Poult. Res. 2017, 26, 130–144. [Google Scholar] [CrossRef]
- Campagnol, P.C.B.; dos Santos, B.A.; Terra, N.N.; Pollonio, M.A.R. Lysine, disodium guanylate and disodium inosinate as flavor enhancers in low-sodium fermented sausages. Meat Sci. 2012, 91, 334–338. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estevez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef]
- Grune, T.; Jung, T.; Merker, K.; Davies, K.J. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int. J. Biochem. Cell Biol. 2004, 36, 2519–2530. [Google Scholar] [CrossRef]
- Rysman, T.; Van Hecke, T.; Van Poucke, C.; De Smet, S.; Van Royen, G. Protein oxidation and proteolysis during storage and in vitro digestion of pork and beef patties. Food Chem. 2016, 209, 177–184. [Google Scholar] [CrossRef]
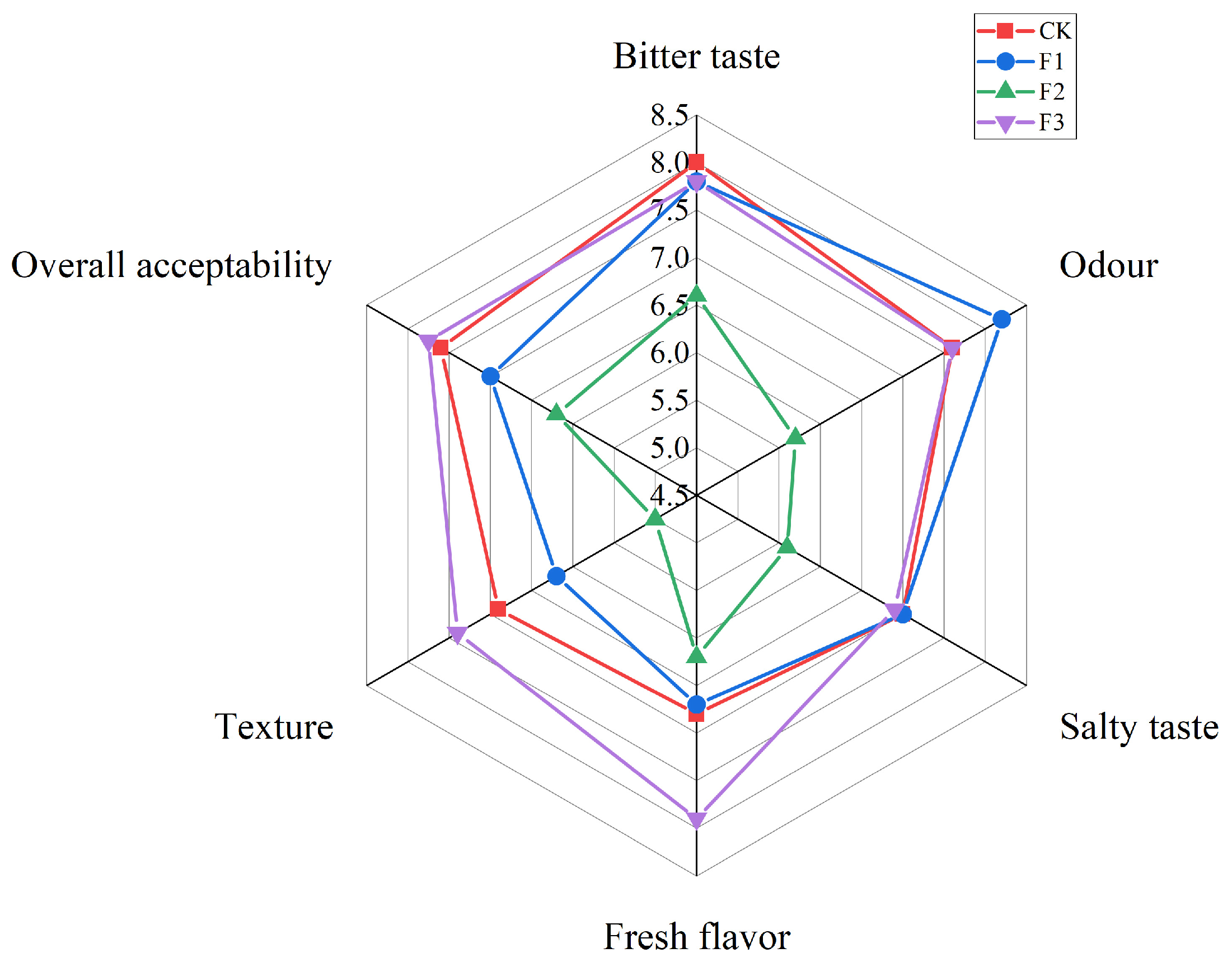


| Sensory | Bitter Taste | Astringent | Salty Taste | Fresh Flavor | Texture | Overall Acceptability |
|---|---|---|---|---|---|---|
| 7–9 | Litter bitter | Little astringent | Moderate saltiness | Moderate flavor | Moderate texture | Very acceptable |
| 4–6 | Bitter | Astringent | Saltier or lighter | Fresher or lighter | Softer or harder | acceptable |
| 1–3 | Very bitter | Very astringent | Too salty or litter salty | Too fresh or litter fresh | Too soft or too hard | inacceptable |
| Hardness | Springiness | Cohesiveness | Gumminess | Chewiness | Resilience | |
|---|---|---|---|---|---|---|
| CK | 5041 ± 410 a | 0.835 ± 0.087 a | 0.852 ± 0.034 a | 4307 ± 483 a | 3556 ± 129 a | 0.693 ± 0.0261 a |
| F1 | 3538 ± 280 b | 0.770 ± 0.019 a | 0.755 ± 0.034 bc | 2659 ± 208 bc | 2039 ± 136 b | 0.576 ± 0.0387 b |
| F2 | 2768 ±251 b | 0.826 ± 0.020 a | 0.782 ± 0.007 b | 2166 ± 214 c | 1785 ± 187 b | 0.537 ± 0.0380 b |
| F3 | 4846 ± 479 a | 0.810 ± 0.046 a | 0.714 ± 0.026 c | 3885 ± 470 ab | 2862 ± 543 a | 0.563 ± 0.0382 b |
| Accession | Gene Name | Description |
|---|---|---|
| F1NX83 | AGL | Glycogen debranching enzyme |
| F1P463 | CNDP2 | CNDP dipeptidase 2 (metallopeptidase M20 family) |
| A0A8V0ZLR4 | HSPB9 | Heat shock protein family B (small) member 9 |
| F1NU17 | PGK2 | Phosphoglycerate kinase |
| C1L370 | pvalb1 | Parvalbumin |
| P02604 | Myosin light chain 1, skeletal muscle isoform | |
| E1BYF1 | CTH | Cystathionine gamma-lyase |
| A6BLM8 | TTN | Titin isoform b11 (Fragment) |
| A0A8V0XA66 | AASS | Aminoadipate-semialdehyde synthase |
| F1NMM5 | ABHD12B | Abhydrolase domain containing 12B |
| P79757 | Connectin/titin (Fragment) | |
| A0A8V0YKB8 | AKR1E2 | Aldo-keto reductase family 1 member E2 |
| P19753 | Parvalbumin, thymic | |
| A0A343CVV5 | ND2 | NADH-ubiquinone oxidoreductase chain 2 |
| A0A8V0ZDH7 | FAHD2A | Fumarylacetoacetate hydrolase domain containing 2A |
| A0A8V0Z2L5 | TRIM63 | Tripartite motif containing 63 |
| A0A8V1A9H4 | LDHD | Lactate dehydrogenase D |
| E1C5M4 | HYKK | Hydroxylysine kinase |
| F1NL83 | COX7B | Cytochrome c oxidase subunit 7B |
| A0A8V0XM57 | SYNPO2 | Synaptopodin 2 |
| A0A8V0YPY7 | MYLK2 | Myosin light chain kinase 2 |
| A0A8V0XI57 | LOC100859645 | Glutathione S-transferase alpha 3 |
| Q9W6J2 | GSTA4 | Glutathione transferase |
| A0A3Q2TYR0 | MYOZ3 | Myozenin 3 |
| E1C312 | CAPN6 | Calpain 6 |
| A0A8V0YZK6 | NMNAT1 | Retinol binding protein 7 |
| P27463 | ALDH1A1 | Aldehyde dehydrogenase 1A1 |
| A0A8V1AEN9 | DPP7 | Dipeptidyl peptidase 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Shi, Z.; Fan, X.; Du, L.; Xia, Q.; Zhou, C.; Sun, Y.; Xu, B.; Pan, D. Characterization of the Effects of Low-Sodium Salt Substitution on Sensory Quality, Protein Oxidation, and Hydrolysis of Air-Dried Chicken Meat and Its Molecular Mechanisms Based on Tandem Mass Tagging-Labeled Quantitative Proteomics. Foods 2024, 13, 737. https://doi.org/10.3390/foods13050737
Li J, Shi Z, Fan X, Du L, Xia Q, Zhou C, Sun Y, Xu B, Pan D. Characterization of the Effects of Low-Sodium Salt Substitution on Sensory Quality, Protein Oxidation, and Hydrolysis of Air-Dried Chicken Meat and Its Molecular Mechanisms Based on Tandem Mass Tagging-Labeled Quantitative Proteomics. Foods. 2024; 13(5):737. https://doi.org/10.3390/foods13050737
Chicago/Turabian StyleLi, Jianhao, Zihang Shi, Xiankang Fan, Lihui Du, Qiang Xia, Changyu Zhou, Yangying Sun, Baocai Xu, and Daodong Pan. 2024. "Characterization of the Effects of Low-Sodium Salt Substitution on Sensory Quality, Protein Oxidation, and Hydrolysis of Air-Dried Chicken Meat and Its Molecular Mechanisms Based on Tandem Mass Tagging-Labeled Quantitative Proteomics" Foods 13, no. 5: 737. https://doi.org/10.3390/foods13050737
APA StyleLi, J., Shi, Z., Fan, X., Du, L., Xia, Q., Zhou, C., Sun, Y., Xu, B., & Pan, D. (2024). Characterization of the Effects of Low-Sodium Salt Substitution on Sensory Quality, Protein Oxidation, and Hydrolysis of Air-Dried Chicken Meat and Its Molecular Mechanisms Based on Tandem Mass Tagging-Labeled Quantitative Proteomics. Foods, 13(5), 737. https://doi.org/10.3390/foods13050737







