Zein Multilayer Electrospun Nanofibers Contain Essential Oil: Release Kinetic, Functional Effectiveness, and Application to Fruit Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gas Chromatography–Mass Spectrometry (GC–MS)
2.3. Solution Preparation
2.4. Electrospinning
2.5. Characterization of Nanofibers
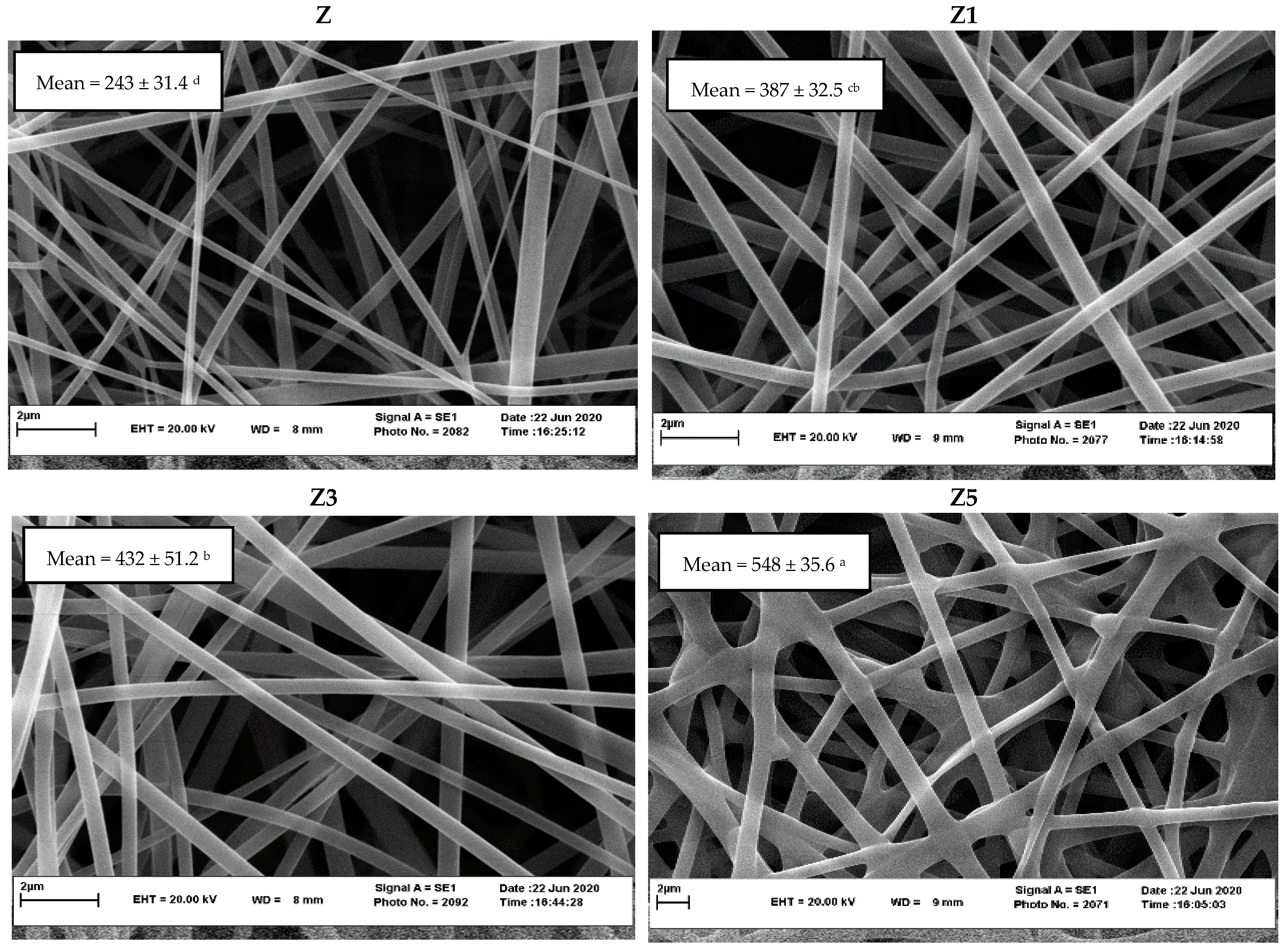
2.5.1. Scanning Electron Microscopy (SEM)
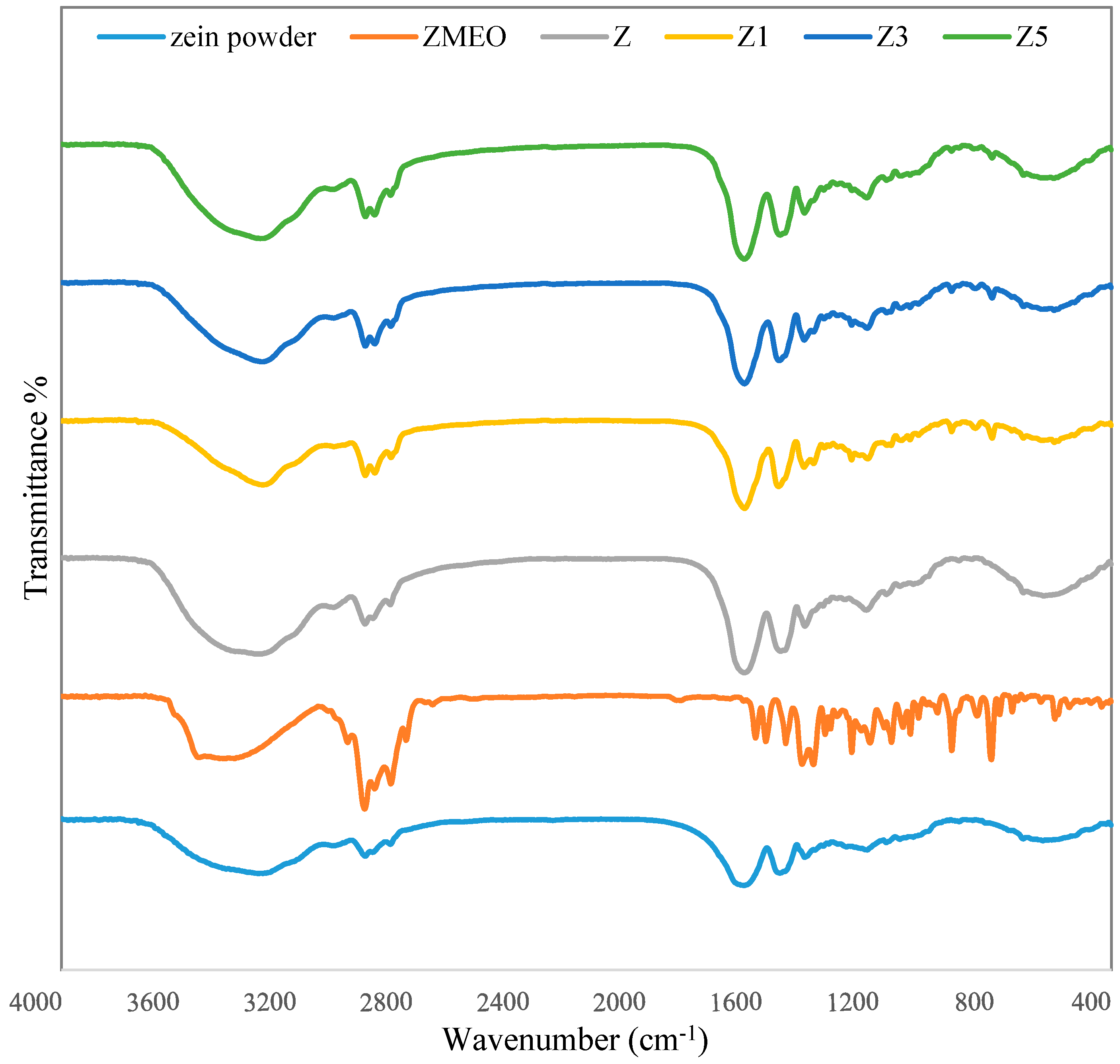
2.5.2. Fourier Transform Infrared (FTIR)
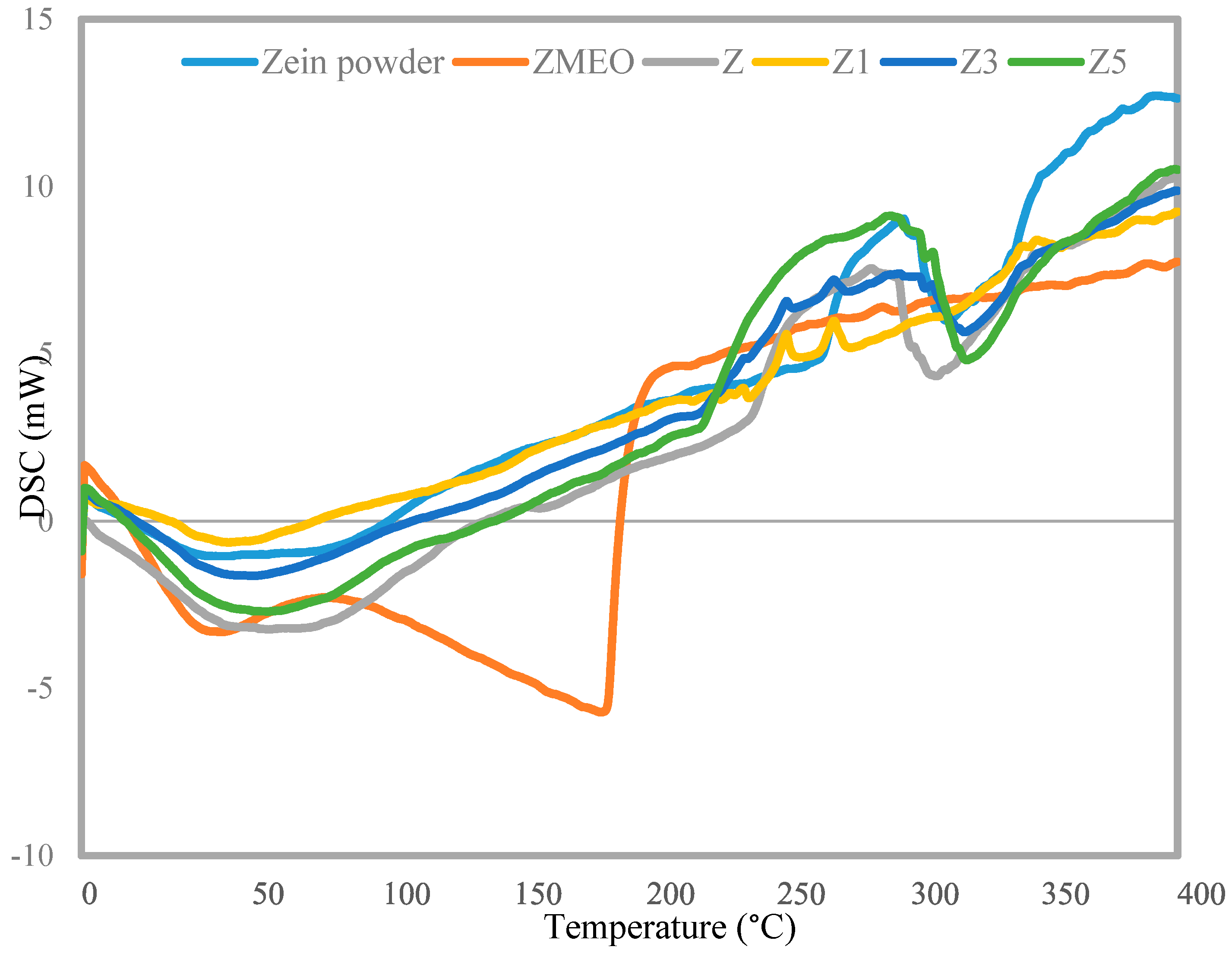
2.5.3. Differential Scanning Calorimetry (DSC)
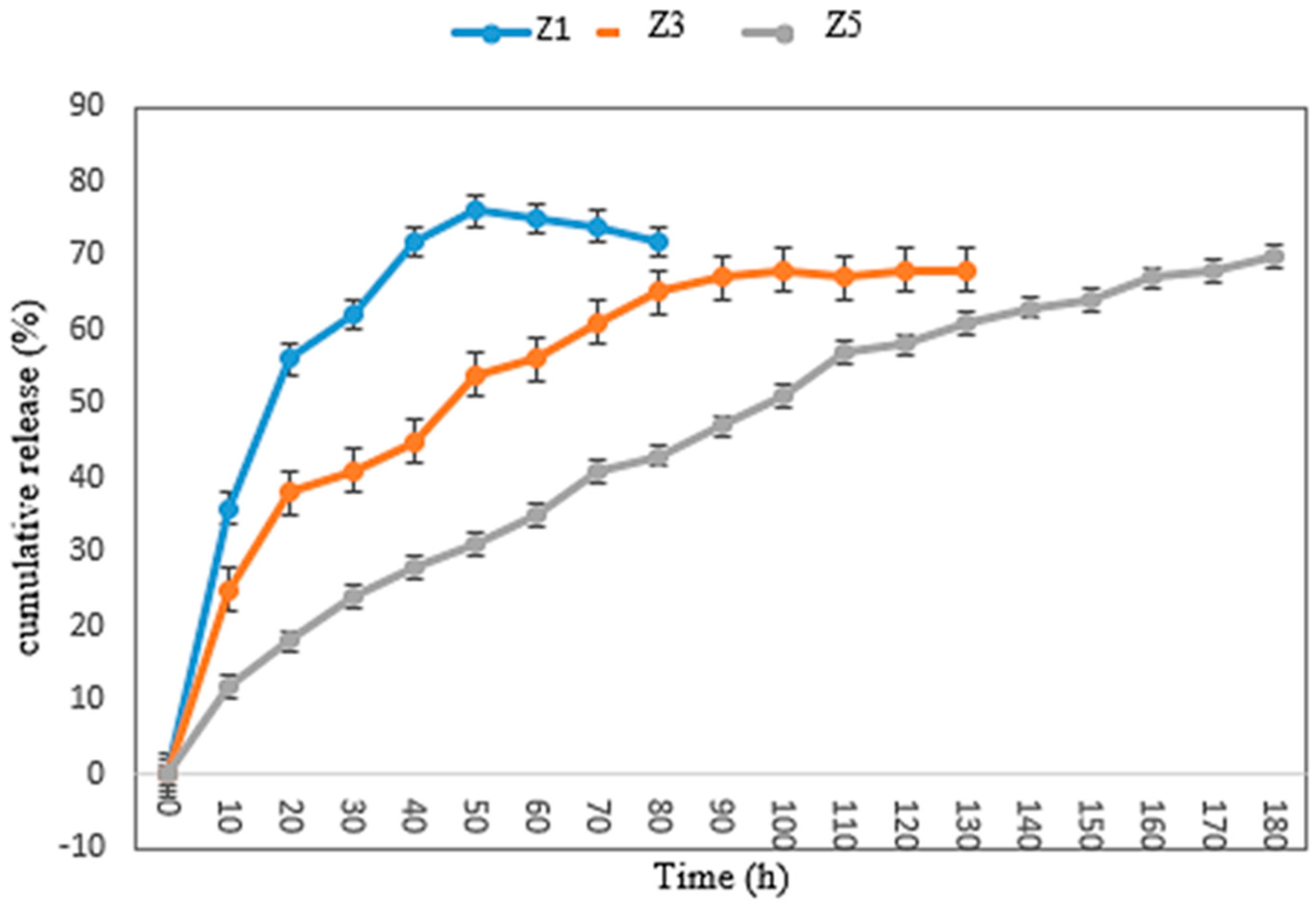
2.5.4. Encapsulation Efficiency and Release of ZMEO from Zein Multilayer Nanofiber
2.6. Fruit Preparation and Active Packaging
2.7. Evaluation of Strawberry Quality
2.8. Statistical Analysis
3. Results and Discussion
3.1. Essential Oil Analysis by GC–MS
3.2. SEM
3.3. FTIR
3.4. DSC
3.5. Encapsulation Efficiency (EE), Release Analysis, and Kinetic Modeling
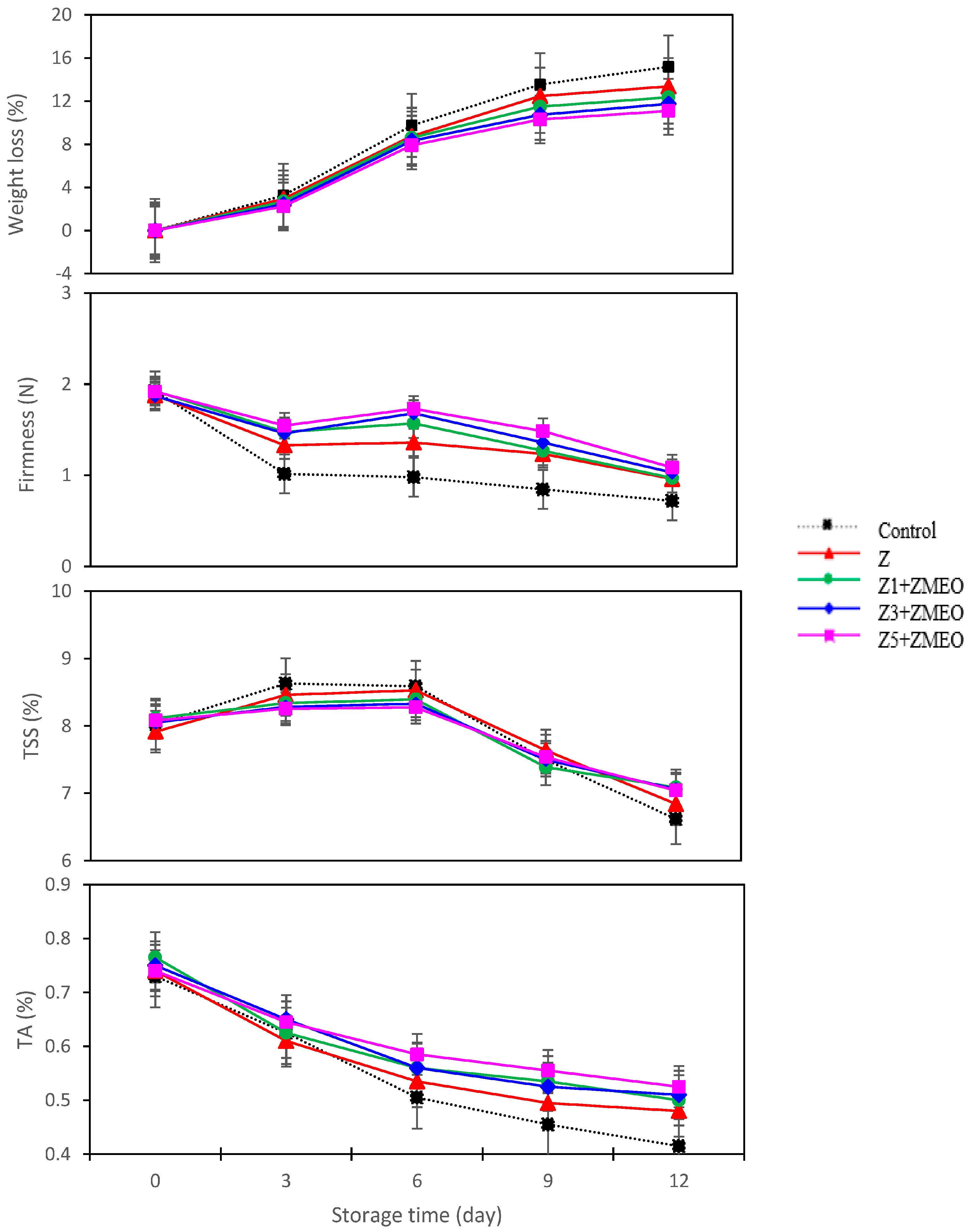
3.6. The Effect of Active Packaging on Weight Loss Percentage
3.7. The Effect of Active Packaging on Firmness (N)
3.8. The Effect of Active Packaging on Total Soluble Solids (TSSs)
3.9. The Effect of Active Packaging on Titratable Acidity (TA)
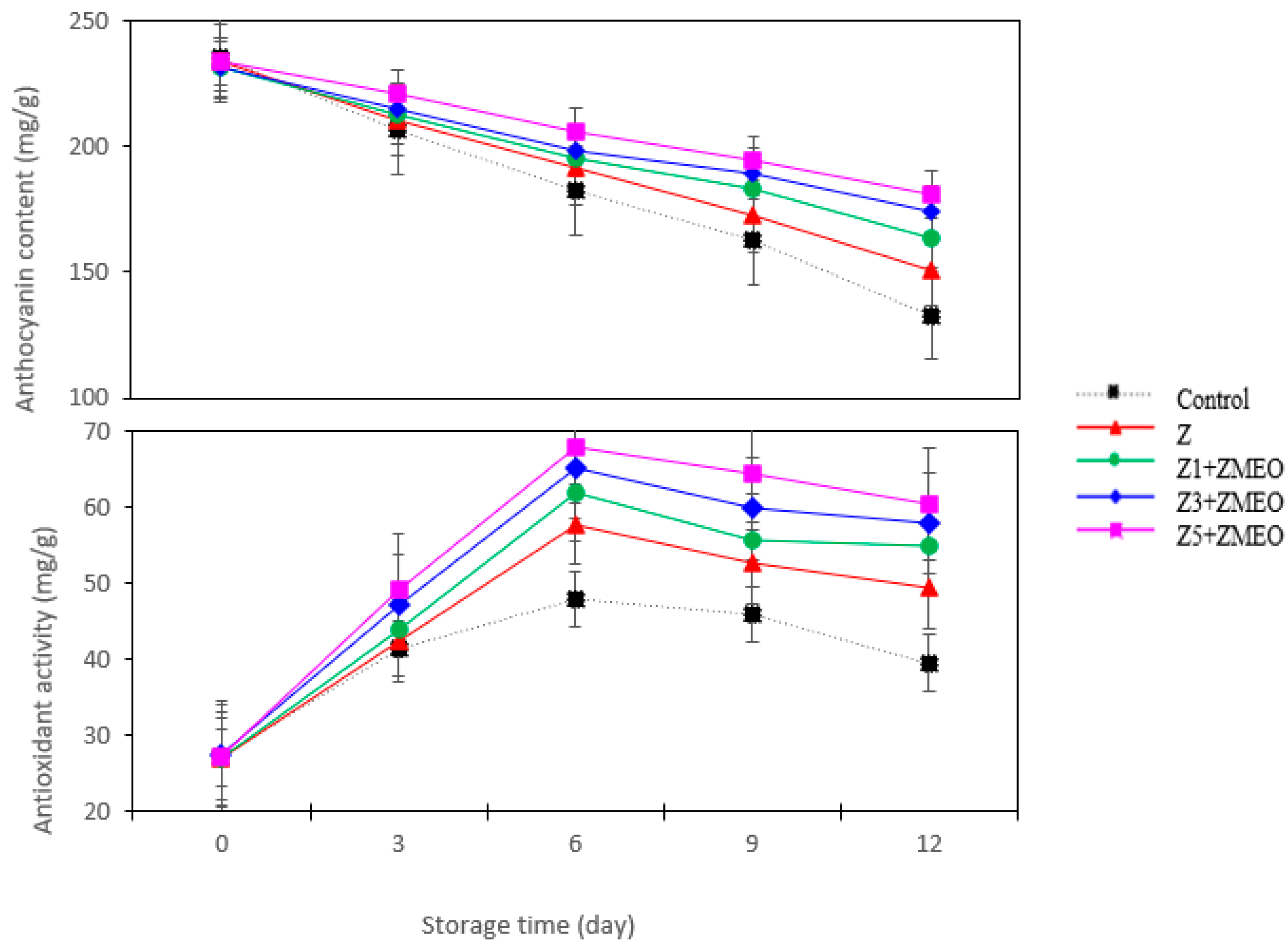
3.10. The Effect of Active Packaging on the Total Anthocyanin Content
3.11. The Effect of Active Packaging on the Antioxidant Activity
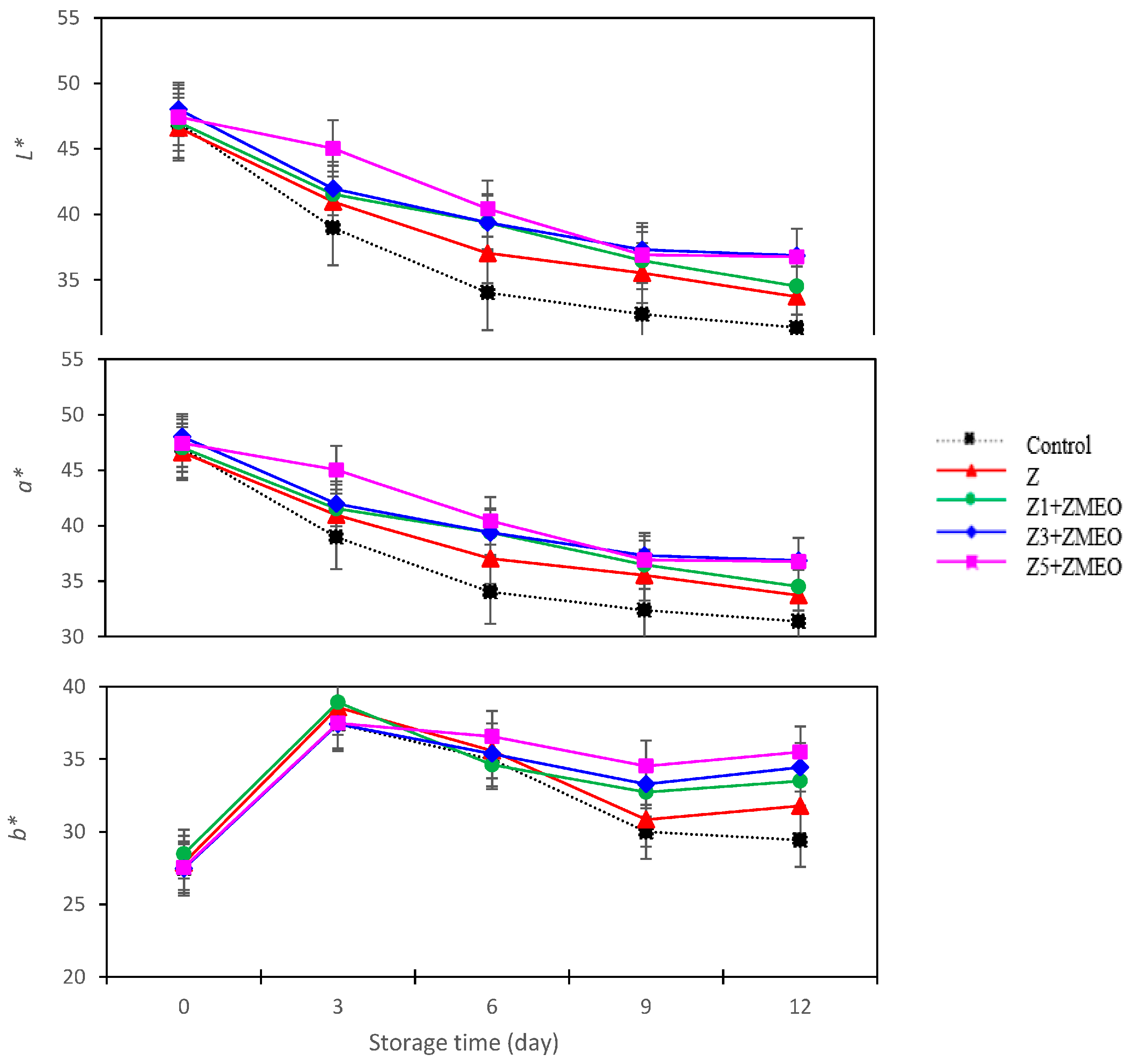
3.12. The Effect of Active Packaging on Color Parameter (L*, a*, and b*)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansarifar, E.; Moradinezhad, F. Encapsulation of thyme essential oil using electrospun zein fiber for strawberry preservation. Chem. Biol. Technol. Agric. 2022, 9, 2. [Google Scholar] [CrossRef]
- Radziejewska-Kubzdela, E. Effect of Ultrasonic, Thermal and Enzymatic Treatment of Mash on Yield and Content of Bioactive Compounds in Strawberry Juice. Appl. Sci. 2023, 13, 4268. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Zhu, Z.; Jiao, X.; Shang, Y.; Wen, Y. Encapsulation of Thymol in Biodegradable Nanofiber via Coaxial Eletrospinning and Applications in Fruit Preservation. J. Agric. Food Chem. 2019, 67, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of cinnamic aldehyde using zein nanofibers by novel needle-less electrospinning: Production, characterization and their application to reduce nitrite in sausages. J. Food Eng. 2020, 288, 110140. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Cui, H. Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT 2018, 97, 711–718. [Google Scholar] [CrossRef]
- Shao, P.; Liu, Y.; Ritzoulis, C.; Niu, B. Preparation of zein nanofibers with cinnamaldehyde encapsulated in surfactants at critical micelle concentration for active food packaging. Food Packag. Shelf Life 2019, 22, 100385. [Google Scholar] [CrossRef]
- Ranjan, S.; Chandrasekaran, R.; Paliyath, G.; Lim, L.-T.; Subramanian, J. Effect of hexanal loaded electrospun fiber in fruit packaging to enhance the post-harvest quality of peach. Food Packag. Shelf Life 2020, 23, 100447. [Google Scholar] [CrossRef]
- Akranmi, F.; Rodríguez-Lafuente, A.; Bentayeb, K.; Pezo, D.; Ghalebi, S.R.; Nerín, C. Antioxidant and antimicrobial active paper based on Zataria (Zataria multiflora) and two cumin cultivars (Cuminum cyminum). LWT-Food Sci. Technol. 2015, 60, 929–933. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Zhang, C.; Feng, F.; Zhang, H. Sequential electrospinning of multilayer ethylcellulose/gelatin/ethylcellulose nanofibrous film for sustained release of curcumin. Food Chem. 2019, 308, 125599. [Google Scholar] [CrossRef] [PubMed]
- Noshad, M.; Mohebbi, M.; Ansarifar, E.; Behbahani, B.A. Quantification of enzymatic browning kinetics of quince preserved by edible coating using the fractal texture Fourier image. J. Food Meas. Charact. 2015, 9, 375–381. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Yang, M.; Liang, Z.; Qi, M.; Dong, Y.; Xu, Y.; Lin, X.; Li, L. The action of RED light: Specific elevation of pelargonidin-based anthocyanin through ABA-related pathway in strawberry. Postharvest Biol. Technol. 2022, 186, 111835. [Google Scholar] [CrossRef]
- Vafania, B.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of thyme essential oil in chitosan-gelatin nanofibers by nozzle-less electrospinning and their application to reduce nitrite in sausages. Food Bioprod. Process. 2019, 116, 240–248. [Google Scholar] [CrossRef]
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef]
- Charpashlo, E.; Ghorani, B.; Mohebbi, M. Multilayered electrospinning strategy for increasing the bioaccessibility of lycopene in gelatin-based sub-micron fiber structures. Food Hydrocoll. 2020, 113, 106411. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Moradinezhad, F.; Ansarifar, E.; Moghaddam, M.M. Extending the shelf life and maintaining quality of minimally-processed pomegranate arils using ascorbic acid coating and modified atmosphere packaging. J. Food Meas. Charact. 2020, 14, 3445–3454. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, W.; Zhong, Y.; Xie, X.; Liu, H.; Huang, H.; Wang, Q.; Xiao, G. Involvement of branched RG-I pectin with hemicellulose in cell–cell adhesion of tomato during fruit softening. Food Chem. 2023, 413, 135574. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Rahimi, R.; ValizadehKaji, B.; Khadivi, A.; Shahrjerdi, I. Effect of chitosan and thymol essential oil on quality maintenance and shelf life extension of peach fruits cv. ‘Zaferani’. J. Hortic. Postharvest Res. 2019, 2, 143–156. [Google Scholar]
- Geransayeh, M.; Sepahvand, S.; Abdossi, V.; Nezhad, R.A. Effect of thymol treatment on decay, postharvest life and quality of strawberry (Fragaria ananassa) Fruit cv.‘Gaviota. Int. J. Agron. Agric. Res. 2015, 6, 151–162. [Google Scholar]
- Shahbazi, Y. Application of carboxymethyl cellulose and chitosan coatings containing Mentha spicata essential oil in fresh strawberries. Int. J. Biol. Macromol. 2018, 112, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Kazemi-Pasarvi, S.; Ebrahimi, N.G.; Shahrampour, D.; Arab-Bafrani, Z. Reducing cytotoxicity of poly (lactic acid)-based/zinc oxide nanocomposites while boosting their antibacterial activities by thymol for biomedical applications. Int. J. Biol. Macromol. 2020, 164, 4556–4565. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Li, W.; Wang, Y.; Cheng, F.; Wu, H.; Sun, Y. Low voltage electrostatic field treatment of fresh-cut pineapples with slightly acidic electrolytic water: Influence on physicochemical changes and membrane stability. Sci. Hortic. 2023, 308, 111602. [Google Scholar] [CrossRef]
- Adelina, F.; Sitanggang, M.; Falah, A.M.F.M. Comparison of Color Quality Measurement Using Chromameter and Image Processing for Dehydrated Strawberry Products. In Proceedings of the 2nd International Conference for Smart Agriculture, Food, and Environment, Online, 4 November 2023. [Google Scholar] [CrossRef]
- Gérard, V.; Ay, E.; Morlet-Savary, F.; Graff, B.; Galopin, C.; Ogren, T.; Mutilangi, W.; Lalevée, J. Thermal and photochemical stability of anthocyanins from black carrot, grape juice, and purple sweet potato in model beverages in the presence of ascorbic acid. J. Agric. Food Chem. 2019, 67, 5647–5660. [Google Scholar] [CrossRef]
| Characteristic | Test Results | Acceptance Limit | Test Method |
|---|---|---|---|
| Appearance | Conform | Yellow to red color | Organoleptic |
| Color Odor | Conform | Thyme odor | |
| Chemical composition of essential oil | Concentration | RI | GC–MS analysis |
| α-Pinene | 1.47 | 930 | |
| Camphene | 0.03 | 944 | |
| β-Pinene | 0.04 | 973 | |
| Myrcene | 0.05 | 987 | |
| p-Cymene | 14.32 | 1022 | |
| Limonene | 0.24 | 1025 | |
| β-Ocimene | 0.09 | 1043 | |
| Terpinene | 22.46 | 1055 | |
| Saninebe hydrate | 0.05 | 1063 | |
| Linalool oxide | 0.1 | 1068 | |
| Terpinolene | 0.02 | 1084 | |
| Linalool | 4.96 | 1100 | |
| 4-Terpineol | 1.39 | 1174 | |
| Thymol | 43.84 | 1290 | |
| Carvacrol | 10.94 | 1300 | |
| Microbial specification | |||
| Total aerobic microbial count (cfu/mL) | ˂10 | ˂100 | USP41 |
| Total fungi and yeast count (cfu/mL) | ˂10 | ˂10 | |
| Pseudomonas aeruginosa | Negative | Negative | |
| Staphylococcus aureus | Negative | Negative | |
| Salmonella sp. | Negative | Negative | |
| Escherichia coli | Negative | Negative | |
| Candida albicans | Negative | Negative |
| EE | Model | R2 | RMSE | Release Kinetic Model’s Data | |
|---|---|---|---|---|---|
| Z1 | 43 | Higuchi | 0.843 | 0.423 | k = 0.235 |
| Korsmeyer–Peppas | 0.963 | 0.186 | k = 4.739 n = 0.065 | ||
| Z3 | 56 | Higuchi | 0.845 | 0.512 | k = 0.332 |
| Korsmeyer–Peppas | 0.966 | 0.234 | k = 2.325 n = 0.083 | ||
| Z5 | 82 | Higuchi | 0.981 | 0.241 | k = 1.734 |
| Korsmeyer–Peppas | 0.968 | 0.321 | k = 0.841 n = 0.612 |
| Active Packaging Treatment (AP) | Weight Loss (%) | Firmness (N) | TSS (%) | TA (%) |
|---|---|---|---|---|
| Control | 8.34 ± 0.74 a | 1.09 ± 0.01 d | 7.87 ± 0.72 a | 0.54 ± 0.01 d |
| Z | 7.50 ± 0.25 b | 1.35 ± 0.12 c | 7.87 ± 0.62 a | 0.57 ± 0.01 c |
| Z1 + ZMEO | 7.02 ± 0.22 c | 1.44 ± 0.14 b | 7.86 ± 0.71 a | 0.59 ± 0.02 b |
| Z3 + ZMEO | 6.64 ± 0.57 d | 1.48 ± 0.15 b | 7.83 ± 0.70 a | 0.59 ± 0.01 b |
| Z5 + ZMEO | 6.30 ± 0.54 e | 1.55 ± 0.12 a | 7.84 ± 0.73 a | 0.61 ± 0.00 a |
| Significance | ** | ** | ns | ** |
| LSD (0.05) | 0.063 | 0.04 | 0.81 | 0.20 |
| Storage time (T) (day) | ||||
| 0 | - | 1.90 ± 0.10 a | 8.03 ± 0.78 b | 0.74 ± 0.02 a |
| 3 | 2.71 ± 0.02 d | 1.36 ± 0.11 c | 8.39 ± 0.54 a | 0.63 ± 0.05 b |
| 6 | 8.66 ± 0.58 c | 1.46 ± 0.11 b | 8.42 ± 0.85 a | 0.54 ± 0.04 c |
| 9 | 11.7 ± 0.84 b | 1.23 ± 0.08 d | 7.50 ± 0.52 c | 0.51 ± 0.02 d |
| 12 | 12.7 ± 0.72 a | 0.95 ± 0.10 e | 6.92 ± 0.24 d | 0.48 ± 0.01 e |
| Significance | ** | ** | ** | ** |
| LSD (0.05) | 0.021 | 0.08 | 0.82 | 0.41 |
| Interaction AP × T | ** | ** | ** | ** |
| Active Packaging Treatment (AP) | Anthocyanin (mg 100 gFW) | Antioxidant Activity (mg/100 g) | Color Parameter | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| Control | 138.99 ± 14.52 e | 40.40 ± 2.25 d | 36.75 ± 4.12 d | 31.85 ± 3.12 c | 19.36 ± 1.10 d |
| Z | 191.46 ± 12.41 d | 45.89 ± 2.54 c | 38.77 ± 2.54 c | 32.92 ± 3.22 bc | 20.38 ± 1.98 c |
| Z1 + ZMEO | 197.04 ± 16.65 c | 48.69 ± 3.12 b | 39.79 ± 3.84 b | 33.64 ± 3.00 ab | 20.67 ± 2.02 c |
| Z3 + ZMEO | 201.66 ± 16.57 b | 51.55 ± 4.24 b | 40.71 ± 4.23 a | 33.59 ± 3.25 ab | 21.38 ± 2.32 b |
| Z5 + ZMEO | 207.22 ± 14.20 a | 53.90 ± 4.98 a | 41.32 ± 3.73 a | 34.32 ± 3.45 a | 22.07 ± 2.00 a |
| Significance | ** | ** | ** | ** | ** |
| LSD (0.05) | 1.39 | 0.84 | 1.45 | 1.13 | 0.68 |
| Storage time (T) (day) | |||||
| 0 | 233.21 ± 19.44 a | 27.16 ± 1.29 e | 47.21 ± 2.78 a | 27.76 ± 2.31 d | 26.61 ± 2.15 a |
| 3 | 213.12 ± 24.09 b | 44.86 ± 3.89 d | 41.70 ± 3.58 b | 37.96 ± 3.24 a | 23.31 ± 2.651 b |
| 6 | 194.52 ± 20.54 c | 60.18 ± 4.22 a | 38.05 ± 3.81 c | 35.42 ± 2.87 b | 20.92 ± 2.42 c |
| 9 | 180.32 ± 18.68 d | 55.76 ± 4.07 b | 35.72 ± 3.30 d | 32.27 ± 3.01 c | 17.05 ± 1.87 d |
| 12 | 160.49 ± 17.45 e | 52.47 ± 5.55 c | 34.65 ± 3.22 e | 32.92 ± 3.24 c | 15.97 ± 1.88 e |
| Significance | ** | ** | ** | ** | ** |
| LSD (0.05) | 1.35 | 1.85 | 1.24 | 2.52 | 0.75 |
| Interaction AP × T | ** | ** | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradinezhad, F.; Aliabadi, M.; Ansarifar, E. Zein Multilayer Electrospun Nanofibers Contain Essential Oil: Release Kinetic, Functional Effectiveness, and Application to Fruit Preservation. Foods 2024, 13, 700. https://doi.org/10.3390/foods13050700
Moradinezhad F, Aliabadi M, Ansarifar E. Zein Multilayer Electrospun Nanofibers Contain Essential Oil: Release Kinetic, Functional Effectiveness, and Application to Fruit Preservation. Foods. 2024; 13(5):700. https://doi.org/10.3390/foods13050700
Chicago/Turabian StyleMoradinezhad, Farid, Majid Aliabadi, and Elham Ansarifar. 2024. "Zein Multilayer Electrospun Nanofibers Contain Essential Oil: Release Kinetic, Functional Effectiveness, and Application to Fruit Preservation" Foods 13, no. 5: 700. https://doi.org/10.3390/foods13050700
APA StyleMoradinezhad, F., Aliabadi, M., & Ansarifar, E. (2024). Zein Multilayer Electrospun Nanofibers Contain Essential Oil: Release Kinetic, Functional Effectiveness, and Application to Fruit Preservation. Foods, 13(5), 700. https://doi.org/10.3390/foods13050700







