Enhancing Bromelain Recovery from Pineapple By-Products: A Sustainable Approach for Value Addition and Waste Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Pineapple By-Products
Cores, Peels, and Crowns
Stems and Basal Stems
Chemicals
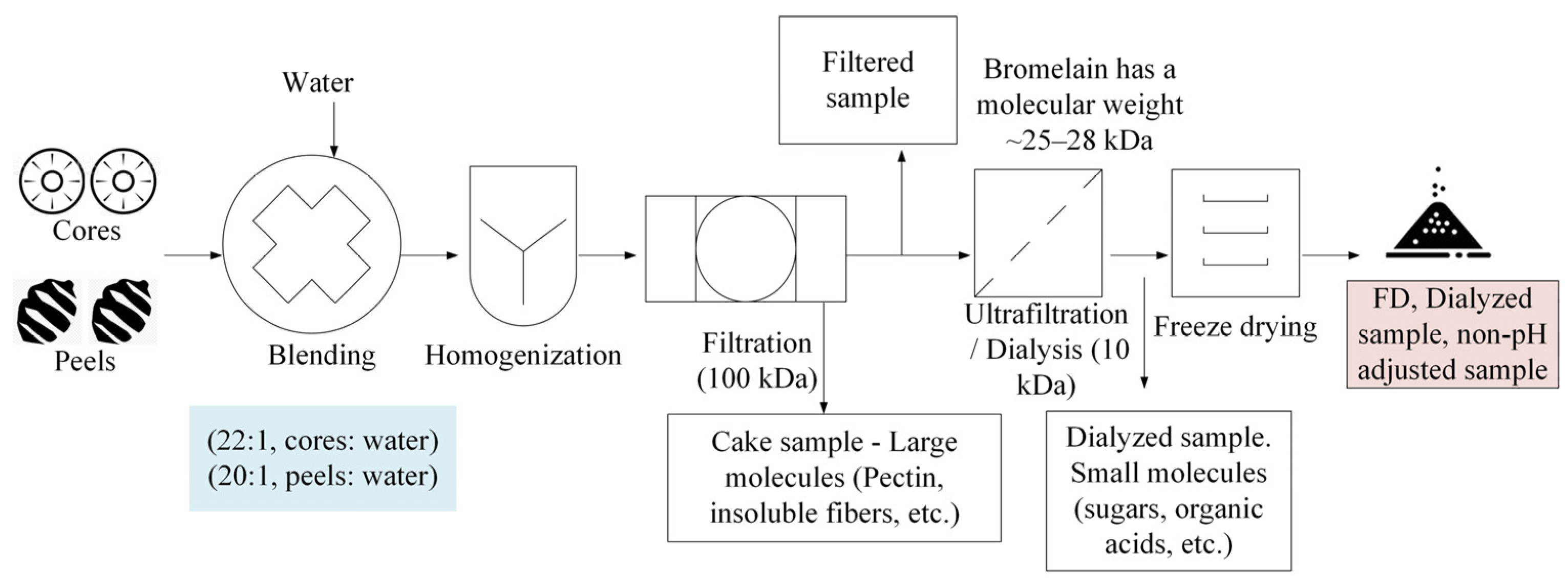
2.2. Experimental Approach
2.2.1. Phase I—Feasibility Studies
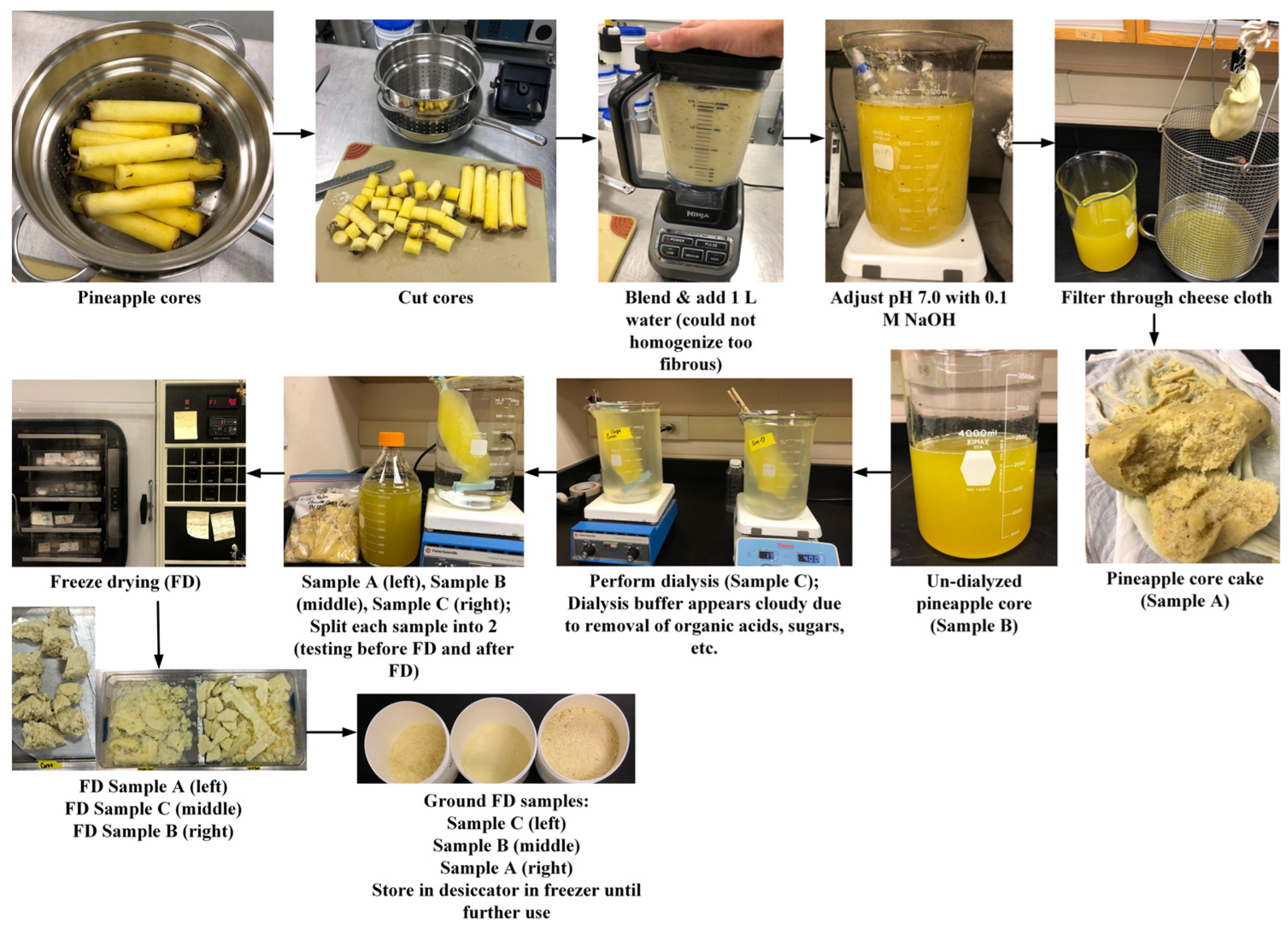
2.2.2. Phase II—Improved Bromelain Extraction
Modified Production of Bromelain Powders from Pineapple Cores
Modified Production of Bromelain Powders from Pineapple Peels
2.2.3. Phase III—Production of Bromelain Powders from Pineapple Stems and Basal Stems
2.3. Analyses
2.3.1. Moisture Content, Total Soluble Solids (TSS), and pH of Pineapple By-Products
2.3.2. Crude Protein Content Determination
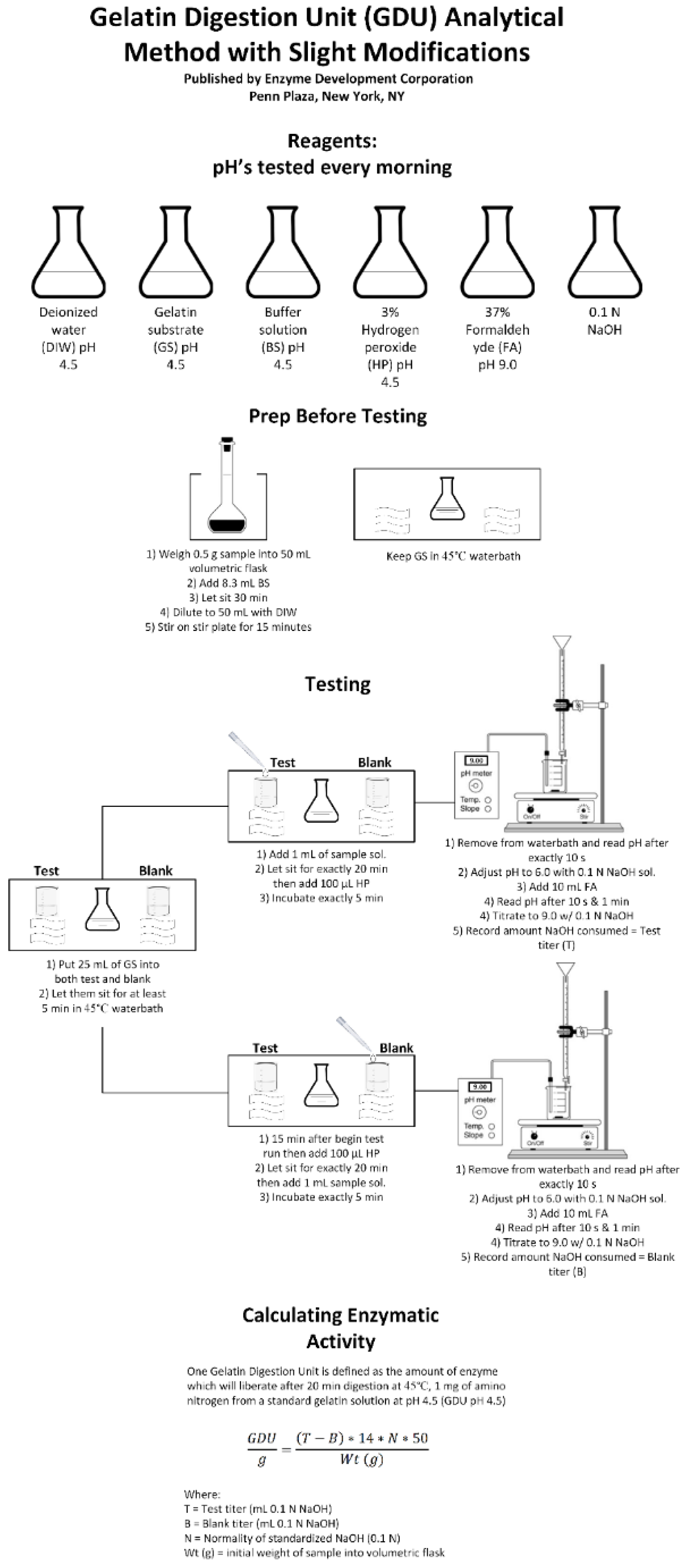
2.3.3. Enzymatic Activity—Gelatin Digestion Method
Reagent Preparation
Sample Testing
Enzymatic Activity Calculation
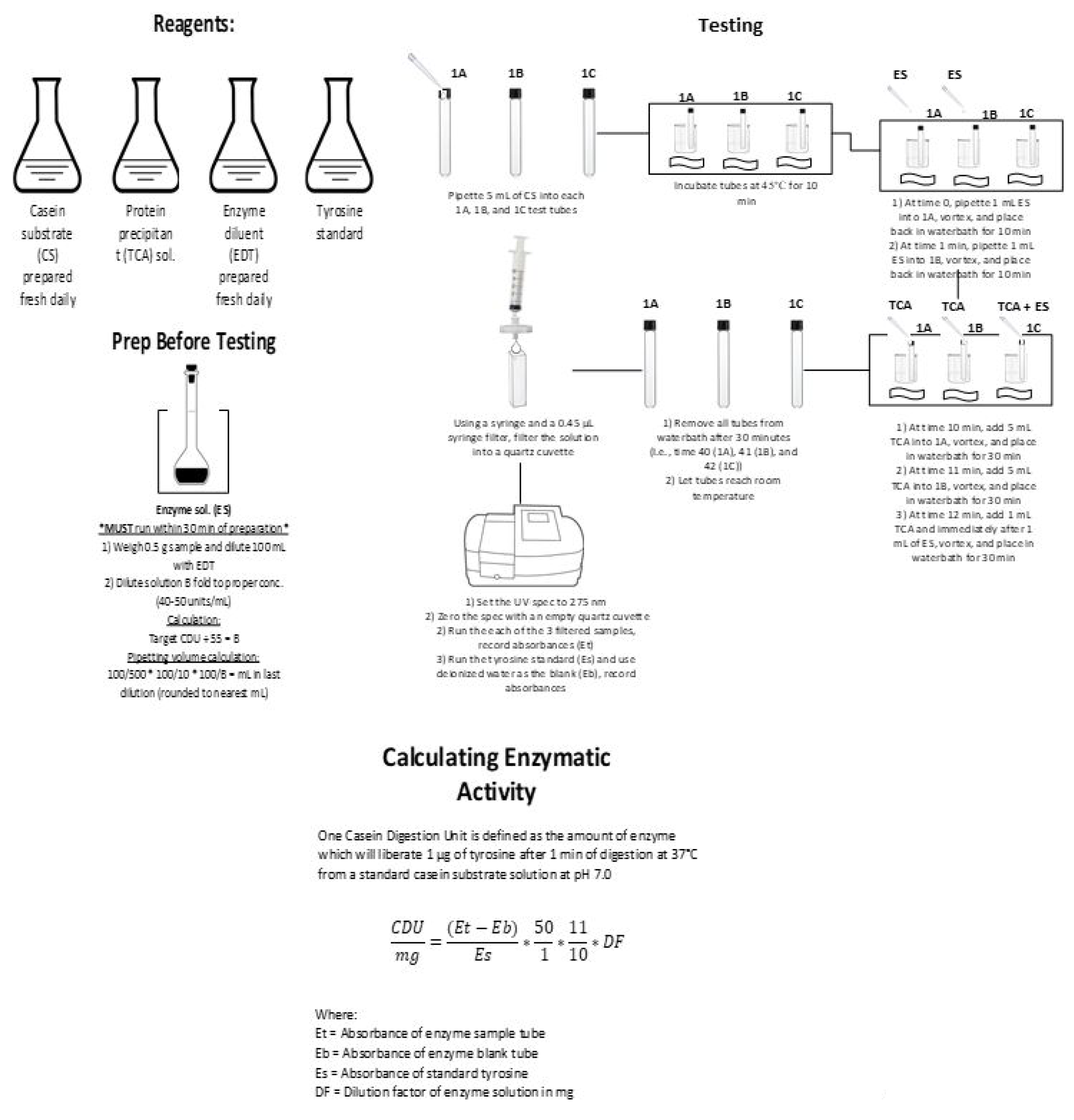
2.3.4. Enzymatic Activity—Casein Digestion
Reagent Preparation
Sample Testing
Enzymatic Activity Calculation
2.4. Statistical Analysis
3. Results and Discussion
3.1. Phase I
3.1.1. Enzymatic Activity of Bromelain Powders Extracted from Pineapple Cores, Peels, and Crowns
Cores
Peels
Crowns
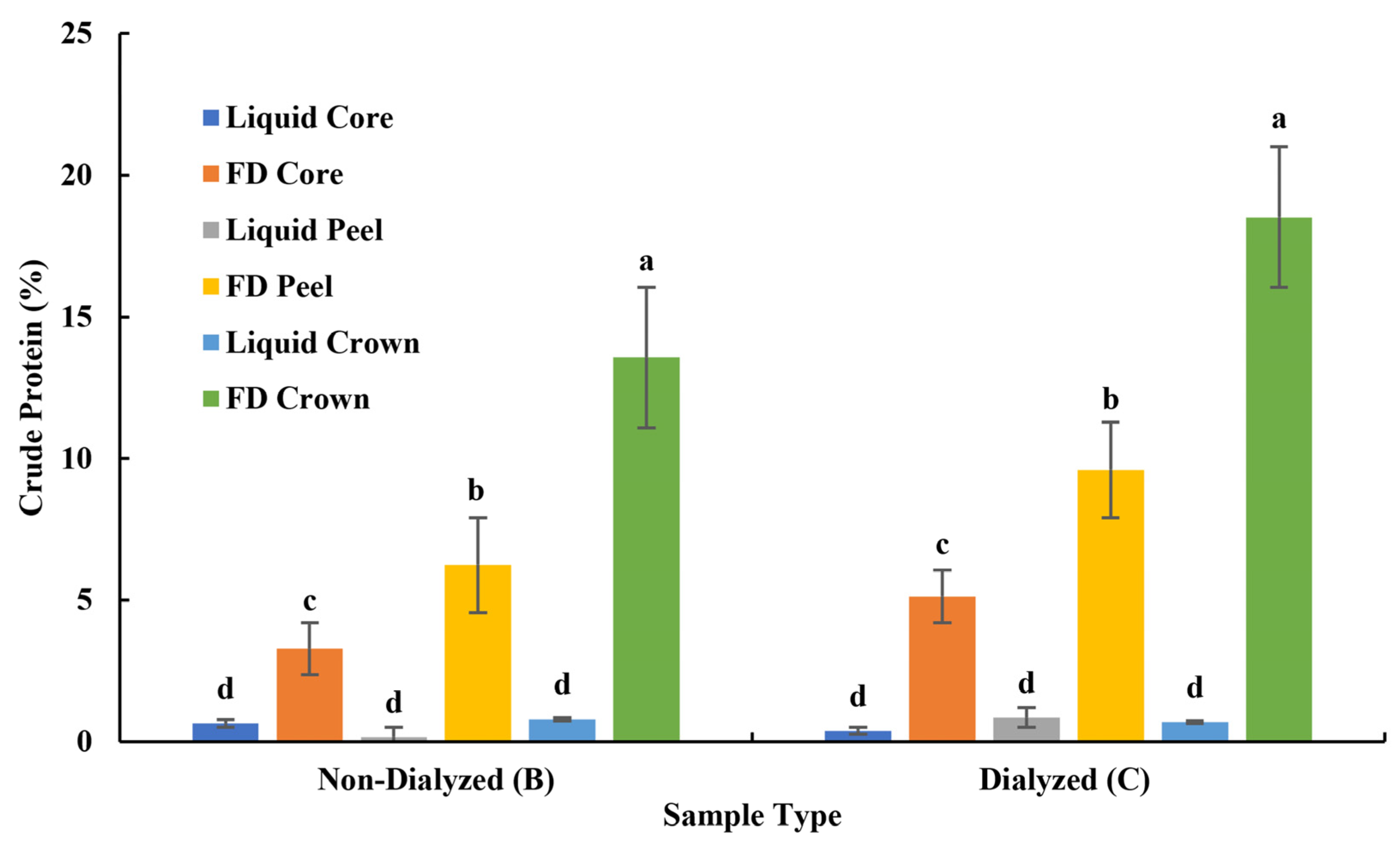
3.1.2. Crude Protein of Bromelain Powders
3.2. Phase II—Improved Bromelain Extraction
3.2.1. Bromelain Powders from Pineapple Cores without pH Adjustment
3.2.2. Bromelain Powders from Pineapple Peels without pH Adjustment
3.3. Phase III—Production of Bromelain Powders from Pineapple Stems and Basal Stems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Nor, M.Z.M.; Ramchandran, L.; Duke, M.; Vasiljevic, T. Characteristic properties of crude pineapple waste extract for bromelain purification by membrane processing. J. Food Sci. Technol. 2015, 52, 7103–7112. [Google Scholar] [CrossRef]
- Banerjee, S.; Arora, A.; Vijayaraghavan, R.; Patti, A.F. Extraction and crosslinking of bromelain aggregates for improved stability and reusability from pineapple processing waste. Int. J. Biol. Macromol. 2020, 158, 318–326. [Google Scholar] [CrossRef]
- Hebbar, H.U.; Sumana, B.; Raghavarao, K.S.M.S. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresour. Technol. 2008, 99, 4896–4902. [Google Scholar] [CrossRef]
- Banerjee, S.; Ranganathan, V.; Patti, A.; Arora, A. Valorisation of pineapple wastes for food and therapeutic applications. Trends Food Sci. Technol. 2018, 82, 60–70. [Google Scholar] [CrossRef]
- Imandi, S.B.; Bandaru, V.V.R.; Somalanka, S.R.; Bandaru, S.R.; Garapati, H.R. Application of statistical experimental designs for the optimization of medium constituents for the production of citric acid from pineapple waste. Bioresour. Technol. 2008, 99, 4445–4450. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Patti, A.F.; Ranganathan, V.; Arora, A. Hemicellulose based biorefinery from pineapple peel waste: Xylan extraction and its conversion into xylooligosaccharides. Food Bioprod. Process. 2019, 117, 38–50. [Google Scholar] [CrossRef]
- Wan, J.; Guo, J.; Miao, Z.; Guo, X. Reverse micellar extraction of bromelain from pineapple peel–effect of surfactant structure. Food Chem. 2016, 197, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Pineapple wastes: A potential source for bromelain extraction. Food Bioprod. Process. 2012, 90, 385–391. [Google Scholar] [CrossRef]
- De Lencastre Novaes, L.C.; Jozala, A.F.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa Junior, A. Stability, purification, and applications of bromelain: A review. Biotechnol. Prog. 2016, 32, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Razali, R.; Fahrudin, F.A.; Subbiah, V.K.; Takano, K.; Budiman, C. Heterologous expression and catalytic properties of codon-optimized small-sized bromelain from MD2 pineapple. Molecules 2022, 27, 6031. [Google Scholar] [CrossRef] [PubMed]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef]
- Manzoor, Z.; Nawaz, A.; Mukhtar, H.; Haq, I. Bromelain: Methods of extraction, purification and therapeutic applications. Braz. Arch. Biol. Technol. 2016, 59, e16150010. [Google Scholar] [CrossRef]
- Zdrojewicz, Z.; Chorbińska, J.; Bieżyński, B.; Krajewski, P. Health-promoting properties of pineapple. Pediatr. Fam. Med. 2018, 14, 133–142. [Google Scholar] [CrossRef]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Extraction of bromelain from pineapple peels. Food Sci. Technol. Int. 2011, 17, 395–402. [Google Scholar] [CrossRef]
- Gil, L.S.; Maupoey, P.F. An integrated approach for pineapple waste valorisation. Bioethanol production and bromelain extraction from pineapple residues. J. Clean. Prod. 2018, 172, 1224–1231. [Google Scholar] [CrossRef]
- Hebbar, U.H.; Sumana, B.; Hemavathi, A.B.; Raghavarao, K.S.M.S. Separation and purification of bromelain by reverse micellar extraction coupled ultrafiltration and comparative studies with other methods. Food Bioprocess Technol. 2012, 5, 1010–1018. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review. part ii. TrAC Trends Anal. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Vasiljevic, T. Pineapple. In Valorization of Fruit Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Chapter 10; pp. 203–225. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of the Association of Official Analytical Chemists: Official Methods of Analysis of AOAC International. In Official Methods of Analysis of AOAC International, 21st ed.; AOAC: Washington DC, USA, 2019. [Google Scholar]
- De Ancos, B.; Sánchez-Moreno, C.; González-Aguilar, G.A. Pineapple composition and nutrition. In Handbook of Pineapple Technology: Production, Postharvest Science, Processing and Nutrition; Lobo, M.G., Paull, R.E., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 221–239. [Google Scholar] [CrossRef]
- Misran, E.; Idris, A.; Sarip, S.H.M.; Ya’akob, H. Properties of bromelain extract from different parts of the pineapple variety Morris. Biocatal. Agric. Biotechnol. 2019, 18, 101095. [Google Scholar] [CrossRef]
- Chaurasiya, R.S.; Hebbar, H.U. Extraction of bromelain from pineapple core and purification by RME and precipitation methods. Sep. Purif. Technol. 2013, 111, 90–97. [Google Scholar] [CrossRef]
- Nadzirah, K.Z.; Zainal, S.; Noriham, A.; Normah, I.; Roha, A.S. Physico-chemical properties of pineapple crown extract variety N36 and bromelain activity in different forms. APCBEE Procedia 2012, 4, 130–134. [Google Scholar] [CrossRef]
- Saptarini, N.M.; Rahayu, D.; Herawati, I.E. Antioxidant activity of crude bromelain of pineapple (Ananas comosus (L.) Merr) crown from Subang District, Indonesia. J. Pharm. Bioallied Sci. 2019, 11, S551–S555. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.S.; Dash, V.; Goyal, A.K.; Rath, G. Comparative study of extraction, purification and estimation of bromelain from stem and fruit of pineapple plant. Thai J. Pharm. Sci. 2010, 34, 67–76. [Google Scholar]
- Mohd Azmi, S.I.; Kumar, P.; Sharma, N.; Sazili, A.Q.; Lee, S.J.; Ismail-Fitry, M.R. Application of plant proteases in meat tenderization: Recent trends and future prospects. Foods 2023, 12, 1336. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a potential bioactive compound: A comprehensive overview from a pharmacological perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef]
- Fissore, A.; Marengo, M.; Santoro, V.; Grillo, G.; Oliaro-Bosso, S.; Cravotto, G.; Piaz, F.D.; Adinolfi, S. Extraction and characterization of bromelain from pineapple core: A strategy for pineapple waste valorization. Processes 2023, 11, 2064. [Google Scholar] [CrossRef]
- Rowan, A.D. Stem bromelain. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; Chapter 424; pp. 1871–1873. [Google Scholar] [CrossRef]
- Misran, E.; Idris, A.; Ya’akob, H. Bromelain extraction using single stage nanofiltration membrane process. J. Food Sci. Technol. 2023, 60, 315–327. [Google Scholar] [CrossRef]



| Moisture (g/100 g, Wet Basis) | TSS (° Bx) | pH | |
|---|---|---|---|
| Cores | 92.2 ± 0.4 a | 9.1 ± 0.1 a | 3.54 c |
| Peels | 92.3 ± 0.4 a | 7.4 ± 0.1 b | 3.72 b |
| Crowns | 18.7 ± 0.3 b | 1.9 ± 0.1 c | 4.24 a |
| Treatment | Enzymatic Activity | |
|---|---|---|
| Gelatin Digestion (GDU/g) | Casein Digestion (CDU/mg) | |
| Cores | 346 a | 115 |
| Peels | 92 a | N.A. |
| Crowns | N.D. | N.D. |
| No. | Treatment | Enzymatic Activity | ||
|---|---|---|---|---|
| Yield (%) | Gelatin Digestion (GDU/g) | Casein Digestion (CDU/mg) | ||
| 1 | Cores, no pH adjustment, non-dialyzed | 5.7 | 204 b | 59 c |
| 2 | Cores, no pH adjustment | 0.7 | 694 b | 124 b |
| 3 | Peels, no pH adjustment | 0.6 | 1179 a | 217 a |
| 4 | Commercial bromelain tablets (Control 1) | N.A. | 424 b | 66 c |
| 5 | Bromelain standard (1200 GDU/g) (Control 2) | N.A. | 553 b | 229 a |
| No. | Treatment | Enzymatic Activity | Crude Protein (%) | ||
|---|---|---|---|---|---|
| Yield (%) | Gelatin Digestion (GDU/g) | Casein Digestion (CDU/mg) | |||
| 1 | Stems | 0.3 | 23 b | 54 b | 10.5 b |
| 2 | Basal stems | 0.7 | 2909 a | 717 a | 27.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarelli, P.G.; Martinez, B.; Nakamura, T.; Mis Solval, K. Enhancing Bromelain Recovery from Pineapple By-Products: A Sustainable Approach for Value Addition and Waste Reduction. Foods 2024, 13, 589. https://doi.org/10.3390/foods13040589
Chiarelli PG, Martinez B, Nakamura T, Mis Solval K. Enhancing Bromelain Recovery from Pineapple By-Products: A Sustainable Approach for Value Addition and Waste Reduction. Foods. 2024; 13(4):589. https://doi.org/10.3390/foods13040589
Chicago/Turabian StyleChiarelli, Peter G., Bismarck Martinez, Takashi Nakamura, and Kevin Mis Solval. 2024. "Enhancing Bromelain Recovery from Pineapple By-Products: A Sustainable Approach for Value Addition and Waste Reduction" Foods 13, no. 4: 589. https://doi.org/10.3390/foods13040589
APA StyleChiarelli, P. G., Martinez, B., Nakamura, T., & Mis Solval, K. (2024). Enhancing Bromelain Recovery from Pineapple By-Products: A Sustainable Approach for Value Addition and Waste Reduction. Foods, 13(4), 589. https://doi.org/10.3390/foods13040589





