Sea Buckthorn Flavonoid Extracted by High Hydrostatic Pressure Inhibited IgE-Stimulated Mast Cell Activation through the Mitogen-Activated Protein Kinase Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Optimization of High Hydrostatic Pressure Extraction
2.3. Purification and HPLC Analysis of Sea Buckthorn
2.4. Mediator and Cytokine Release Assays of RBL-2H3 Cells
2.5. Measurement and Observation of Intracellular Ca2+ Levels ([Ca2+]i)
2.6. Western Bolt
2.7. Statistical Analysis
3. Results
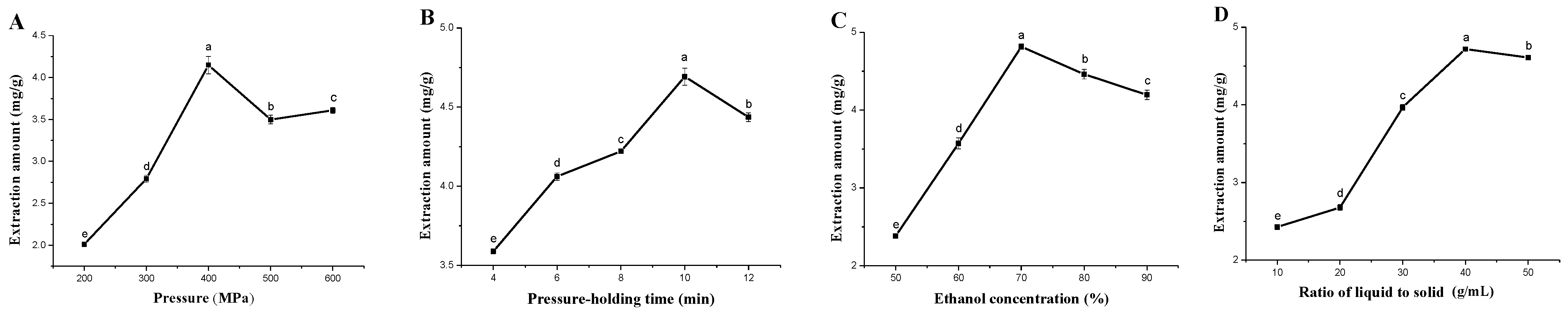
3.1. Effect of HHP Condition on SBF Amount
3.2. Optimization of the HHP Condition and Validation of the Model
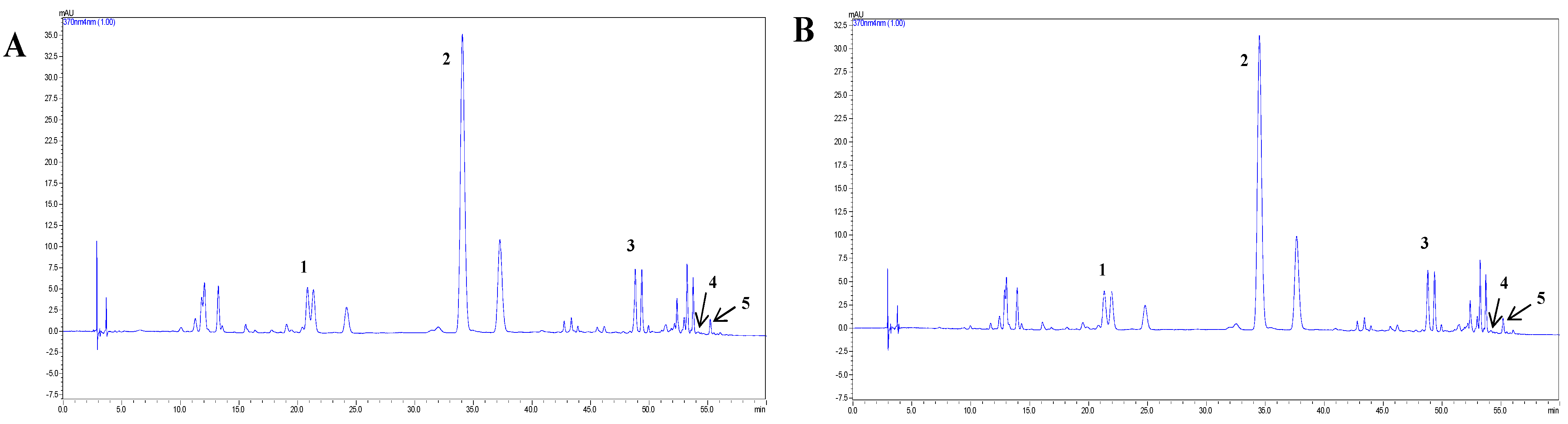
3.3. Identification and Quantified of SBF and PSBF
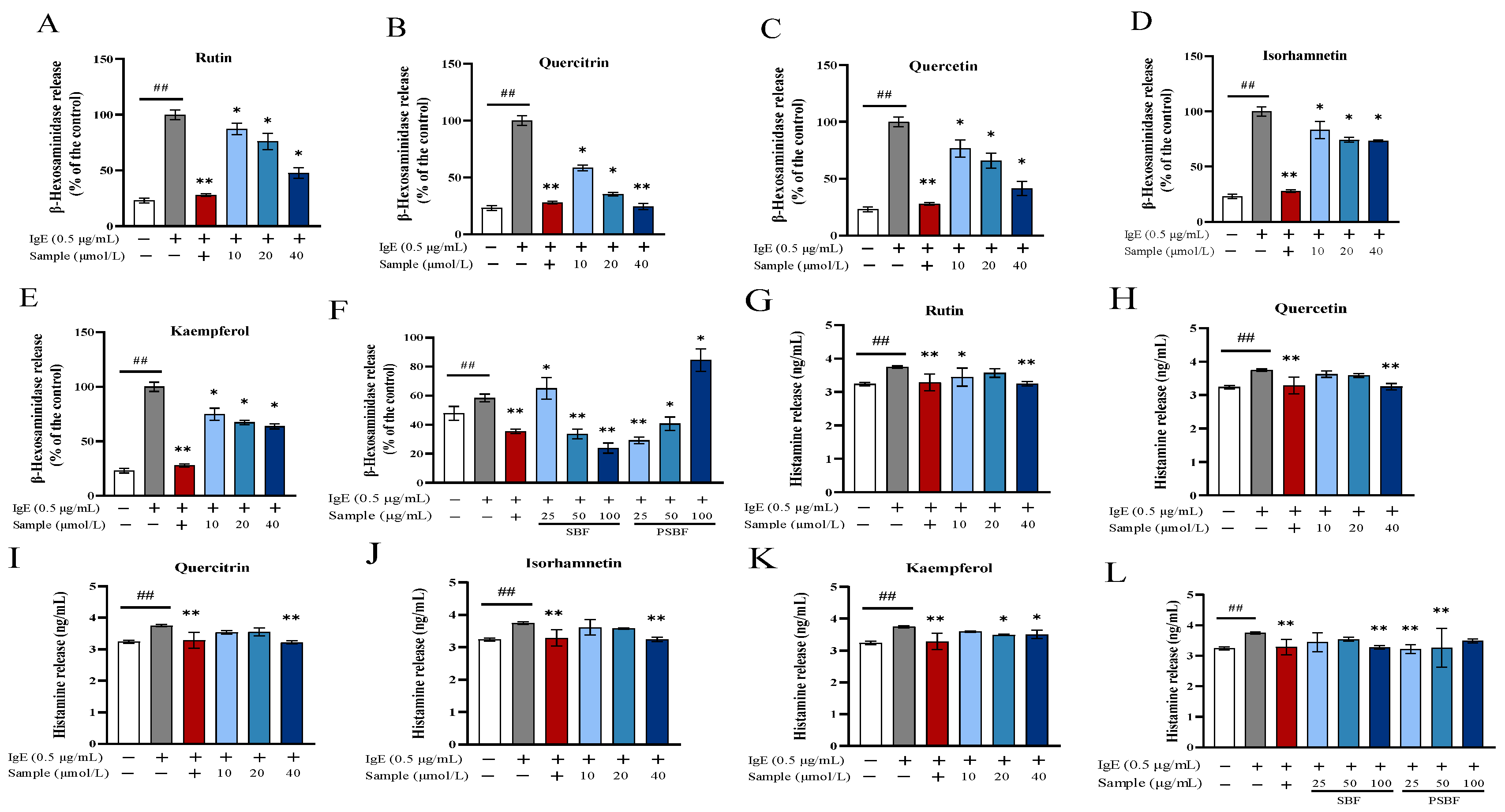
3.4. SBF, PSBF, and Its Active Compounds Inhibit Degranulation in RBL-2H3 Cells
3.5. SBF, PSBF, and Its Active Compounds Regulate Release of IL-4 and IFN-γ
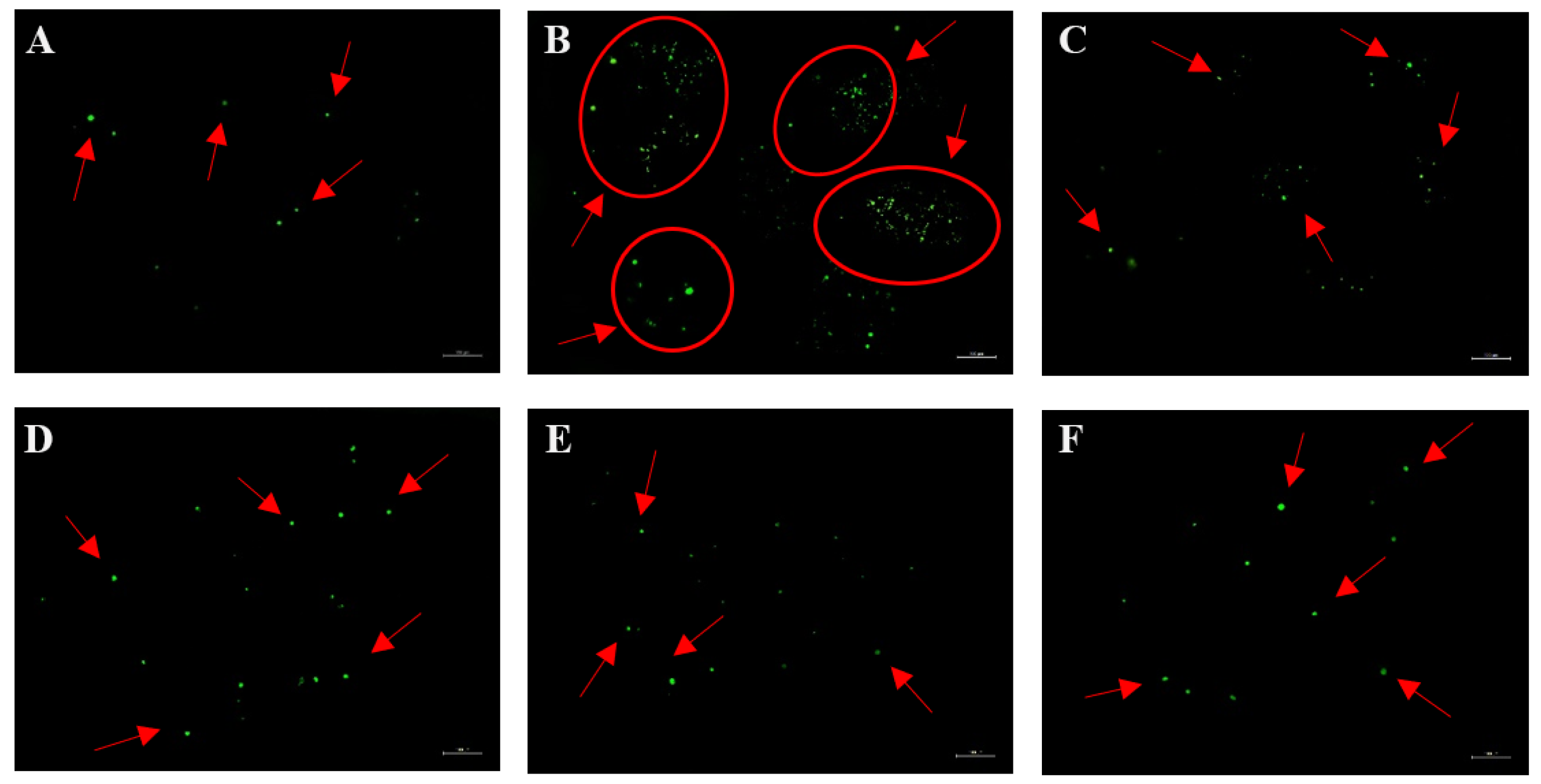
3.6. SBF, PSBF, and Its Active Compounds Inhibit Intracellular [Ca2+]i Influx
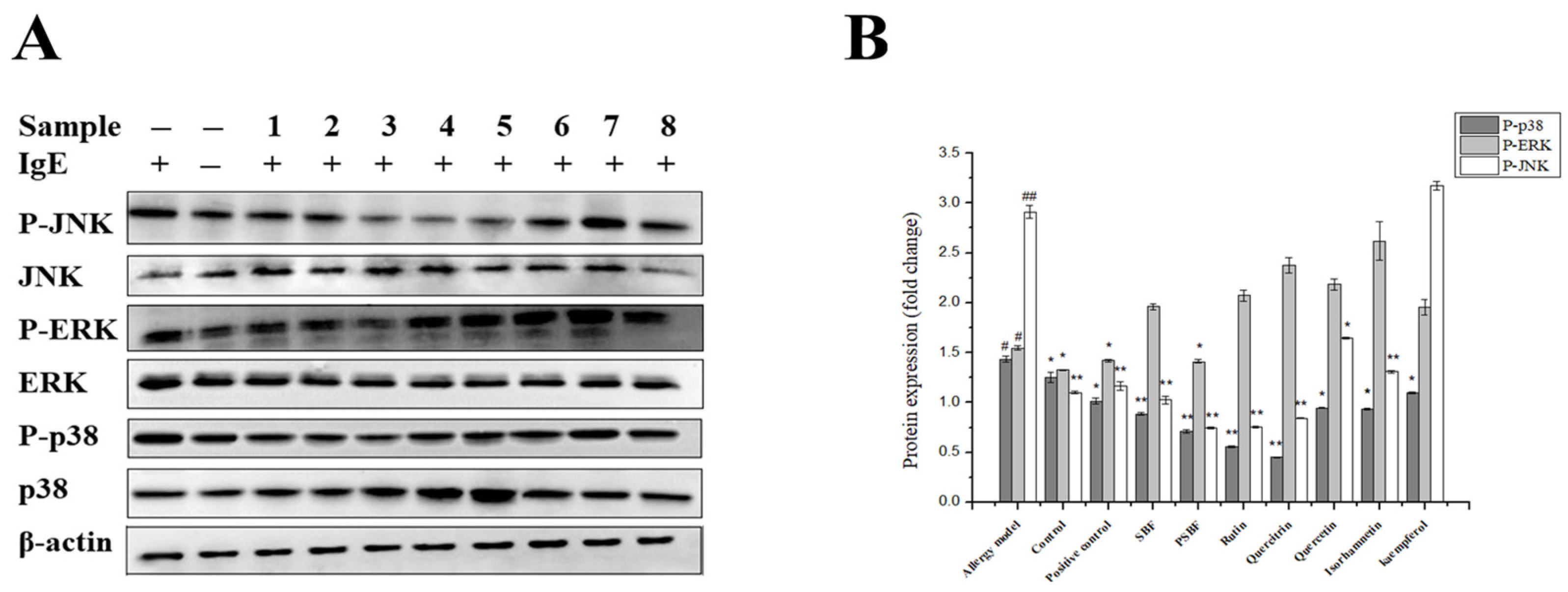
3.7. SBF, PSBF, and Its Active Compounds Inhibit the MAPK Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Dang, E.V. Novel mechanistic insights underlying fungal allergic inflammation. PLoS Pathog. 2023, 19, e1011623. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Lin, H.; Xu, L.; Li, S.; Costa, J.; Mafra, I.; Chen, G.; Gao, X.; Li, Z. Immunomodulatory Effect of Laccase/Caffeic Acid and Transglutaminase in Alleviating Shrimp Tropomyosin (Mete 1) Allergenicity. J. Agric. Food Chem. 2020, 68, 7765–7778. [Google Scholar] [CrossRef] [PubMed]
- Do, H.J.; Oh, T.W.; Park, K.I. Ethanol Extract of Sesamum indicum Linn. Inhibits FcepsilonRI-Mediated Allergic Reaction via Regulation of Lyn/Syk and Fyn Signaling Pathways in Rat Basophilic Leukemic RBL-2H3 Mast Cells. Mediat. Inflamm. 2019, 2019, 5914396. [Google Scholar] [CrossRef]
- Hussain, M.; Borcard, L.; Walsh, K.P.; Rodriguez, M.P.; Mueller, C.; Kim, B.S.; Kubo, M.; Noti, M. Basophil-derived IL-4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. J. Allergy Clin. Immunol. 2018, 141, 223–234. [Google Scholar] [CrossRef]
- Kim, M.; Lim, S.J.; Kang, S.W.; Um, B.H.; Nho, C.W. Aceriphyllum rossii Extract and Its Active Compounds, Quercetin and Kaempferol Inhibit IgE-mediated Mast Cell Activation and Passive Cutaneous Anaphylaxis. J. Agric. Food Chem. 2014, 62, 3750–3758. [Google Scholar] [CrossRef] [PubMed]
- Han, E.H.; Hwang, Y.P.; Kim, H.G.; Park, J.H.; Choi, J.H.; Im, J.H.; Khanal, T.; Park, B.H.; Yang, J.H.; Choi, J.M.; et al. Ethyl acetate extract of Psidium guajava inhibits IgE-mediated allergic responses by blocking FcεRI signaling. Food Chem. Toxicolo. 2011, 49, 100–108. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.; Liu, L.; Zou, K. Anti-allergic activity of emodin on IgE-mediated activation in RBL-2H3 cells. Pharmacol. Rep. 2012, 64, 1216–1222. [Google Scholar] [CrossRef]
- Ma, J.; Tong, P.Y.; Chen, Y.J.; Wang, Y.; Ren, H.; Gao, Z.P.; Yue, T.; Long, F.Y. The inhibition of pectin oligosaccharides on degranulation of RBL-2H3 cells from apple pectin with high hydrostatic pressure assisted enzyme treatment. Food Chem. 2022, 371, 131097. [Google Scholar] [CrossRef]
- Kumari, P.; Gaur, S.S.; Tiwari, R.K. Banana and its by-products: A comprehensive review on its nutritional composition and pharmacological benefits. eFood. 2023, 4, e110. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit Rev Food Sci. 2022, 62, 7730–7742. [Google Scholar] [CrossRef]
- Mulati, A.; Ma, S.; Zhang, H.; Ren, B.; Zhao, B.; Wang, L.; Liu, X.; Zhao, T.; Kamanova, S.; Sair, A.T.; et al. Sea-Buckthorn Flavonoids Alleviate High-Fat and High-Fructose Diet-Induced Cognitive Impairment by Inhibiting Insulin Resistance and Neuroinflammation. J. Agric. Food Chem. 2020, 68, 5835–5846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Guan, H.; Liu, D.; Wu, X.; Fan, M.; Han, J. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct. 2017, 8, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, X.; Liu, F.; Zhang, J.; Zhang, X.; Liu, J.; Li, S.; Wang, D.; Guan, H.; Hou, D. Total flavonoids of sea buckthorn (Hippophae rhamnoides L.) improve MC903-induced atopic dermatitis-like lesions. J. Ethnopharmacol. 2022, 292, 115195. [Google Scholar] [CrossRef]
- Ren, Q.; Li, X.; Li, Q.; Yang, H.; Wang, H.; Zhang, H.; Zhao, L.; Jiang, Y.S.; Meng, X.; Zhang, Y.; et al. Total flavonoids from sea buckthorn ameliorates lipopolysaccharide/cigarette smoke-induced airway inflammation. Phytother Res. 2019, 33, 2102–2117. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov.Today 2016, 21, 632–639. [Google Scholar] [CrossRef]
- Lee, E.-J.; Ji, G.-E.; Sung, M.-K. Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells. Inflamm. Res. 2010, 59, 847–850. [Google Scholar] [CrossRef]
- Wu, S.S.; Zhang, R.; Liu, Y.; Gao, J.; Wu, Y.; Tu, C.; Chen, H.; Yuan, J. In Vitro Effect of Flavonoids on Basophils Degranulation and Intestinal Epithelial Barrier Damage Induced by ω-5 Gliadin-Derived Peptide. Foods 2022, 11, 3857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, P.; Li, L.; Huang, Y.; Pu, Y.; Hou, X.; Song, L. Identification and Antioxidant Activity of Flavonoids Extracted from Xinjiang Jujube (Ziziphus jujube Mill.) Leaves with Ultra-High Pressure Extraction Technology. Molecules 2018, 24, 122. [Google Scholar] [CrossRef]
- Xi, J.; He, L.; Yan, L. Kinetic modeling of pressure-assisted solvent extraction of polyphenols from green tea in comparison with the conventional extraction. Food Chem. 2015, 166, 287–291. [Google Scholar] [CrossRef]
- Xi, J.; Luo, S. Pressure-enhanced solid–liquid extraction of rutin from Chinese scholar-tree flower: Kinetic modeling of influential factors. Sep. Purif. Technol. 2015, 156, 809–816. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Chen, J.B.; Zhao, C.N.; Liu, X.J.; Chen, Y.Y.; Liang, J.J.; Cao, J.P.; Wang, Y.; Sun, C.D. Advances in extraction and purification of citrus flavonoids. Food Front. 2023, 4, 750–781. [Google Scholar] [CrossRef]
- Fu, S.; Ni, S.; Wang, D.; Fu, M.; Hong, T. Berberine suppresses mast cell-mediated allergic responses via regulating FcɛRI-mediated and MAPK signaling. Int. Immunopharmacol. 2019, 71, 1–6. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.Y.; Randy, A.; Lim, S.J.; Dorjsembe, B.; Nho, C.W. Inhibitory effect of the Larix sibirica and its various flavonoids on the IgE-stimulated mast cell activation and anaphylaxis. J. Funct. Food 2016, 27, 631–644. [Google Scholar] [CrossRef]
- Antonio, M.; Juan, M.; Mohsen, G.; Buenaventura, G.; Felipe, P.; Carmen, L. Effect of HHP and UHPH High-Pressure Techniques on the Extraction and Stability of Grape and Other Fruit Anthocyanins. Antioxidants 2023, 12, 1746. [Google Scholar]
- Ma, X.; Laaksonen, O.; Zheng, J.; Yang, W.; Trépanier, M.; Kallio, H.; Yang, B. Flavonol glycosides in berries of two major subspecies of sea buckthorn (Hippophaë rhamnoides L.) and influence of growth sites. Food Chem. 2016, 200, 189–198. [Google Scholar] [CrossRef]
- Nada, O. Allergic Inflammation: Effect of Propolis and Its Flavonoids. Molecules 2022, 27, 6694. [Google Scholar]
- Cruz, E.A.; Da -Silva, S.A.; Muzitano, M.F.; Silva, P.M.; Costa, S.S.; Rossi -Bergmann, B. Immunomodulatory pretreatment with Kalanchoe pinnata extract and its quercitrin flavonoid effectively protects mice against fatal anaphylactic shock. Int. Immunopharmacol. 2008, 8, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Lin, H.; Li, Z.; Xu, L.; Qazi, I.M.; Luo, C.; Gao, X.; Khan, M.U.; Iqbal, A.; Guo, Y.; et al. Tyrosinase/caffeic acid cross-linking alleviated shrimp (Metapenaeus ensis) tropomyosin-induced allergic responses by modulating the Th1/Th2 immunobalance. Food Chem. 2021, 340, 127948. [Google Scholar] [CrossRef]
- Jiang, T.; He, F.; Han, S.; Chen, C.; Zhang, Y.; Che, H. Characterization of cAMP as an anti-allergic functional factor in Chinese jujube (Ziziphus jujuba Mill.). J. Funct Food. 2019, 60, 103414. [Google Scholar] [CrossRef]
- Sang, C.W.; Bai, Q.; Feng, X.P.; Wu, C.Y.; Liu, Y.; Gao, Z.P.; Long, F.Y. Optimized Extraction of cAMP From Jujube by Ultra-High Pressure Technology and the Anti-allergic Effect for Peanut Allergy Mouse. Front. Nutr. 2022, 9, 862900. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Guo, X.; Han, D.; Xi, H.; Rao, L.; Liao, X.; Hu, X.; Wu, J. Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: A comparison. Carbohydr. Polym. 2012, 88, 441–448. [Google Scholar] [CrossRef]
- Wang, R.; Chang, Y.; Tan, Z.; Li, F. A novel combined process for extracting, separating and recovering flavonoids from flos sophorae immaturus. Sep. Purif. Technol. 2017, 172, 422–432. [Google Scholar] [CrossRef]
- Gabriele, R.; Luigi, L.; José, G.E. Cellular assays combined with metabolomics highlight the dual face of phenolics: From high permeability to morphological cell damage. Food Chem. 2024, 430, 137081. [Google Scholar]
- Sirine, A.; Emilie, D.; Eric, L. Optimization of supercritical fluid extraction of polar flavonoids from Robinia pseudoacacia L. heartwood. J. CO2 Util. 2023, 70, 102440. [Google Scholar]
- Li, D.; Sun, C.; Yang, J.; Ma, X.; Jiang, Y.; Qiu, S.; Wang, G. Ionic Liquid-Microwave-Based Extraction of Biflavonoids from Selaginella sinensis. Molecules 2019, 13, 2507. [Google Scholar] [CrossRef] [PubMed]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, Y.M.; Han, Y.Y.; Yang, B.; Lin, H.; Li, Z.X. The natural substances with anti-allergic properties in food allergy. Trends Food Sci. Technol. 2022, 128, 53–67. [Google Scholar] [CrossRef]
- Itoh, T.; Ohguchi, K.; Nakajima, C.; Oyama, M.; Iinuma, M.; Nozawa, Y.; Akao, Y.; Ito, M. Inhibitory effects of flavonoid glycosides isolated from the peel of Japanese persimmon (Diospyros kaki Fuyu) on antigen-stimulated degranulation in rat basophilic leukaemia RBL-2H3 cells. Food Chem. 2011, 126, 289–294. [Google Scholar] [CrossRef]
- Beáta, Č.; Beáta, H.; Vladimíra, T.; Anna, B. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar]
- Park, S.H.; Park, E.K.; Kim, D.H. Passive Cutaneous Anaphylaxis-Inhibitory Activity of Flavanones from Citrus unshiu and Poncirus trifoliata. Planta Med. 2005, 71, 24–27. [Google Scholar] [CrossRef]
- Abd Rani, N.Z.; Kumolosasi, E.; Jasamai, M.; Jamal, J.A.; Lam, K.W.; Husain, K. In vitro anti-allergic activity of Moringa oleifera Lam. extracts and their isolated compounds. BMC Complement. Med. Ther. 2019, 19, 361. [Google Scholar] [CrossRef]
- Romagnani, S. Immunologic influences on allergy and the TH1/TH2 balance. J. Allergy Clin. Immunol. 2004, 113, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Meng, H.C.; Tsai, W.J. Regulation of cell proliferation, inflammatory cytokine production and calcium mobilization in primary human T lymphocytes by emodin from Polygonum hypoleucum Ohwi. Inflamm. Res. 2001, 50, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Lee, S.R.; Jeong, Y.J.; Park, D.W.; Cho, Y.M.; Joo, H.M.; Kim, I.; Seu, Y.B.; Sohn, E.H.; Kang, S.C. Antiallergic Activity of Ethanol Extracts of Arctium lappa L. Undried Roots and Its Active Compound, Oleamide, in Regulating FcεRI-Mediated and MAPK Signaling in RBL-2H3 Cells. J. Agric. Food Chem. 2016, 64, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Wei, J.; Li, X.; Jin, Y.; Shi, X. Inhibitory activity of narirutin on RBL-2H3 cells degranulation. Immunopharmacol. Immunotoxicol. 2021, 43, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Miguel, R.; Beatriz, V.; Milagros, G.; Marta, T.; Julio, G.; Francisco, P.; Juan, D. Protective vascular effects of quercitrin in acute TNBS-colitis in rats: The role of nitric oxide. Food Funct. 2017, 8, 2702–2711. [Google Scholar]
- Xing, L.; Ni, H.; Wang, Y. Quercitrin attenuates osteoporosis in ovariectomized rats by regulating mitogen-activated protein kinase (MAPK) signaling pathways. Biomed. Pharmacother. 2017, 89, 1136–1141. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, C.; Tian, Y.; Zeng, M.; Wang, Z.; Chen, Q.; Chen, J.; Hen, Z. Miquelianin, a main functional flavonoid of lotus leaf, induces thermogenic signature via p38-PINK1-PARKIN-mediated mitophagy and mimicking NRF2 signaling during brown adipocyte differentiation. Food Front. 2023, 4, 1831–1844. [Google Scholar] [CrossRef]
- Chung, M.Y.; Shin, H.S.; Choi, D.W.; Shon, D.H. Citrus Tachibana Leaf Extract Mitigates Symptoms of Food Allergy by Inhibiting Th2-Associated Responses. J. Food Sci. 2016, 81, H1537–H1545. [Google Scholar] [CrossRef]
- Shingnaisui, K.; Dey, T.; Manna, P.; Kalita, J. Therapeutic potentials of Houttuynia cordata Thunb. against inflammation and oxidative stress: A review. J. Ethnopharmacol. 2018, 220, 35–43. [Google Scholar] [CrossRef] [PubMed]



| Code | Factor | |||
|---|---|---|---|---|
| A Pressure (MPa) | B Pressure-Holding Time (min) | C Ethanol Concentration (%) | D Liquid-to-Solid Ratio (mL/g) | |
| −1 | 300 | 8 | 60 | 30 |
| 0 | 400 | 10 | 70 | 40 |
| 1 | 500 | 12 | 80 | 50 |
| Compound | Concentration (μg/mL) | |
|---|---|---|
| SBF | PSBF | |
| Rutin | 123.72 ± 8.02 | 521.83 ± 18.73 |
| Quercitrin | 945.28 ± 20.32 | 4105.66 ± 192.15 |
| Quercetin | 23.23 ± 1.35 | 100.30 ± 8.34 |
| Isorhamnetin | 15.15 ± 0.87 | 75.10 ± 6.12 |
| Kaempferol | 0.82 ± 0.01 | 3.45 ± 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Feng, X.; Li, X.; Gao, Z.; Wang, Z.; Ren, G.; Long, F. Sea Buckthorn Flavonoid Extracted by High Hydrostatic Pressure Inhibited IgE-Stimulated Mast Cell Activation through the Mitogen-Activated Protein Kinase Signaling Pathway. Foods 2024, 13, 560. https://doi.org/10.3390/foods13040560
Yan Z, Feng X, Li X, Gao Z, Wang Z, Ren G, Long F. Sea Buckthorn Flavonoid Extracted by High Hydrostatic Pressure Inhibited IgE-Stimulated Mast Cell Activation through the Mitogen-Activated Protein Kinase Signaling Pathway. Foods. 2024; 13(4):560. https://doi.org/10.3390/foods13040560
Chicago/Turabian StyleYan, Zhuomin, Xiaoping Feng, Xinian Li, Zhenpeng Gao, Zhouli Wang, Guangxu Ren, and Fangyu Long. 2024. "Sea Buckthorn Flavonoid Extracted by High Hydrostatic Pressure Inhibited IgE-Stimulated Mast Cell Activation through the Mitogen-Activated Protein Kinase Signaling Pathway" Foods 13, no. 4: 560. https://doi.org/10.3390/foods13040560
APA StyleYan, Z., Feng, X., Li, X., Gao, Z., Wang, Z., Ren, G., & Long, F. (2024). Sea Buckthorn Flavonoid Extracted by High Hydrostatic Pressure Inhibited IgE-Stimulated Mast Cell Activation through the Mitogen-Activated Protein Kinase Signaling Pathway. Foods, 13(4), 560. https://doi.org/10.3390/foods13040560






