Effects of Quality Enhancement of Frozen Tuna Fillets Using Ultrasound-Assisted Salting: Physicochemical Properties, Histology, and Proteomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Salting and Ultrasound Pretreatment
2.3. Measurement of Physicochemical Indicators
2.3.1. Yield, Water Content, and Salt Content
2.3.2. WHC
Centrifuging Loss (CL)
Thawing Loss (TL)
2.3.3. Texture Properties and Color
2.3.4. TBARS
2.3.5. TVBN
2.4. LF-NMR and Magnetic Resonance Imaging (MRI)
2.5. Microstructural Observation
2.5.1. Light Microscopy (LM)
2.5.2. Scanning Electron Microscopy (SEM)
2.6. Proteomic Analysis
2.6.1. Protein Extraction and Digestion
2.6.2. LC-MS/MS Analysis
2.6.3. Protein Identification and Bioinformatics Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes in Physicochemical Indicators of SS and UAS
3.1.1. Changes in Quality-Related Indicators of SS and UAS
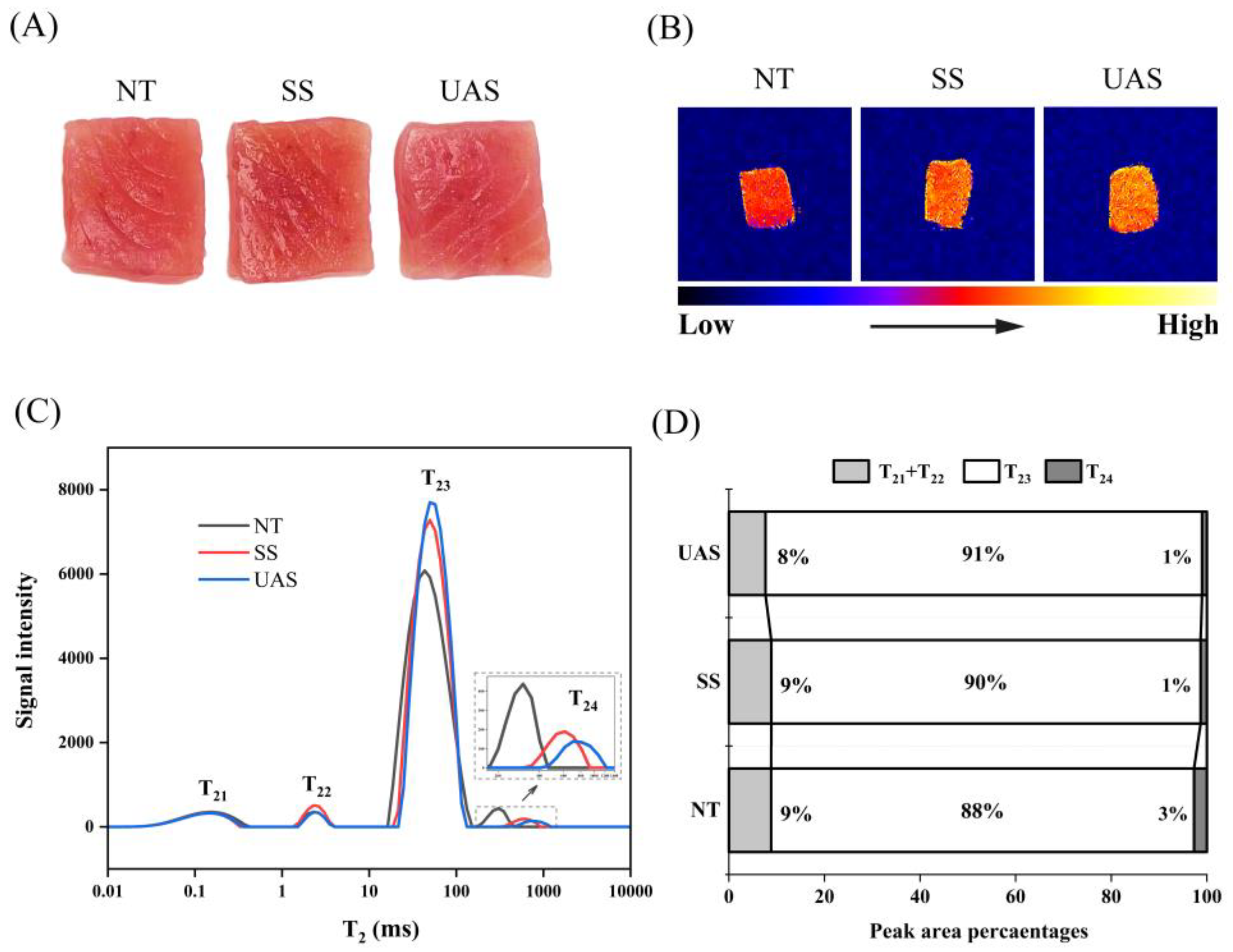
3.1.2. Changes in LF-NMR and MRI of SS and UAS
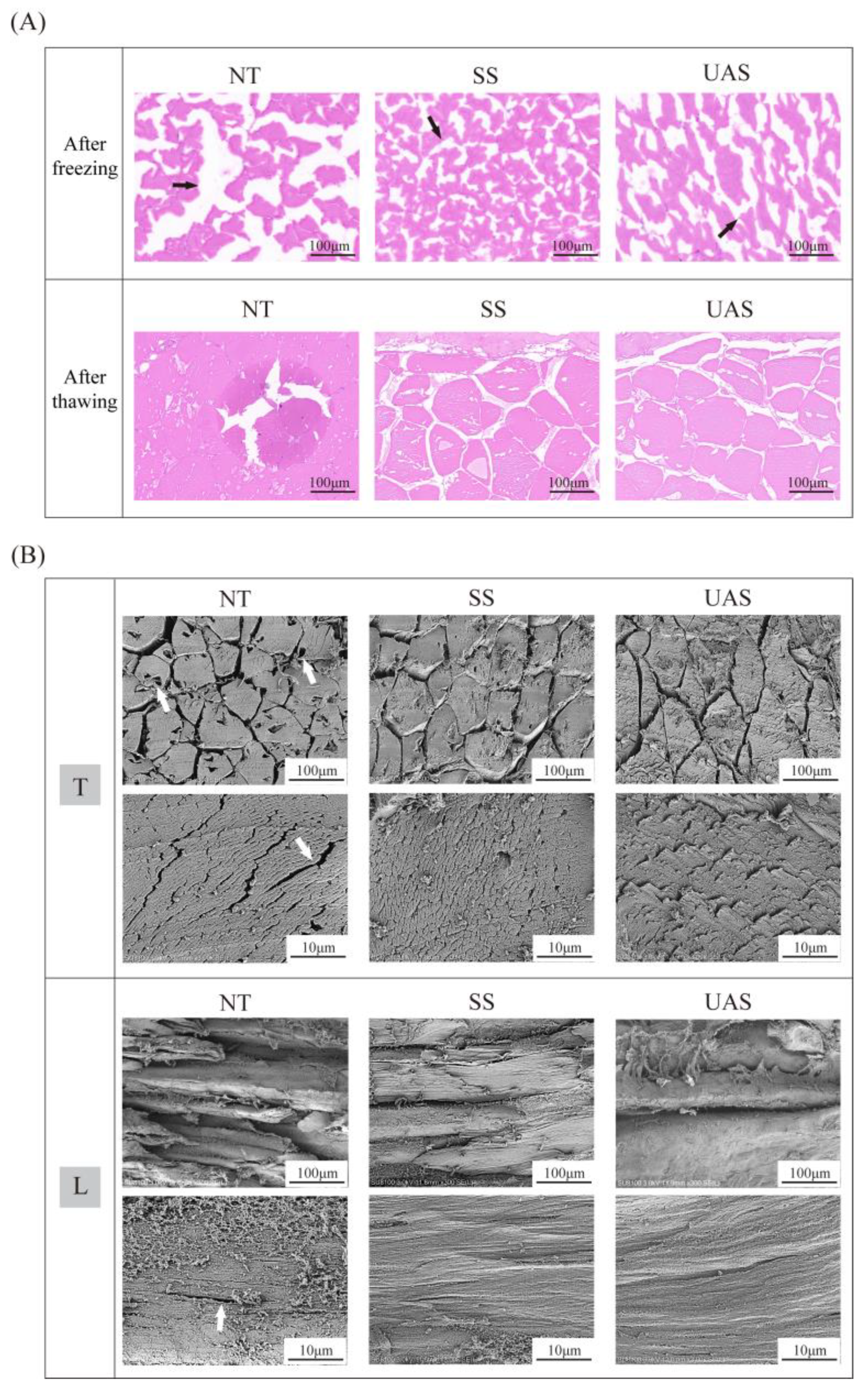
3.2. Changes in Histological Observations of SS and UAS
3.3. Changes in Proteomics Analyses of SS and UAS
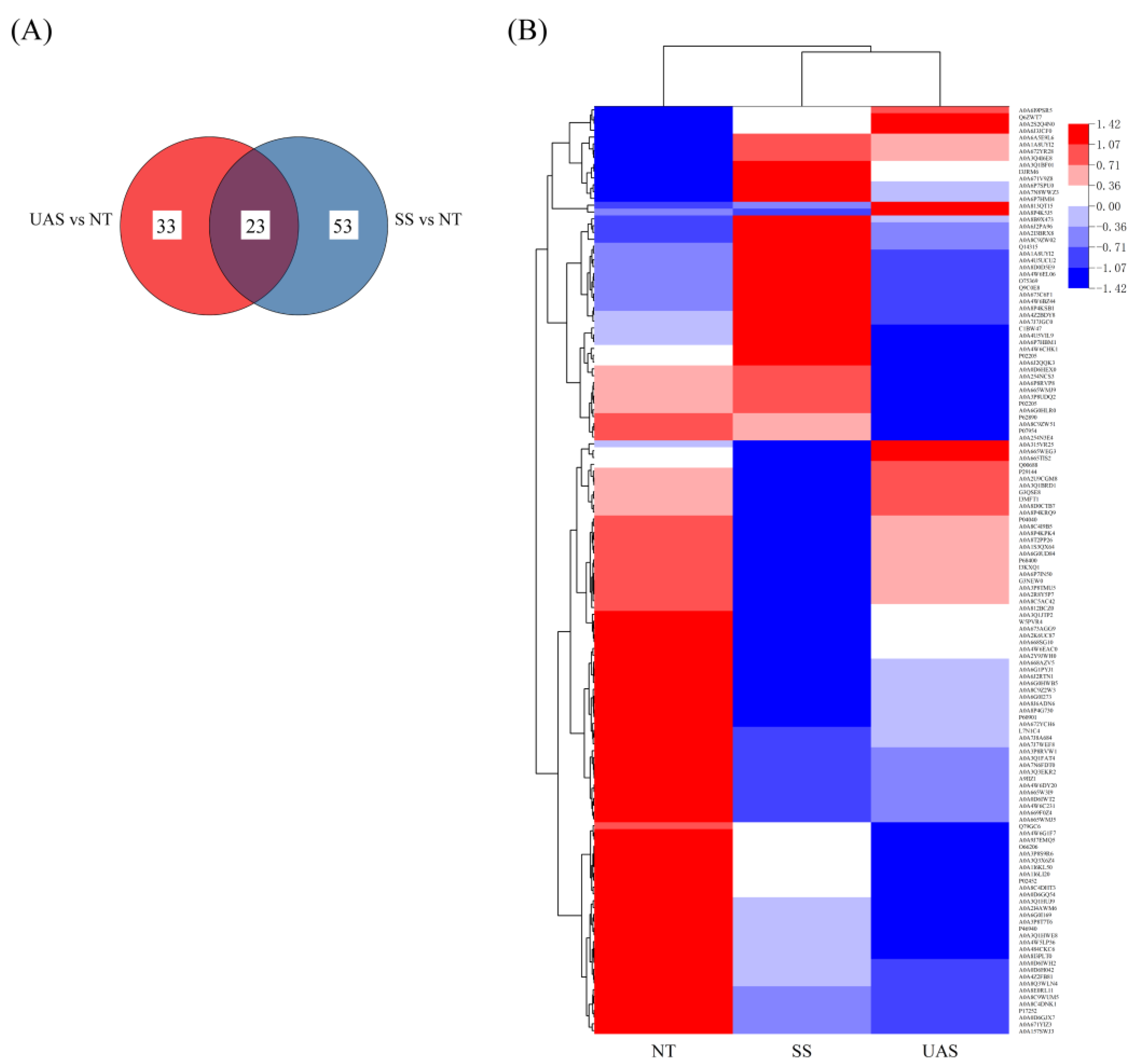
3.3.1. Identification of Proteins
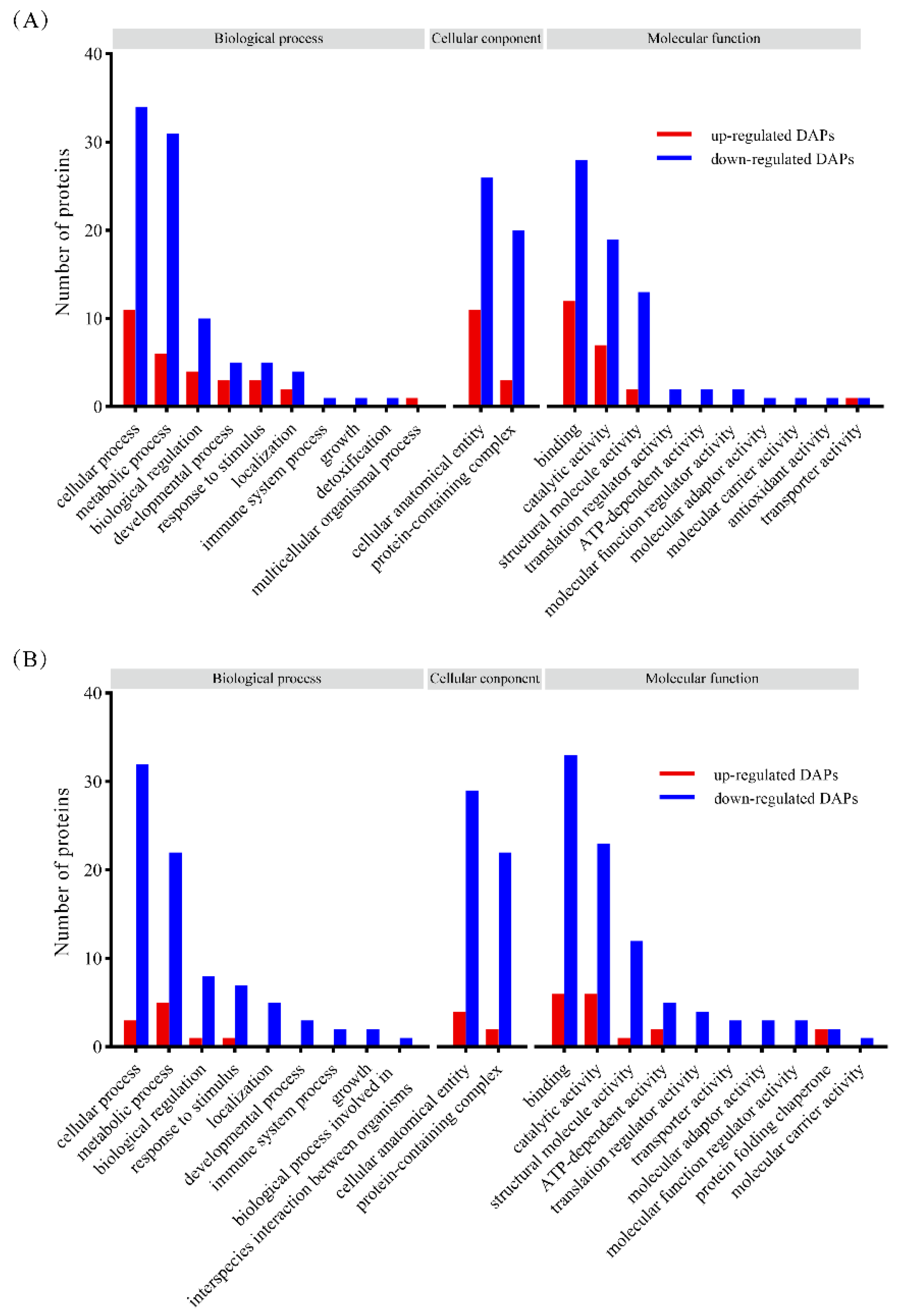
3.3.2. GO Annotation Analysis
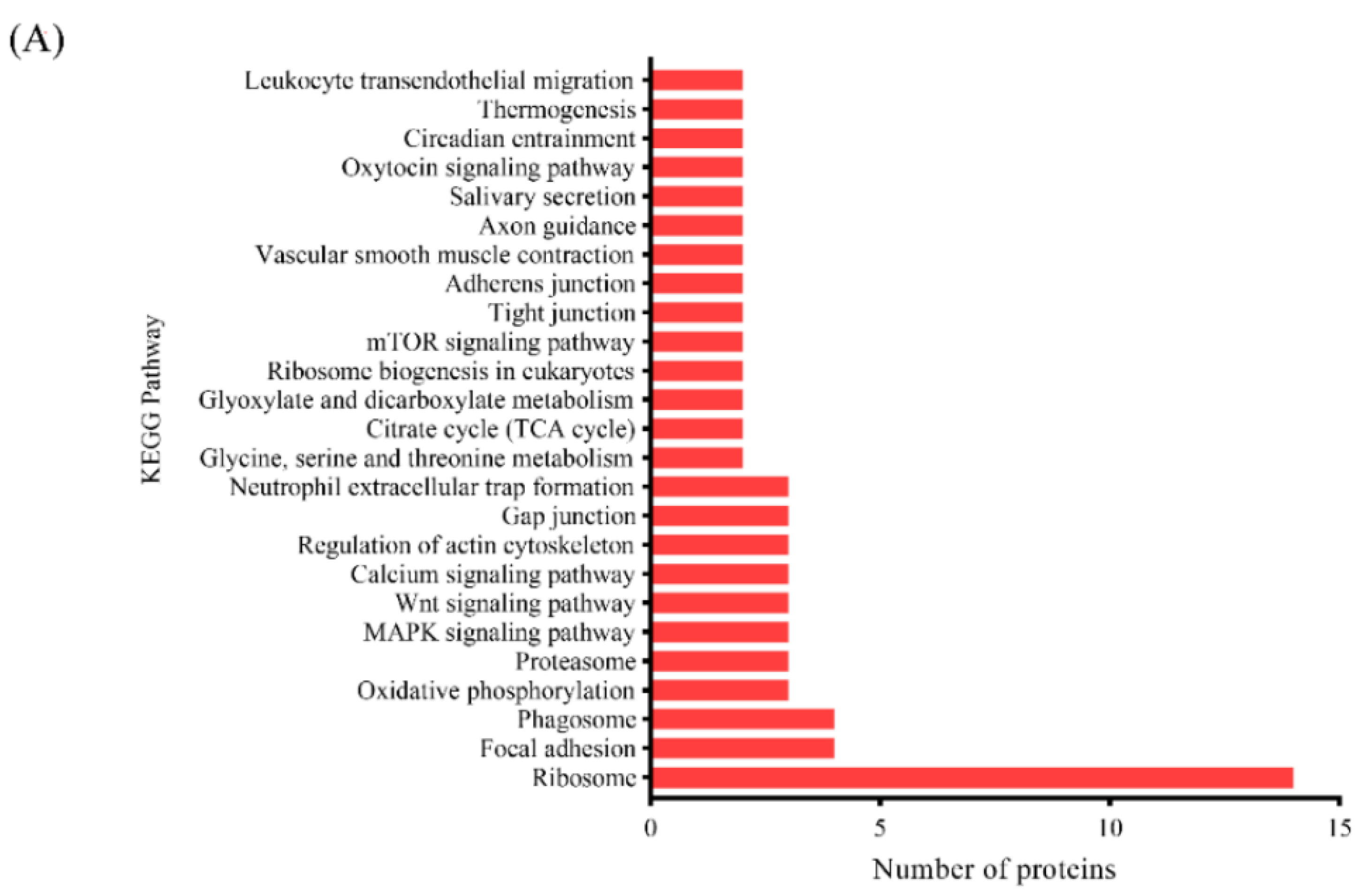
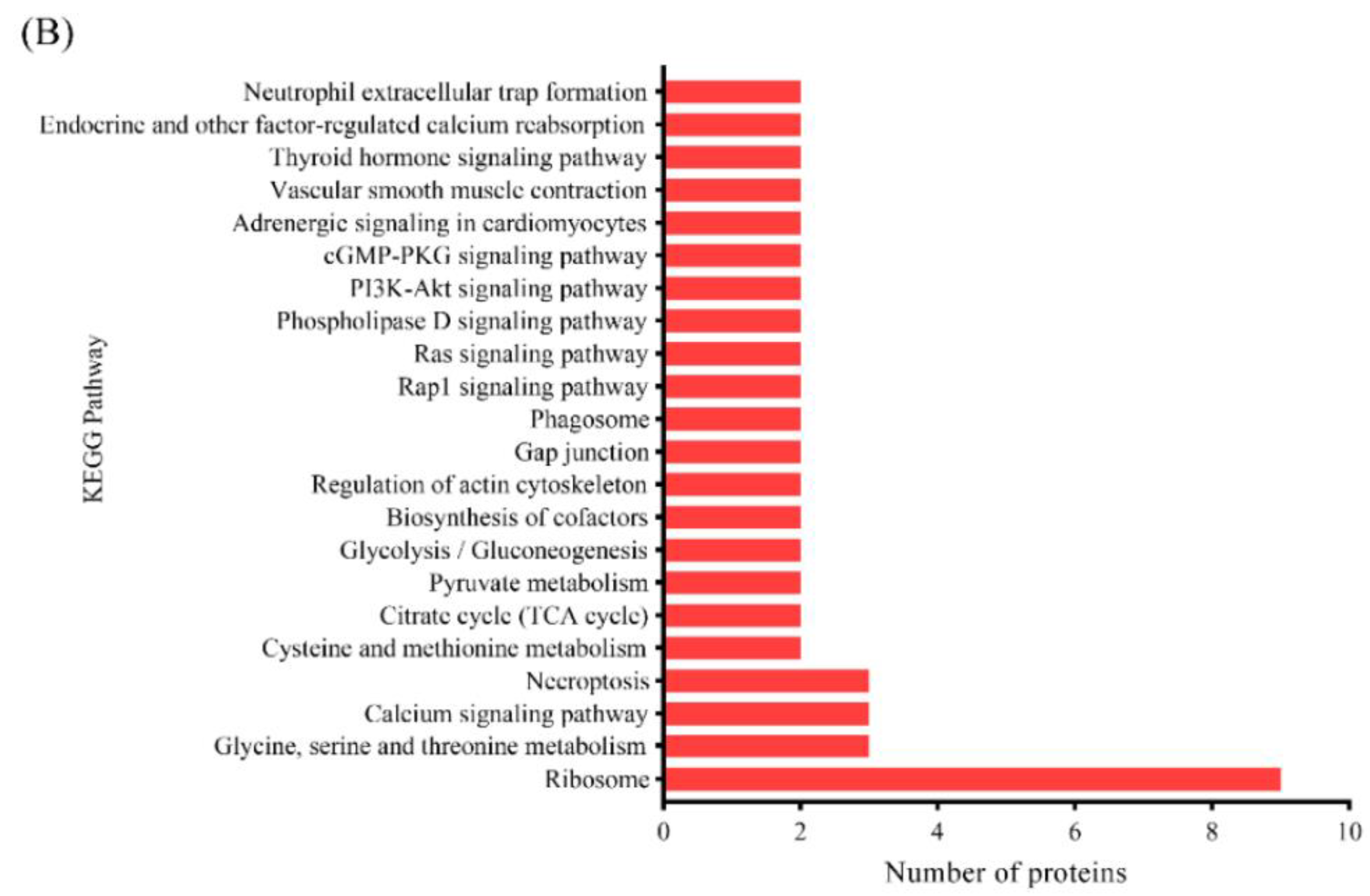
3.3.3. KEGG Pathway Analysis
3.3.4. Analysis of DAPs Associated with Quality Changes
Changes in DAPs Associated with Protein Structure
Changes in DAPs Associated with Energy Metabolism
Changes in DAPs Associated with Protein Turnover
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FishStat. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/fishery/statistics-query/en/capture/capture_quantity (accessed on 20 December 2023).
- Jiang, Q.Q.; Nakazawa, N.; Hu, Y.Q.; Osako, K.; Okazaki, E. Microstructural modification and its effect on the quality attributes of frozen-thawed bigeye tuna (Thunnus obesus) meat during salting. LWT 2019, 100, 213–219. [Google Scholar] [CrossRef]
- Yao, Y.; Han, R.; Li, F.; Tang, J.; Jiao, Y. Mass transfer enhancement of tuna brining with different NaCl concentrations assisted by ultrasound. Ultrason. Sonochem. 2022, 85, 105989. [Google Scholar] [CrossRef]
- Tan, M.; Ye, J.; Chu, Y.; Xie, J. The effects of ice crystal on water properties and protein stability of large yellow croaker (Pseudosciaena crocea). Int. J. Refrig. 2021, 130, 242–252. [Google Scholar] [CrossRef]
- Ge, L.H.; Xu, Y.S.; Xia, W.S.; Zhao, N.; Jiang, Q.X. Contribution of myofibril filament disassembly to textural deterioration of ice-stored grass carp fillet: Significance of endogenous proteolytic activity, loss of heat shock protein and dephosphorylation of myosin light chain. Food Chem. 2018, 269, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Puolanne, E.; Halonen, M. Theoretical aspects of water-holding in meat. Meat Sci. 2010, 86, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Petit, G.; Jury, V.; de Lamballerie, M.; Duranton, F.; Pottier, L.; Martin, J.L. Salt Intake from Processed Meat Products: Benefits, Risks and Evolving Practices. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1453–1473. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, L.; Wu, Y.; Chen, S.; Zhao, Y.; Cai, Q.; Wang, Y. Ultrasound improves the low-sodium salt curing of sea bass: Insights into the effects of ultrasound on texture, microstructure, and flavor characteristics. Ultrason. Sonochem. 2023, 100, 106597. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Jiang, Q.; Jiang, X.; Feng, Q.; Shi, W. Changes in physicochemical properties and myofibrillar protein properties in grass carp salted by brining and injection. Int. J. Food Sci. Technol. 2021, 56, 5674–5687. [Google Scholar] [CrossRef]
- Jin, G.F.; Liu, Y.Y.; Zhang, Y.; Li, C.L.; He, L.C.; Zhang, Y.M.; Wang, Y.; Cao, J.X. Underlying formation mechanisms of ultrasound-assisted brined porcine meat: The role of physicochemical modification, myofiber fragmentation and histological organization. Ultrason. Sonochem. 2023, 94, 106318. [Google Scholar] [CrossRef]
- Kang, D.C.; Gao, X.Q.; Ge, Q.F.; Zhou, G.H.; Zhang, W.G. Effects of ultrasound on the beef structure and water distribution during curing through protein degradation and modification. Ultrason. Sonochem. 2017, 38, 317–325. [Google Scholar] [CrossRef]
- Hou, X.H.; Liu, Q.F.; Meng, Q.S.; Wang, L.G.; Yan, H.; Zhang, L.C.; Wang, L.X. TMT-based quantitative proteomic analysis of porcine muscle associated with postmortem meat quality. Food Chem. 2020, 328, 127133. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, W.; Wan, X.; Wu, L.; Zang, J.; Jiang, Q.; Xu, Y.; Xia, W. The protective pattern of chitosan-based active coating on texture stabilization of refrigerated carp fillets from the perspective of proteolysis. Food Chem. 2023, 404, 134633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Mao, J.-L.; Yao, H.; Aubourg, S.P. Label-free based proteomics analysis of protein changes in frozen whiteleg shrimp (Litopenaeus vannamei) pre-soaked with sodium trimetaphosphate. Food Res. Int. 2020, 137, 109455. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis; Association of Official Analytical Chemists Inc.: Washington, DC, USA, 1999. [Google Scholar]
- Bu, Y.; Han, M.; Tan, G.; Zhu, W.; Li, X.; Li, J. Changes in quality characteristics of southern bluefin tuna (Thunnus maccoyii) during refrigerated storage and their correlation with color stability. LWT 2022, 154, 112715. [Google Scholar] [CrossRef]
- Wei, Z.; Dai, C.; Bassey, A.P.; Tang, C.; Han, Y.; Wang, C.; Zhou, G. Identification of Potential Peptide Marker(s) for Evaluating Pork Meat Freshness via Mass Spectrometry-Based Peptidomics during Storage under Different Temperatures. Foods 2022, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Cordray, J.C.; Ahn, D.U. Effect of NaCl, Myoglobin, Fe(II), and Fe(III) on Lipid Oxidation of Raw and Cooked Chicken Breast and Beef Loin. J. Agric. Food Chem. 2010, 58, 600–605. [Google Scholar] [CrossRef]
- Jiang, Q.; Du, Y.; Nakazawa, N.; Hu, Y.; Shi, W.; Wang, X.; Osako, K.; Okazaki, E. Effects of frozen storage temperature on the quality and oxidative stability of bigeye tuna flesh after light salting. Int. J. Food Sci. Technol. 2022, 57, 3069–3077. [Google Scholar] [CrossRef]
- Pan, J.; Li, C.; Liu, X.; He, L.; Zhang, M.; Huang, S.; Huang, S.; Liu, Y.; Zhang, Y.; Jin, G. A multivariate insight into the organoleptic properties of porcine muscle by ultrasound-assisted brining: Protein oxidation, water state and microstructure. LWT 2022, 159, 113136. [Google Scholar] [CrossRef]
- Du, X.; Li, H.J.; Nuerjiang, M.; Shi, S.; Kong, B.H.; Liu, Q.; Xia, X.F. Application of ultrasound treatment in chicken gizzards tenderization: Effects on muscle fiber and connective tissue. Ultrason. Sonochem. 2021, 79, 105786. [Google Scholar] [CrossRef]
- Yao, Y. Radio Frequency Tempering of Brined Thunnus albacares and its Quality Evaluation. Master’s Thesis, Food Science and Engineering, Shanghai Ocean University, Shanghai, China, 2022. Available online: https://shorturl.asia/dAEaC/ (accessed on 20 December 2023).
- Youle, R.J.; van der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Shui, S.; Yan, H.; Tu, C.; Benjakul, S.; Aubourg, S.P.; Zhang, B. Cold-induced denaturation of muscle proteins in hairtail (Trichiurus lepturus) during storage: Physicochemical and label-free based proteomics analyses. Food Chem. X 2022, 16, 100479. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997, 4, 429–434. [Google Scholar] [CrossRef]
- Gao, S.; Zhuang, S.; Zhang, L.; Lametsch, R.; Tan, Y.; Li, B.; Hong, H.; Luo, Y. Proteomic evidence of protein degradation and oxidation in brined bighead carp fillets during long-term frozen storage. Food Chem. 2024, 433, 137312. [Google Scholar] [CrossRef] [PubMed]
- Ertbjerg, P.; Puolanne, E. Muscle structure, sarcomere length and influences on meat quality: A review. Meat Sci. 2017, 132, 139–152. [Google Scholar] [CrossRef]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postep. Hig. I Med. Dosw. 2016, 70, 1150–1165. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.A.; Goldberg, A.L. The Logic of the 26S Proteasome. Cell 2017, 169, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Rape, M. Building ubiquitin chains: E2 enzymes at work. Nat. Rev. Mol. Cell Biol. 2009, 10, 755–764. [Google Scholar] [CrossRef]
- Attaix, D.; Ventadour, S.; Codran, A.; Béchet, D.; Taillandier, D.; Combaret, L. The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem. 2005, 41, 173–186. [Google Scholar] [CrossRef]
- Li, X.; Zhang, N.; Jiao, X.; Zhang, W.; Yan, B.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Insight into Ionic Strength-Induced Solubilization of Myofibrillar Proteins from Silver Carp (Hypophthalmichthys molitrix): Structural Changes and 4D Label-Free Proteomics Analysis. J. Agric. Food Chem. 2023, 71, 13920–13933. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhang, R.; Liu, L.; Zhu, Z.; Xu, M.; Shi, L. Molecular mechanism of protein dynamic change for Hengshan goat meat during freezing storage based on high-throughput proteomics. Food Res. Int. 2021, 143, 110289. [Google Scholar] [CrossRef] [PubMed]
| Quality Parameters | NT | SS | UAS |
|---|---|---|---|
| Water content (%) | 71.48 ± 0.10 c | 73.81 ± 0.09 b | 74.44 ± 0.18 a |
| Salt content (%) | 1.10 ± 0.02 c | 2.01 ± 0.02 b | 2.10 ± 0.02 a |
| Yield (%) | 98.49 ± 0.44 c | 104.76 ± 0.31 b | 106.47 ± 0.26 a |
| L* | 37.82 ± 1.00 a | 36.53 ± 0.04 a | 37.32 ± 0.92 a |
| a* | 9.10 ± 0.32 a | 8.06 ± 0.11 b | 6.83 ± 0.14 c |
| b* | 2.52 ± 0.24 a | 1.21 ± 0.09 b | 0.64 ± 0.04 c |
| Hardness (g) | 570.99 ± 5.41 a | 523.15 ± 7.37 b | 535.80 ± 11.24 b |
| Springiness | 0.51 ± 0.01 b | 0.59 ± 0.02 a | 0.59 ± 0.02 a |
| Adhesiveness (g·sec) | 6.49 ± 0.86 b | 7.37 ± 1.40 ab | 11.24 ± 1.73 a |
| CL (%) | 31.30 ± 1.36 a | 27.71 ± 2.40 b | 24.09 ± 1.14 c |
| TL (%) | 1.36 ± 0.12 a | 0.87 ± 0.09 ab | 0.65 ± 0.11 b |
| TBARS (mg MDA/kg) | 0.74 ± 0.07 b | 0.89 ± 0.13 ab | 1.03 ± 0.06 a |
| TVBN (mg/100 g) | 15.96 ± 0.74 a | 11.48 ± 0.28 b | 9.80 ± 0.28 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zhao, Z.; Wu, Y.; Lu, Z.; Zhao, C.; Xiao, J.; Guo, Z. Effects of Quality Enhancement of Frozen Tuna Fillets Using Ultrasound-Assisted Salting: Physicochemical Properties, Histology, and Proteomics. Foods 2024, 13, 525. https://doi.org/10.3390/foods13040525
He Y, Zhao Z, Wu Y, Lu Z, Zhao C, Xiao J, Guo Z. Effects of Quality Enhancement of Frozen Tuna Fillets Using Ultrasound-Assisted Salting: Physicochemical Properties, Histology, and Proteomics. Foods. 2024; 13(4):525. https://doi.org/10.3390/foods13040525
Chicago/Turabian StyleHe, Yuke, Zhou Zhao, Yaogang Wu, Zhiyuan Lu, Caibo Zhao, Juan Xiao, and Zhiqiang Guo. 2024. "Effects of Quality Enhancement of Frozen Tuna Fillets Using Ultrasound-Assisted Salting: Physicochemical Properties, Histology, and Proteomics" Foods 13, no. 4: 525. https://doi.org/10.3390/foods13040525
APA StyleHe, Y., Zhao, Z., Wu, Y., Lu, Z., Zhao, C., Xiao, J., & Guo, Z. (2024). Effects of Quality Enhancement of Frozen Tuna Fillets Using Ultrasound-Assisted Salting: Physicochemical Properties, Histology, and Proteomics. Foods, 13(4), 525. https://doi.org/10.3390/foods13040525






