Plant-Based Oil-in-Water Food Emulsions: Exploring the Influence of Different Formulations on Their Physicochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Fabrication of O/W Emulsions
2.3. Fabrication of O/W Emulsions with Thickener
2.4. Determination of Critical Micelle Concentration (CMC) between Surfactant Dispersions and Flaxseed Oil
2.5. Determination of Physicochemical Properties of O/W Emulsions
2.5.1. Microstructure and Particle Size Distribution
2.5.2. ζ-Potential of Emulsions
2.5.3. Rheological Properties of Emulsions
2.5.4. Physical Stability of Emulsions
2.6. Statistical Analysis of Data
3. Results and Discussion
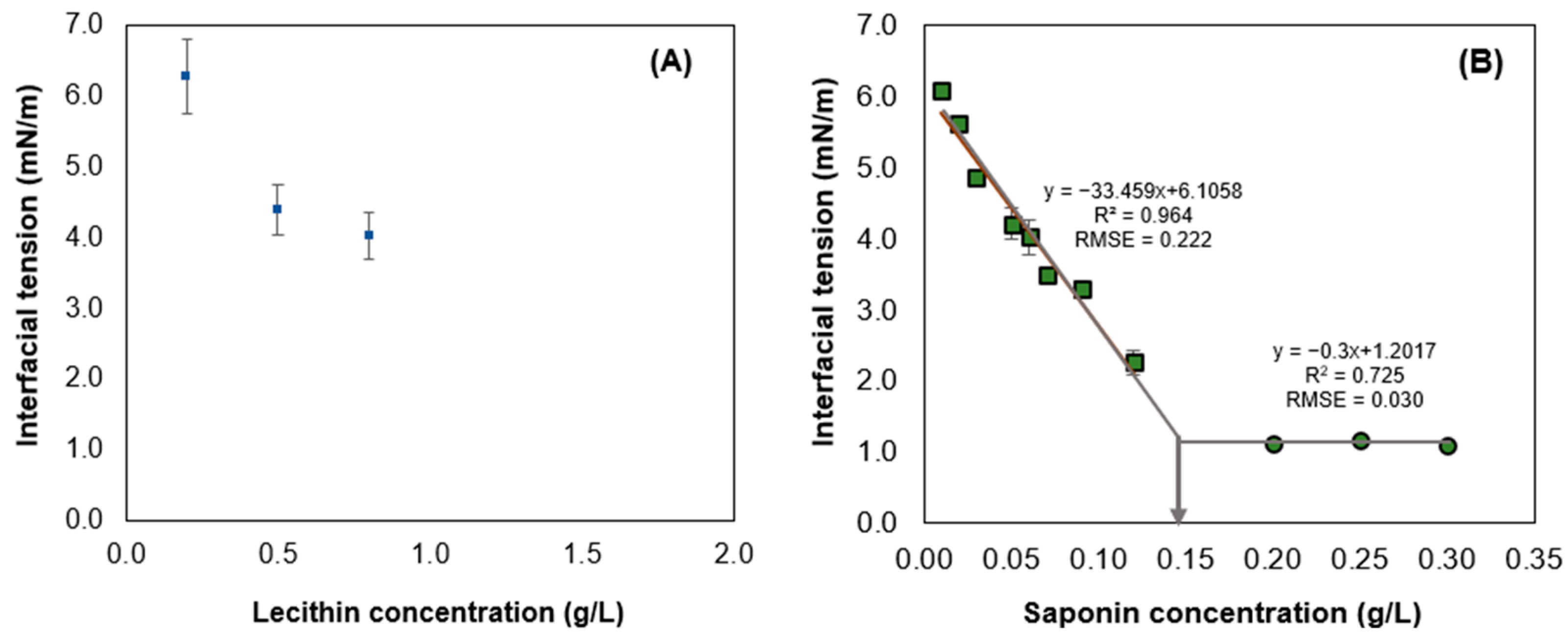
3.1. Critical Micellar Concentration and Interfacial Tension
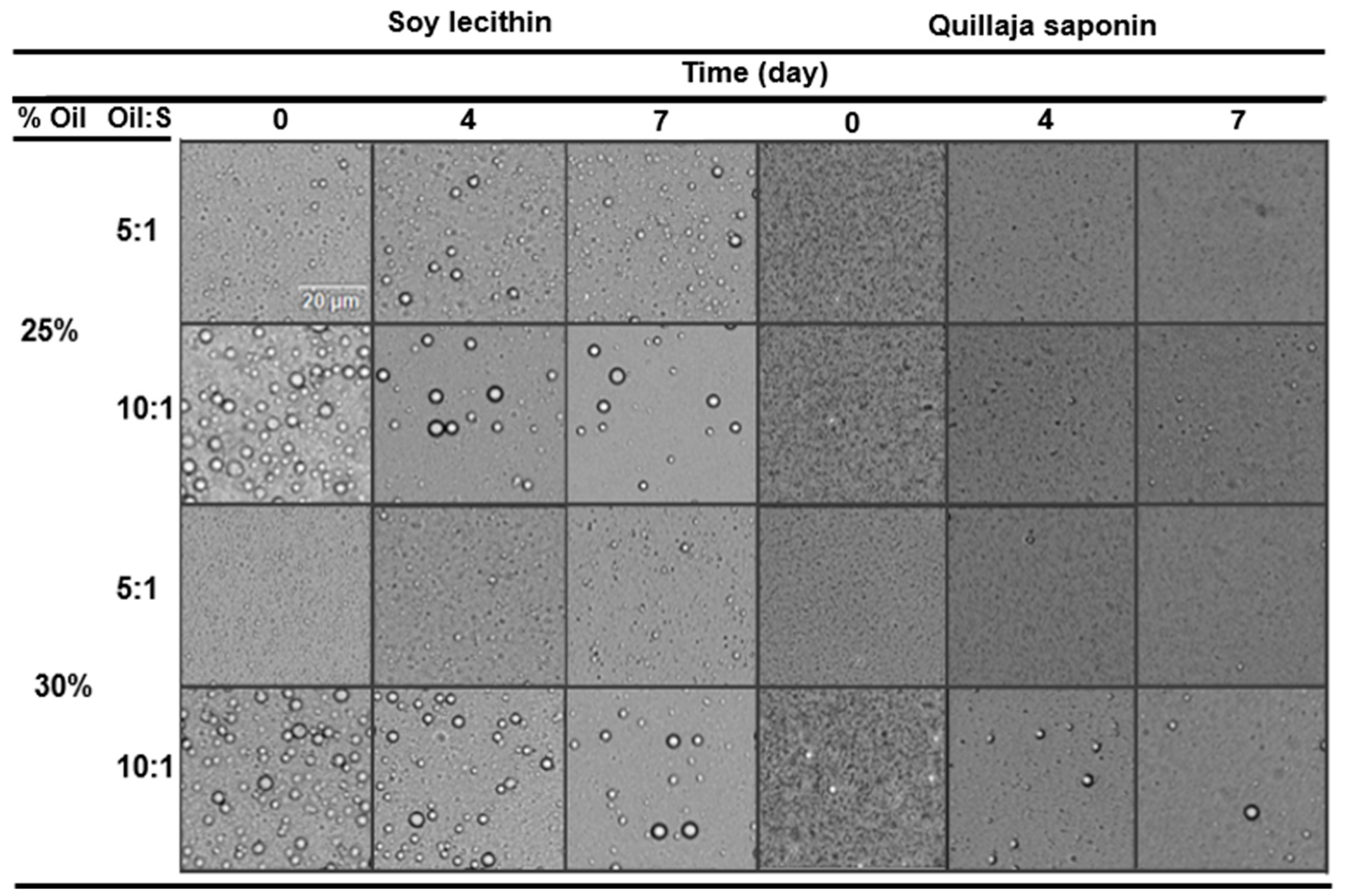
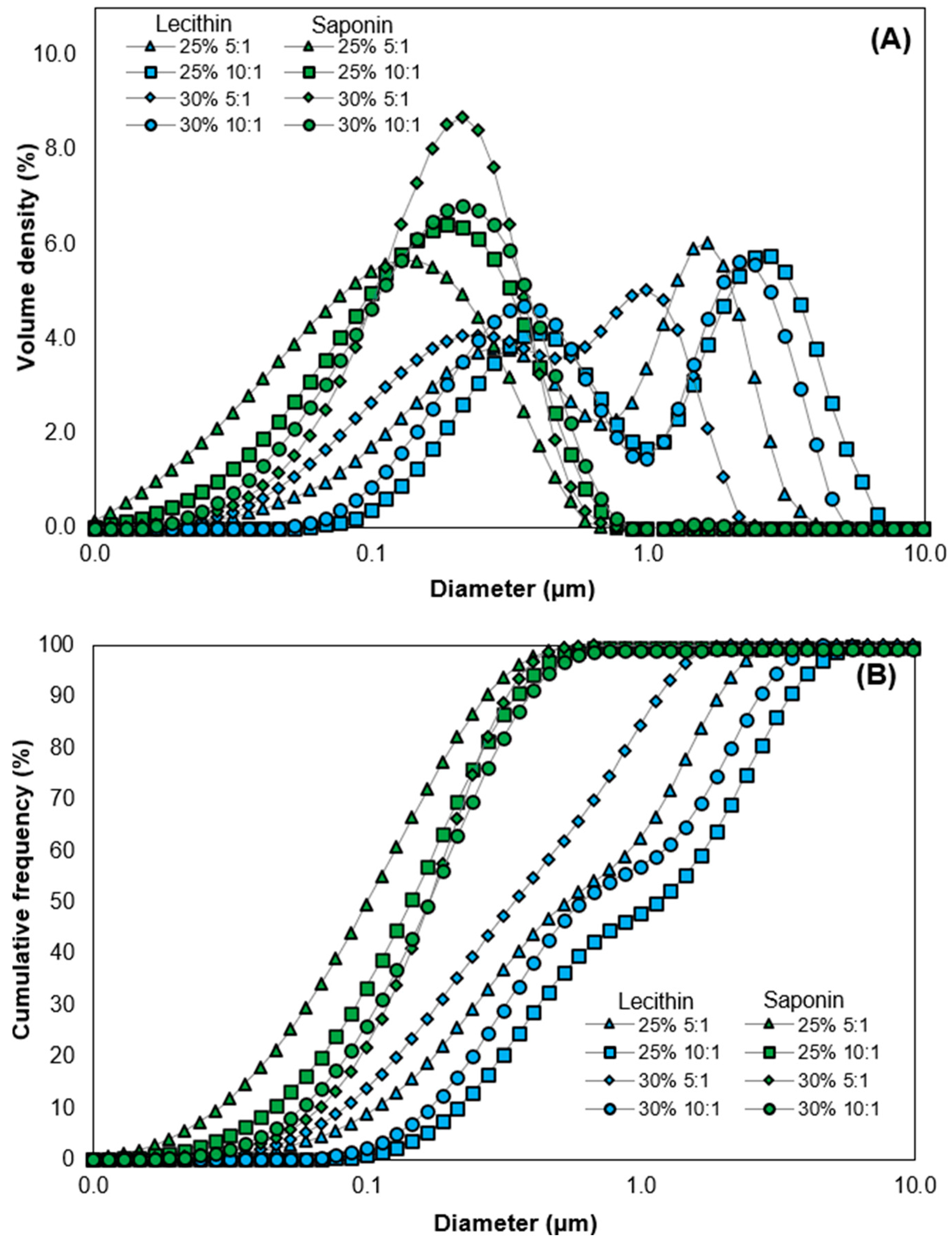
3.2. Particle Size and Microstructure
3.3. Measurements of ζ-Potential of O/W Emulsions Stabilized by Plant-Based Surfactants
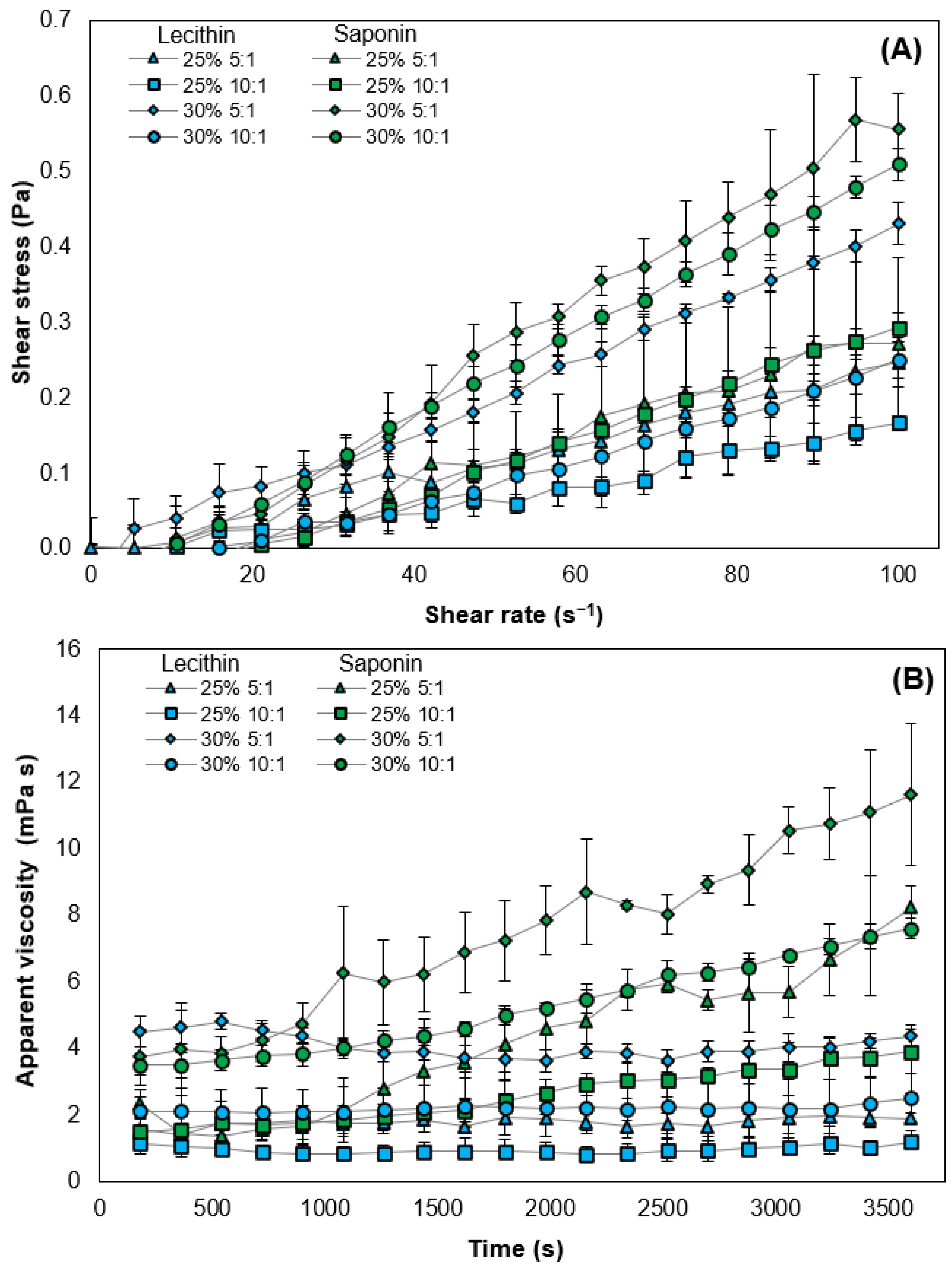
3.4. Flow Curve and Rheological Behavior of O/W Emulsions Stabilized by Plant-Based Surfactant
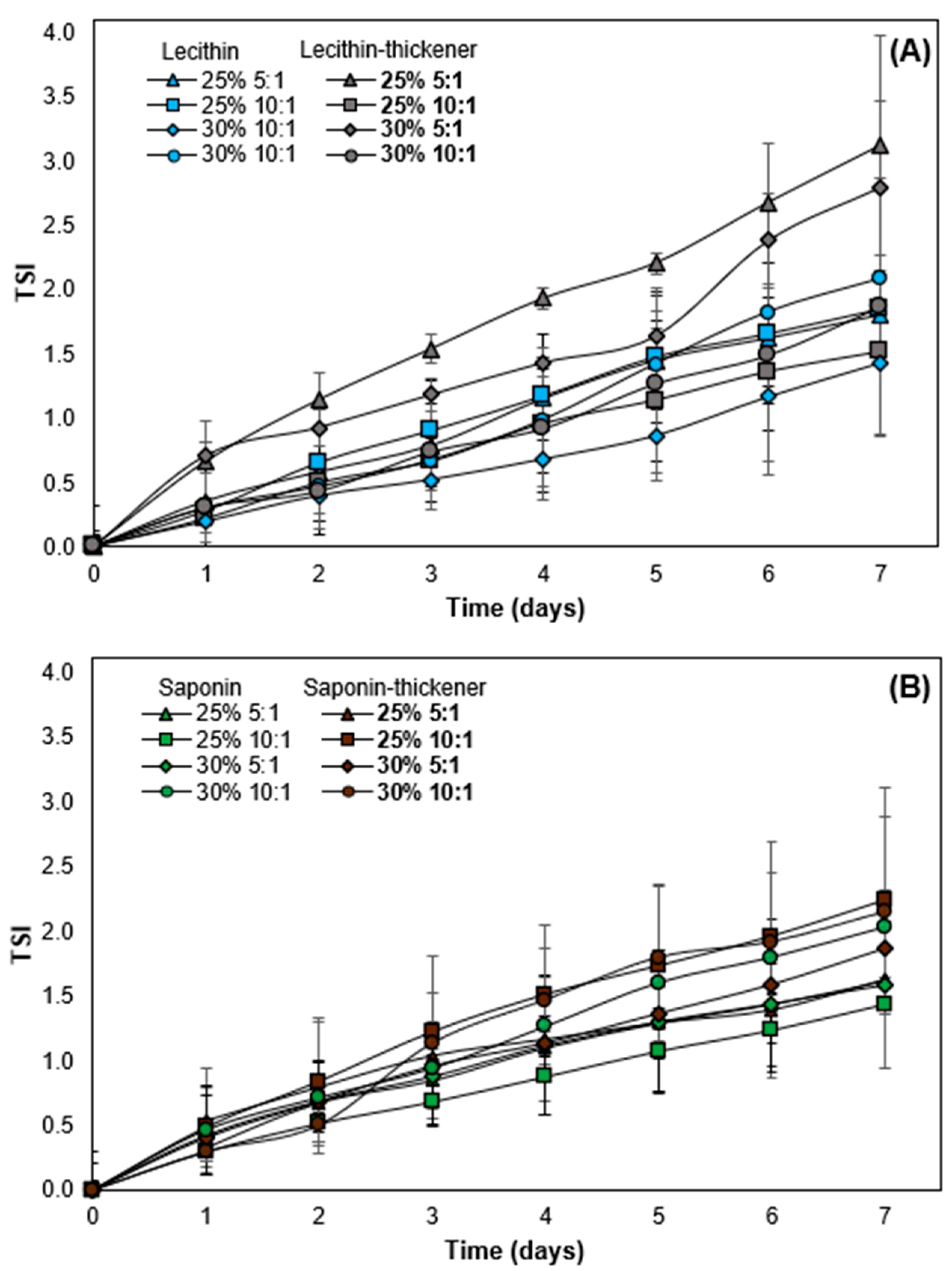
3.5. Stabilizing Effect of the Modified Starch in O/W Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hariharan, D.; Vellanki, K.; Kramer, H. The western diet and chronic kidney disease. Curr. Hyper. Repo. 2015, 17, 16. [Google Scholar] [CrossRef]
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the Western diet: How we got here. Missou. Medic. 2020, 117, 536–538. [Google Scholar]
- Cerqueira, M.A.; McClements, D.J.; Pastrana, L.M. Nutrition, health and well-being in the world: The role of food structure design. In Food Structure Engineering and Design for Improved Nutrition, Health and Well-Being; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 1. [Google Scholar]
- Horie, N.H.; Serrao, V.T.; Simon, S.S.; Gascon, M.R.P.; Santos, A.X.D.; Zambone, M.A.; de Freitas, M.M.M.D.B.; Cunha-Neto, E.; Marques, E.L.; Halpern, A.; et al. Cognitive effects of international weight loss in elderly obese individuals with mild cognitive impairment. J. Clinic. Endo. Metab. 2016, 101, 1104–1112. [Google Scholar] [CrossRef]
- Chung, C.; Sher, A.; Rousset, P.; Decker, E.A.; McClements, D.J. Formulation of food emulsions using natural emulsifiers: Utilization of quillaja saponin and soy lecithin to fabricate liquid coffee whiteners. J. Food Eng. 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Khoury, C.K.; Bjorkman, A.D.; Dempewolf, H.; Ramirez-Villegas, J.; Guarino, L.; Jarvis, A.; Rieseberg, L.H.; Struik, P.C. Increasing homogeneity in global food supplies and the implications for food security. Pro. Natl. Acad. Sci. USA 2014, 111, 4001–4006. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, L.; Disdier, A.C.; Etile, F. Globalisation and national trends in nutrition and health: Agrouped fixed-effects approach to intercountry heterogeneity. Health Econ. 2017, 26, 1146–1161. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Decker, E.A. Phospholipids in foods: Prooxidants or antioxidants? J. Sci. Food Agric. 2016, 96, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega 6/omega 3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Carraro, J.C.C.; Dantas, M.I.D.S.; Espeschit, A.C.R.; Martino, H.S.D.; Ribeiro, S.M.R. Flaxseed and human health: Reviewing benefits and adverse effects. Food Rev. Int. 2012, 28, 203–230. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, G.; Ahonen, E.; Damerau, A.; Gudmundsson, H.G.; Haraldsson, G.G.; Linderborg, K.M. Lipid structure influences the digestion and oxidation behavior of docosahexaenoic and eicosapentaenoic acids in the simulated digestion system. J. Agric. Food Chem. 2023, 71, 10087–10096. [Google Scholar] [CrossRef]
- Prieto, C.; Calvo, L. The encapsulation of low viscosity omerga-3-rich fish oil in polycaprolactone by supercritical fluid extraction of emulsions. J. Supercrit. Fluids 2017, 128, 227–234. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Singh, A.K.; Arora, S.; Lal, D.; Sabikhi, L. Development of stable flaxseed oil emulsions as a potential delivery system of ω-3 fatty acids. J. Food Sci. Technol. 2015, 52, 4256–4265. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Cho, H.T.; McClements, D.J.; Decker, E.A.; Park, Y. Effects of salts on oxidative stability of lipids in tween-20 stabilized oil-in-water emulsions. Food Chem. 2016, 197 Pt B, 1130–1135. [Google Scholar] [CrossRef]
- Kmiecik, D.; Fedko, M.; Malecka, J.; Siger, A.; Kowalczewski, P.L. Effect of heating temperature of high-quality arbequina, picual, manzanilla and cornicabra olive oils on changes in nutritional indices of lipid, tocopherol content and triacylglycerol polymerization process. Molecules 2023, 28, 4247. [Google Scholar] [CrossRef] [PubMed]
- Zajac, M.; Kulawik, P.; Tkaczewska, J.; Migdal, W. Increasing meat product functionality by the addition of milled flaxseed Linum usitatissimum. J. Sci. Food Agric. 2017, 97, 2865–2874. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; McClements, D.J.; Hou, T.; Geng, F.; Chen, P.; Chen, H.; Xie, B.; Sun, Z.; Tang, H.; et al. Composition, processing, and quality control of whole flaxseed products used to fortify foods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 587–614. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; McClements, D.J.; Bourlieu-Lacanal, C.; Durand, E.; Figueroa-Espinoza, M.C.; Lecomte, J.; Villeneuve, P. Hurdles in predicting antioxidant efficacy in oil-in-water emulsions. Trends Food Sci. Technol. 2017, 67, 183–194. [Google Scholar] [CrossRef]
- Zhou, L.; Elias, R.J. Antioxidant and pro-oxidant activity of (-) epigalloca-techin-3-gallate in food emulsions: Influence of pH and phenolic concentration. Food Chem. 2013, 138, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Fomuso, L.B.; Corredig, M.; Akoh, C.C. Effect of emulsifier on oxidation properties of fish oil-based structured lipid emulsions. J. Agric. Food Chem. 2002, 50, 2957–2961. [Google Scholar] [CrossRef]
- Gutiérrez, G.; Matos, M.; Barrero, P.; Pando, D.; Iglesias, O.; Pazos, C. Iron-entrapped niosomes and their potential application for yogurt fortification. LWT 2016, 74, 550–556. [Google Scholar] [CrossRef]
- Dickinson, E. Food emulsions and foams: Stabilization by particles. Curr. Opin. Coll. Int. Sci. 2010, 15, 40–49. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2015; ISBN 1498726690. [Google Scholar]
- Falkeborg, M.; Gou, Z. Dodecenyl succinylated alginate (DSA) as a novel dual-function emulsifier for improved fish oil-in-water emulsions. Food Hydrocoll. 2015, 46, 10–18. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Yesiltas, B.; García-Moreno, P.J.; Sørensen, A.D.M.; Jacobsen, C. Physical and oxidative stability of high fat fish oil-in-water emulsions stabilized with combinations of sodium caseinate and sodium alginate. Eur. J. Lipid Sci. Technol. 2017, 119, 1600484. [Google Scholar] [CrossRef]
- Alicja, M.; Fernando, A.; Riccardo, C.; Alessandro, D.D.; Birgit, D.; Jose, F.M.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of polyglycerol polyricinoleate (E 476) as a food additive. EFSA J. 2017, 15, 4743. [Google Scholar] [CrossRef]
- García-González, D.O.; Yáñez-Soto, B.; Dibildox-Alvarado, E.; Ornelas-Paz, J.J.; Pérez-Martínez, J.D. The effect of interfacial interactions on the rheology of water in oil emulsions oleogelled by candelilla wax and saturated triacylglycerols. LWT-Food Sci. Technol. 2021, 146, 111405. [Google Scholar] [CrossRef]
- Ozturk, B.; McClements, D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Wang, M.; Yan, W.; Zhou, Y.; Fan, L.; Liu, Y.; Li, J. Progress in the application of lecithin in water-in-oil emulsions. Trends Food Sci. Technol. 2021, 118 Pt A, 388–398. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2015, 13, 945–977. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, C.; Li, S.; Yan, H.; Zou, H.; Yu, C. Improving emulsifying properties using mixed natural emulsifiers: Tea saponin and golden pompano protein. Colloid Surf. A Physicochem. Eng. Asp. 2023, 656 Pt A, 130311. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Ghanadian, S.M.; Bahrami, G.; Mansouri, K.; Iranpanah, A.; Farzaei, M.H.; Mojarrab, M. Inhibiting angiogenesis by anti-cancer saponins: From phytochemistry to cellular signaling pathways. Metabolites 2023, 13, 323. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Singh, N.; Kaur, A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017, 233, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Tippel, J.; Lehmann, M.; Von Kiltzing, R.; Drusch, S. Interfacial properties of Quillaja saponins and its use for micellisation of lutein esters. Food Chem. 2016, 212, 35–42. [Google Scholar] [CrossRef]
- Góral, I.; Wojciechowski, K. Surface activity and foaming properties of saponin-rich plants extracts. Adv. Colloid Interface Sci. 2020, 279, 102145. [Google Scholar] [CrossRef] [PubMed]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Tippel, J.; Gies, K.; Harbaum-Piayda, B.; Steffen-Heins, A.; Drusch, S. Composition of Quillaja saponin extract affects lipid oxidation in oil-in-water emulsions. Food Chem. 2017, 221, 386–394. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I. Advances in the design and production of reduced-fat and reduced-cholesterol salad dressing and mayonnaise: A review. Food Bioprocess Technol. 2013, 6, 648–670. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Emadzadeh, B.; Kadkhodaee, R. Effects of pectin and xanthan gum on induced-flocculation phenomenon in an acidic model emulsion system. J. Dispers. Sci. Technol. 2019, 40, 256–263. [Google Scholar] [CrossRef]
- Müller, L.E.; Schiedeck, G. Physical properties of botanical surfactants. Sci. Total Environ. 2018, 610–611, 1133–1137. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Prieto, G.; Rega, C.; Varela, L.M.; Sarmiento, F.; Mosquera, V. A comparative study of the determination of the critical micelle concentration by conductivity and dielectric constant measurements. Langmuir 1998, 14, 4422–4426. [Google Scholar] [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Olesen, K.; Fogang, L.T.; Palm-Henriksen, G.P.; Alyafei, N.; Sølling, T.I. How a range of metal ions influence the interfacial tension of n-decane/carboxylic acid/water systems: The impact of concentration, molecular- and electronic structure. J. Pet. Sci. Eng. 2019, 182, 106307. [Google Scholar] [CrossRef]
- Malvern Panalytical. 2010. Selecting an Appropriate Particle Absorption for Laser Diffraction Particle Size Calculations. Available online: https://www.malvernpanalytical.com/es/learn/knowledge-center/technical-notes/tn101104selectingparticleabsorbtionlaserdiffractio (accessed on 31 December 2023).
- Han, Z.; Xu, S.; Sun, J.; Yue, X.; Wu, Z.; Shao, J. Effects of fatty acid saturation degree on salt-soluble pork protein conformation and interfacial adsorption characteristics at the oil/water interface. Food Hydrocoll. 2021, 113, 106472. [Google Scholar] [CrossRef]
- Bansal, N.; Truong, T.; Bhandari, B. Feasibility study of lecithin nanovesicles as spacers to improve the solubility of milk protein concentrate powder during storage. Dairy Sci. Technol. 2017, 96, 861–872. [Google Scholar] [CrossRef]
- Bukrejewski, P.; Jarczewski, Z.; Matuszewska, A. Evaluation of the chemical stability of diesel oil with using Turbiscan Stability Index (TSI). Arch. Automot. Eng. 2020, 88, 5–18. [Google Scholar] [CrossRef]
- Saari, H.; Wahlgren, M.; Rayner, M.; Sjöö, M.; Matos, M. A comparison of emulsion stability for different OSA-modified waxy maize emulsifiers: Granules, disolved starch, and non-solvent precipitates. PLoS ONE 2019, 14, e0210690. [Google Scholar] [CrossRef]
- Cho, S.J.; Won, J.W.; Park, K.M.; Chang, P. A new method for determining the emulsion stability index by backscattering light detection. J. Food Process Eng. 2014, 37, 229–236. [Google Scholar] [CrossRef]
- Zalewska, A.; Kowalik, J.; Grubecki, I. Application of turbiscan lab to study the effect of emulsifier content on the stability of plant origin dispersion. Chem. Process Eng. 2019, 40, 399–409. [Google Scholar] [CrossRef]
- Yang, Y.; Leser, M.E.; Sher, A.A.; McClements, D.J. Formation and stability of emulsions using a natural small molecule surfactant: Quillaja saponin (Q-Naturale®). Food Hydrocoll. 2013, 30, 589–596. [Google Scholar] [CrossRef]
- Homma, R.; Suzuki, K.; Cui, L.; McClements, D.J.; Decker, E.A. Impact of association colloids on lipid oxidation in triacylglycerols and fatty acid ethyl esters. J. Agric. Food Chem. 2015, 63, 10161–10169. [Google Scholar] [CrossRef]
- Chaiyasit, W.; Elias, R.J.; McClements, D.J.; Decker, E.A. Role of physical structures in bulk oils on lipid oxidation. Crit. Rev. Food Sci. Nutr. 2007, 47, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Kittipongpittaya, K.; Panya, A.; McClements, D.J.; Decker, E.A. Impact of free fatty acids and phospholipids on reverse micelles formation and lipid oxidation in bulk oil. J. Am. Oil Chem. Soc. 2014, 91, 453–462. [Google Scholar] [CrossRef]
- Lee, P.; Choo, W. Characterization of flaxseed oil emulsions. J. Food Sci. Technol. 2015, 52, 4378–4386. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef]
- Hunter, R.J. Foundations of Colloid Science; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Varona, S.; Martín, Á.; Cocero, M.J. Formulation of a natural biocide based on lavandin essential oil by emulsification using modified starches. Chem. Eng. Process. Process Intensif. 2009, 48, 1121–1128. [Google Scholar] [CrossRef]
- Güςlü-Üstündag, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-Based, biological-active surfactants from plants. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: London, UK, 2017; Chapter 6; pp. 184–198. [Google Scholar]
- Wang, L.; Yu, X.; Geng, F.; Cheng, C.; Yang, J.; Deng, Q. Effects of tocopherols on the stability of flaxseed oil-in-water emulsions stabilized by different emulsifiers: Interfacial partitioning and interaction. Food Chem. 2022, 374, 131691. [Google Scholar] [CrossRef]
- Zheng, G.; Selvam, A.; Wong, J.W.C. Enhanced solubilization and desorption of organochlorine pesticides (OCPs) from soil by oil-swollen micelles formed with a nonionic surfactant. Environ. Sci. Technol. 2012, 46, 12062–12068. [Google Scholar] [CrossRef]
- Schreiner, T.B.; Dias, M.M.; Barreiro, M.F.; Pinho, S.P. Saponins as natural emulsifiers for nanoemulsions. J. Agric. Food Chem. 2022, 70, 6573–6590. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Farias, C.B.B.; Campos-Takaki, G.M. Co-Utilization of canola oil and glucose on the production of a surfactant by Candida lipolytica. Curr. Microbio. 2007, 54, 68–73. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Nanoemulsion delivery systems for oil-soluble vitamins: Influence of carrier oil type on lipid digestion and vitamin D-3 bioaccessibility. Food Chem. 2015, 187, 499–506. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Y.; Bai, L.; Liu, F.; Zhang, R.; Zhang, Z.; Zheng, B.; Deng, Y.; McClements, D.J. Production of highly concentrated Oil-in-Water emulsions using dual-channel microfluidization: Use of individual and mixed natural emulsifiers (saponin and lecithin). Food Res. Int. 2017, 96, 103–112. [Google Scholar] [CrossRef]
- Fuentes, K.; Matamala, C.; Martínez, N.; Zúñiga, R.N.; Troncoso, E. Comparative study of physicochemical properties of nanoemulsions fabricated with natural and synthetic surfactants. Processes 2021, 9, 2002. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Emulsions and microemulsions for topical and transdermal drug delivery. In Handbook on Non-Invasive Drug Delivery Systems. Non-Invasive and Minimally-Invasive Drug Delivery Systems for Pharmaceutical and Personal Care Products; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. [Google Scholar]
- Xu, Q.; Nakajima, M.; Liu, Z.; Shiina, T. Soybean-based surfactants and their applications. In Soybean—Applications and Technology; Ng, T.B., Ed.; InTech: London, UK, 2011; pp. 125–140. [Google Scholar]
- Okuro, P.K.; Gomes, A.; Costa, A.L.R.; Adame, M.A.; Cunha, R.L. Formation and stability of W/O-high internal phase emulsions (HIPEs) and derived O/W emulsions stabilized by PGPR and lecithin. Food Res. Int. 2019, 122, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pedersem, J.N.; Anankanbil, S.; Guo, Z. Enhanced fish oil-in-water emulsions enabled by rapeseed lecithins obtained under different processing conditions. Food Chem. 2018, 264, 233–240. [Google Scholar] [CrossRef]
- Yussof, N.S.; Ping, T.C.; Boon, T.T.; Utra, U.; Ramli, M.E. Influence of soy lecithin and sodium caseinate on the stability and in vitro bioaccessibility of lycopene nanodispersion. Food Technol. Biotechnol. 2023, 61, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Ralla, T.; Salminen, H.; Tuosto, J.; Weiss, J. Formation and stability of emulsions stabilised by yucca saponin extract. Int. J. Food Sci. Technol. 2018, 53, 1381–1388. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, R.; Gu, J.; McClements, D.J.; Zhang, Y.; Tan, H. Vitamin E encapsulation in plant-based nanoemulsions fabricated using dual-channel microfluidization: Formation, stability, and bioaccessibility. J. Agric. Food Chem. 2018, 66, 10532–10542. [Google Scholar] [CrossRef] [PubMed]
- Taarji, N.; Rabelo da Silva, C.A.; Khalid, N.; Gadhi, C.; Hafidi, A.; Kobayashi, L.; Neves, M.A.; Isoda, H.; Nakajima, M. Formulation and stabilization of oil-in-water nanoemulsions using a saponins rich extract from argan oil press-cake. Food Chem. 2018, 246, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.B.; Santamaria-Echart, A.; Riveiro, A.; Peres, A.M.; Dias, M.M.; Pinho, S.P.; Barreiro, M.F. Formulation and optimization of nanoemulsions using the natural surfactant saponin from quillaja bark. Molecules 2020, 25, 1538. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Urquidi, J.; Singh, S.; Wilse Robinson, G. Thermal offset viscosities of liquid H2O, D2O, and T2O. J. Phys. Chem. 1999, 103, 1991–1994. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Nogueira, V.M.; Santiago, D.F.; Machado, F.A.; Fernandes, F.A.N.; Santiago-Aguiar, R.S.; de Sant’Ana, H.B. Density and viscosity of binary systems containing (linseed or corn) oil, (linseed or corn) biodiesel and diesel. J. Chem. Eng. Data 2015, 60, 3120–3131. [Google Scholar] [CrossRef]
- Maranzano, D.J.; Wagner, N.J. The effects of interparticle interactions and particle sizer on reversible shear thickening: Hard-sphere colloid dispersion. J. Rheol. 2001, 45, 1205–1222. [Google Scholar] [CrossRef]
- Pang, B.; Wang, S.; Chen, W.; Hassan, M.; Lu, H. Effects of flow behavior index and consistency coefficient on hydrodynamics of power-law fluids and particles in fluidized beds. Powder Technol. 2020, 366, 249–260. [Google Scholar] [CrossRef]
- Zhang, F.; Proctor, A. Rheology and Stability of Phospholipid-Stabilized Emulsions. J. Am. Oil Chem. Soc. 1997, 74, 869–874. [Google Scholar] [CrossRef]
- Rapp, B.E. Fluids. In Microfluidics: Modelling Mechanics and Mathematics; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 9. [Google Scholar]
- Sochi, T. Non-Newtonian flow in porous media. Polymer 2010, 51, 5007–5023. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, Y.; Ji, Z.; Chen, J. Lubrication and sensory properties of emulsion systems and effects of droplet size distribution. Foods 2021, 10, 3024. [Google Scholar] [CrossRef]
- Ramírez, M.; Bullón, J.; Andérez, J.; Mira, I.; Salager, J. Drop size distribution bimodality and its effect on O/W emulsion viscosity. J. Disp. Sci. Technol. 2002, 23, 309–321. [Google Scholar] [CrossRef]
- Hong, I.K.; Kim, S.I.; Lee, S.B. Effects of HLB value on oil-in-water emulsions: Droplet size, rheological behavior, zeta-potential, and creaming index. J. Ind. Eng. Chem. 2018, 67, 123–131. [Google Scholar] [CrossRef]
- Princen, H.M. The Structure, Mechanics and Rheology of Concentrated Emulsions and Fluid Foams. In Encyclopedic Handbook of Emulsion Technology; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Rivas, H.; Silva, F.; Gutierrez, X.; Nunez, G. Bitumen in water bimodal emulsions stabilized by natural surfactants. J. Colloid Int. Sci. 2002, 23, 405–418. [Google Scholar] [CrossRef]
- Querol, N.; Cabeza, L.F.; Barreneche, C. Rheological properties of bimodal bitumen emulsions: New approach. Afinidad 2017, 74, 580. [Google Scholar]
- Kronberg, B.; Holmberg, K.; Lindman, B. Surface Chemistry of Surfactants and Polymers. In Colloidal Stability; John Wiley & Sons, Ltd.: West Sussex, UK, 2014; pp. 335–360. [Google Scholar]
- Wang, K.; Li, G.; Zhang, B. Opposite results of emulsion stability evaluated by the TSI and the phase separation proportion. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 402–409. [Google Scholar] [CrossRef]
- Santos, J.; Calero, N.; Guerrero, A.; Muñoz, J. Relationship of rheological and microstructural properties with physical stability of potato protein-based emulsions stabilized by guar gum. Food Hydrocoll. 2015, 44, 109–114. [Google Scholar] [CrossRef]
- Riquelme, N.; Robert, P.; Troncoso, E.; Arancibia, C. Influence of the particle size and hydrocolloid type on lipid digestion of thickened emulsions. Food Funct. 2020, 11, 5955–5964. [Google Scholar] [CrossRef]
- Felix, M.; Romero, A.; Guerrero, A. Influence of pH and Xanthan Gum on long-term stability of crayfish-based emulsions. Food Hydrocoll. 2017, 72, 372–380. [Google Scholar] [CrossRef]
- Gou, Q.; Zhu, X.; Zhen, W.; Li, Z.; Kang, J.; Sun, X.; Wang, S.; Cui, S.W. Rheological properties and stabilizing effects of high-temperature extracted flaxseed gum on oil/wáter emulsion systems. Food Hydrocoll. 2021, 112, 106289. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhlouffi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lidman, B.; Edlund, H.; Norgren, M. Emulsion formation and stabilization by biomolecules: The leading role of cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, C.; Bayarri, S.; Costell, E. Comparing carboxymethyl cellulose and starch as thickeners in oil/water emulsions. Implications on rheological and structural properties. Food Biophys. 2013, 8, 122–136. [Google Scholar] [CrossRef]



| Surfactant | Flaxseed Oil Concentration (% w/w) | Oil:Surfactant Ratio | d10 (µm) | d50 (µm) | d90 (µm) | PDI |
|---|---|---|---|---|---|---|
| Soy lecithin | 25 | 5:1 | 0.14 ± 0.07 Aa | 0.66 ± 0.27 Aa | 2.19 ± 0.16 Aa | 3.11 ± 2.40 Aa |
| 10:1 | 0.25 ± 0.05 Ab | 1.30 ± 0.49 Ab | 3.99 ± 0.57 Ab | 3.36 ± 1.96 Aa | ||
| 30 | 5:1 | 0.10 ± 0.02 Aa | 0.40 ± 0.09 Aa | 1.30 ± 0.08 Aa | 3.09 ± 0.59 Aa | |
| 10:1 | 0.19 ± 0.02 Ab | 0.72 ± 0.14 Ab | 3.08 ± 0.03 Ab | 4.13 ± 0.91 Aa | ||
| Quillaja saponin | 25 | 5:1 | 0.03 ± 0.01 Ba | 0.11 ± 0.02 Ba | 0.31 ± 0.02 Ba | 2.41 ± 0.33 Ba |
| 10:1 | 0.06 ± 0.01 Bb | 0.18 ± 0.01 Bb | 0.41 ± 0.02 Bb | 2.04 ± 0.14 Ba | ||
| 30 | 5:1 | 0.08 ± 0.02 Ba | 0.19 ± 0.01 Ba | 0.37 ± 0.03 Ba | 1.53 ± 0.30 Ba | |
| 10:1 | 0.07 ± 0.00 Bb | 0.18 ± 0.01 Bb | 0.42 ± 0.02 Bb | 1.84 ± 0.16 Ba |
| Surfactant | Flaxseed Oil Concentration (% w/w) | Oil:Surfactant Ratio | Consistency Coefficient, K (Pa·s) (×10−3) | Flow Behavior Index, n (Dimensionless) | R2 | RMSE |
|---|---|---|---|---|---|---|
| Soy lecithin | 25 | 5:1 | 1.70 ± 0.00 Aa | 1.09 ± 0.12 a | 0.97 | 0.02 |
| 10:1 | 0.54 ± 0.00 Ab | 1.41 ± 0.39 b | 0.93 | 0.11 | ||
| 30 | 5:1 | 2.90 ± 0.00 Ba | 1.09 ± 0.11 a | 0.99 | 0.02 | |
| 10:1 | 0.19 ± 0.00 Bb | 1.57 ± 0.16 b | 0.98 | 0.02 | ||
| Quillaja saponin | 25 | 5:1 | 0.70 ± 0.00 Aa | 1.42 ± 0.31 a | 0.97 | 0.09 |
| 10:1 | 0.20 ± 0.00 Ab | 1.57 ± 0.09 b | 0.98 | 0.02 | ||
| 30 | 5:1 | 1.90 ± 0.00 Ba | 1.30 ± 0.06 a | 0.99 | 0.10 | |
| 10:1 | 2.00 ± 0.00 Bb | 1.21 ± 0.09 b | 0.99 | 0.02 |
| Surfactant | Flaxseed Oil Concentration (% w/w) | Oil:Surfactant Ratio | Apparent Viscosity without Thickener (mPa·s) | Apparent Viscosity with Thickener (mPa·s) |
|---|---|---|---|---|
| Soy lecithin | 25 | 5:1 | 1.89 ± 0.70 A | 691.0 ± 32.7 B |
| 10:1 | 1.19 ± 0.18 A | 370.6 ± 6.1 B | ||
| 30 | 5:1 | 4.36 ± 0.32 A | 1991.4 ± 164.6 B | |
| 10:1 | 2.50 ± 0.72 A | 759.4 ± 34.2 B | ||
| Quillaja saponin | 25 | 5:1 | 8.23 ± 0.63 A | 789.4 ± 16.3 B |
| 10:1 | 3.90 ± 0.65 A | 679.7 ± 32.1 B | ||
| 30 | 5:1 | 11.61 ± 2.14 A | 2117.3 ± 110.4 B | |
| 10:1 | 7.59 ± 0.30 A | 2133.2 ± 40.1 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quezada, C.; Urra, M.; Mella, C.; Zúñiga, R.N.; Troncoso, E. Plant-Based Oil-in-Water Food Emulsions: Exploring the Influence of Different Formulations on Their Physicochemical Properties. Foods 2024, 13, 513. https://doi.org/10.3390/foods13040513
Quezada C, Urra M, Mella C, Zúñiga RN, Troncoso E. Plant-Based Oil-in-Water Food Emulsions: Exploring the Influence of Different Formulations on Their Physicochemical Properties. Foods. 2024; 13(4):513. https://doi.org/10.3390/foods13040513
Chicago/Turabian StyleQuezada, Carolina, Matías Urra, Camila Mella, Rommy N. Zúñiga, and Elizabeth Troncoso. 2024. "Plant-Based Oil-in-Water Food Emulsions: Exploring the Influence of Different Formulations on Their Physicochemical Properties" Foods 13, no. 4: 513. https://doi.org/10.3390/foods13040513
APA StyleQuezada, C., Urra, M., Mella, C., Zúñiga, R. N., & Troncoso, E. (2024). Plant-Based Oil-in-Water Food Emulsions: Exploring the Influence of Different Formulations on Their Physicochemical Properties. Foods, 13(4), 513. https://doi.org/10.3390/foods13040513






