Cereal β-d-Glucans in Food Processing Applications and Nanotechnology Research
Abstract
1. Introduction
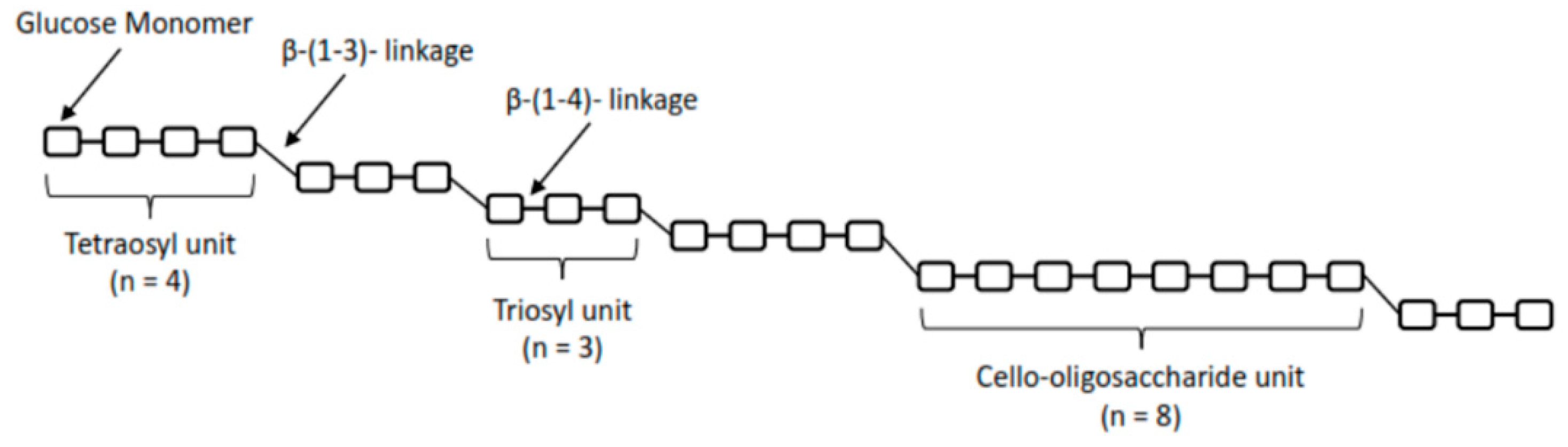
2. Production of β-d-Glucans from Cereal Grains
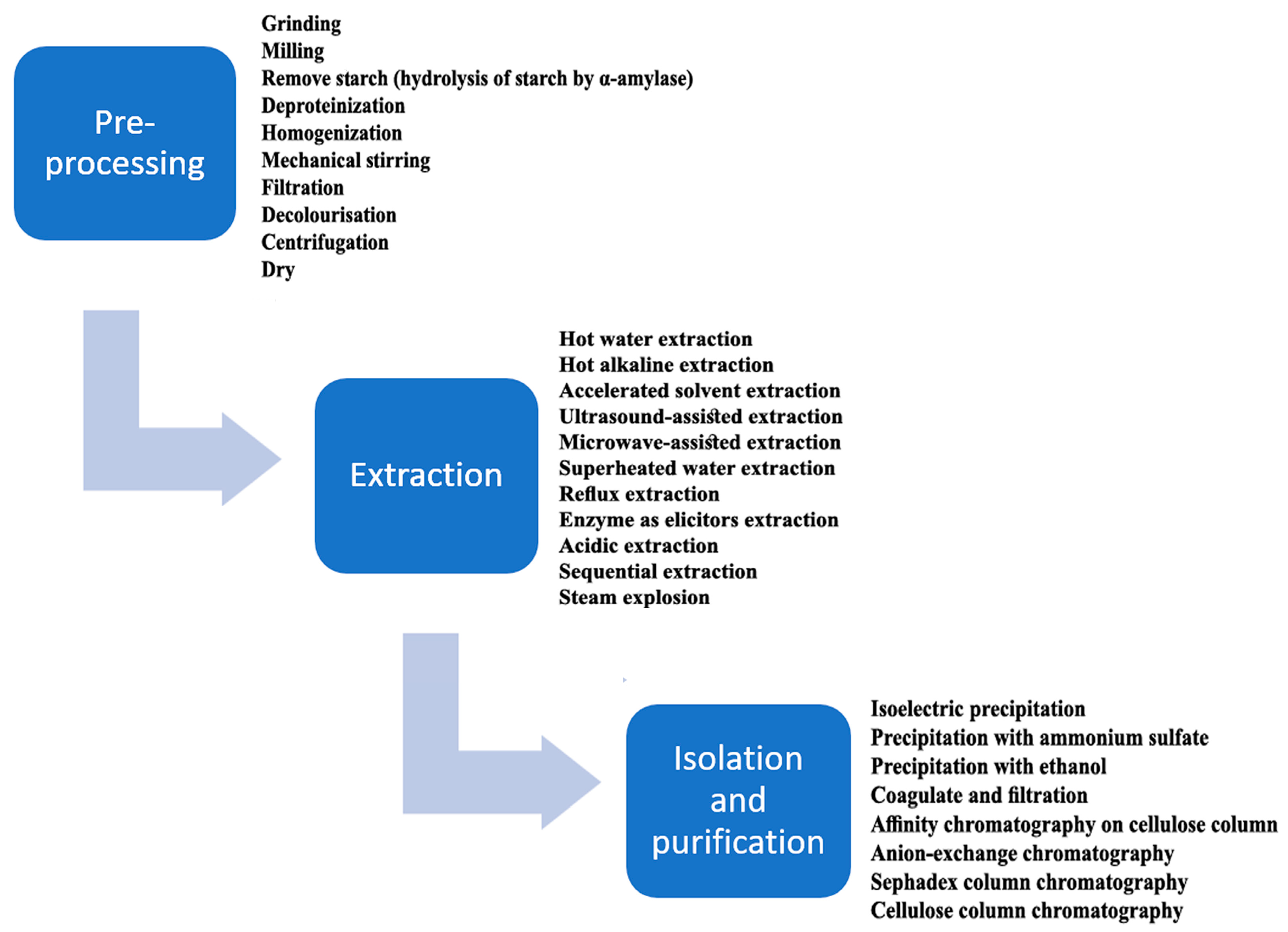
2.1. Extraction Procedures for β-d-Glucans from Cereals
2.2. Separation Techniques Used in β-d-Glucans Production from Cereals
2.3. Purification of β-d-Glucans from the Cereal Matrix
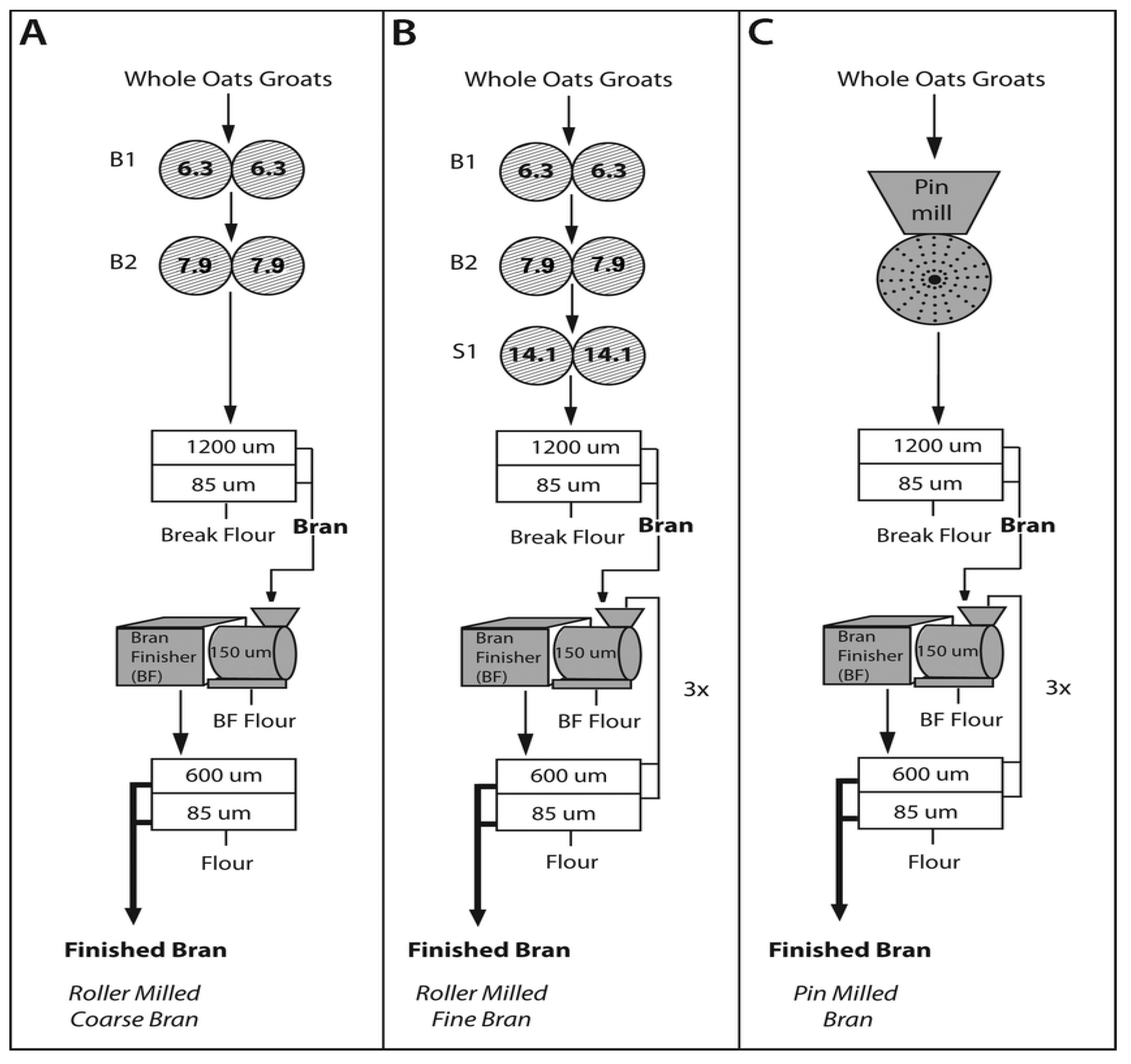
3. Effect of the Milling Process and Grain Pearling on Cereal β-d-Glucans
4. Impact of Dough Preparation, Fermentation, and Baking on β-d-Glucans
5. The Influence of Germination on β-d-Glucans in Cereals
6. Effect of Extrusion
7. The Range of Applications of β-d-Glucans in Food Products
| Application | Addition | Source | Aim | Product | References |
|---|---|---|---|---|---|
| Cereal processing | 2.5 and 5% | Barley | Fortification with soluble fiber, increasing loaf volume | Bread | [102] |
| Cereal processing | 0.2, 0.6, 1.0, and 1.4% | Barley | Fortification with soluble fiber, resistance to deformation | Bread | [71] |
| Cereal processing | 10, 12, and 14% soluble oat fiber (70% β-d-glucans) | Oat | Replacing of flour in wheat bread | Bread | [113] |
| Cereal processing | 2.6 and 5.6% | Oat | Addition of functional ingredients | Bread | [114] |
| Cereal processing | 30% high-beta-D-glucan barley flour | Barley | Fortification–health claim linking the consumption of barley beta-D-glucan | Pasta | [115] |
| Cereal processing | 10% | Oat | Fortification | Yellow alkaline noodles | [117] |
| Cereal processing | 20 and 30% high-beta-D-glucan barley flour | Barley | Fortification-functional couscous | Couscous | [116] |
| Cereal processing | 100% barley flour (3.4–4.4% β-d-glucans) | Barley | Fortification, replacing of wheat flour | Sponge cake | [118] |
| Cereal processing | 5.2% | Barley | Fortification with dietary fiber, reduction in energy | Biscuit bar | [119] |
| Milk processing | 0.5% | Barley | Fat replacement | Yogurt | [121] |
| Milk processing | 0.2–0.8% | Yeast | Thickening | Yogurt | [127] |
| Milk beverage production | 3% | Oat | Stabilizing | Chocolate-flavored milk | [107] |
| Cheese production | 0.7 and 1.4% | Oat | Fat replacement | White-brined cheese | [125] |
| Cheese production | Fat replacement 3.47% and 6.84% | Yeast | Fat replacement | Cheddar cheese | [128] |
| Cheese production | 0.5% | Barley | Fat replacement | Dahi cheese | [129] |
| Cheese production | 5% | Barley | Fat replacement | Labneh | [130] |
| Cheese production | 0.2% | Barley | Fat replacement | Mozzarella | [131] |
| Milk processing | 0.5% | Cereal (not specified) | Fat replacement | Cottage cheese | [132] |
8. Potential of Cereal β-d-Glucans in Nanotechnology
8.1. Characteristics of β-Glucans from Different Sources for Use in Nanotechnology
8.2. Biological Activities of β-Glucans Applied in Nanotechnology
8.3. Modifications of the β-Glucans Molecules for Nanotechnology
8.4. β-Glucan Nanotechnology in the Food Industry
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiozzi, V.; Eliopoulos, C.; Markou, G.; Arapoglou, D.; Agriopoulou, S.; El Enshasy, H.A.; Varzakas, T. Biotechnological Addition of β-Glucans from Cereals, Mushrooms and Yeasts in Foods and Animal Feed. Processes 2021, 9, 1889. [Google Scholar] [CrossRef]
- Li, X.; Cheung, P.C.K. Application of Natural β-Glucans as Biocompatible Functional Nanomaterials. Food Sci. Hum. Wellness 2019, 8, 315–319. [Google Scholar] [CrossRef]
- Tosh, S.M.; Brummer, Y.; Wood, P.J.; Wang, Q.; Weisz, J. Evaluation of Structure in the Formation of Gels by Structurally Diverse (1→3)(1→4)-β-d-Glucans from Four Cereal and One Lichen Species. Carbohydr. Polym. 2004, 57, 249–259. [Google Scholar] [CrossRef]
- Cui, W.; Wood, P.J. Relationships between Structural Features, Molecular Weight, and Rheological Properties of Cereal β-d-Glucans. In Hydrocoll; Nishinari, K., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2000; pp. 159–168. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Macri, L.J.; MacGregor, A.W. Structure and Physicochemical Properties of Barley Non-Starch Polysaccharides—I. Water-Extractable β-Glucans and Arabinoxylans. Carbohydr. Polym. 1998, 35, 249–258. [Google Scholar] [CrossRef]
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Kagimura, F.Y.; da Cunha, M.A.A.; Theis, T.V.; Malfatti, C.R.M.; Dekker, R.F.H.; Barbosa, A.M.; Teixeira, S.D.; Salomé, K. Carboxymethylation of (1→6)-β-Glucan (Lasiodiplodan): Preparation, Characterization and Antioxidant Evaluation. Carbohydr. Polym. 2015, 127, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Biliaderis, C.G.; Lazaridou, A.; Izydorczyk, M.S. Structure and Rheological Properties of Water Soluble β-Glucans from Oat Cultivars of Avena sativa and Avena bysantina. J. Cereal Sci. 2003, 38, 15–31. [Google Scholar] [CrossRef]
- Mao, H.; Xu, M.; Ji, J.; Zhou, M.; Li, H.; Wen, Y.; Wang, J.; Sun, B. The Utilization of Oat for the Production of Wholegrain Foods: Processing Technology and Products. Food Front. 2022, 3, 28–45. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M. Cereal Polysaccharides as Sources of Functional Ingredient for Reformulation of Meat Products: A Review. J. Funct. Foods 2019, 62, 103527. [Google Scholar] [CrossRef]
- Lukinac, J.; Jukić, M. Barley in the Production of Cereal-Based Products. Plants 2022, 11, 3519. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Beta-Glucans from Oats and Barley and Maintenance of Normal Blood LDL-Cholesterol Concentrations (ID 1236, 1299), Increase in Satiety Leading to a Reduction in Energy Intake (ID 851, 852), Reduction of Post-Prandial Glycaemic Responses (ID 821, 824), and “Digestive Function” (ID 850) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2207. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Rahn, M.; Dioum, E.H.; Spruill, S.E.; Ezatagha, A.; Campbell, J.E.; Jenkins, A.L.; Chu, Y.F. An Oat β-Glucan Beverage Reduces LDL Cholesterol and Cardiovascular Disease Risk in Men and Women with Borderline High Cholesterol: A Double-Blind, Randomized, Controlled Clinical Trial. J. Nutr. 2021, 151, 2655–2666. [Google Scholar] [CrossRef]
- Sandford, P.A.; Baird, J. 7—Industrial Utilization of Polysaccharides. In The Polysaccharides; Aspinall, G.O., Ed.; Academic Press: Cambridge, MA, USA, 1983; pp. 411–490. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M. Chapter 11—β-Glucan as a Food Ingredient. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 351–381. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Cheung, P.C. Fungal β-Glucan-Based Nanotherapeutics: From Fabrication to Application. J. Fungi 2023, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, G.; Arumugam, S.; Doble, M. Industrial Production and Applications of α/β Linear and Branched Glucans. Indian Chem. Eng. 2021, 63, 533–547. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B.A. Critical Review on Production and Industrial Applications of Beta-Glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Brennan, C.S.; Cleary, L.J. The Potential Use of Cereal (1→3,1→4)-β-d-Glucans as Functional Food Ingredients. J. Cereal Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Sushytskyi, L.; Synytsya, A.; Čopíková, J.; Lukáč, P.; Rajsiglová, L.; Tenti, P.; Vannucci, L.E. Perspectives in the Application of High, Medium, and Low Molecular Weight Oat β-d-Glucans in Dietary Nutrition and Food Technology—A Short Overview. Foods 2023, 12, 1121. [Google Scholar] [CrossRef]
- Wang, Q.; Sheng, X.; Shi, A.; Hu, H.; Yang, Y.; Liu, L.; Fei, L.; Liu, H. β-Glucans: Relationships between Modification, Conformation and Functional Activities. Molecules 2017, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Sibakov, J.; Abecassis, J.; Barron, C.; Poutanen, K. Electrostatic Separation Combined with Ultra-Fine Grinding to Produce β-Glucan Enriched Ingredients from Oat Bran. IFSET 2014, 26, 445–455. [Google Scholar] [CrossRef]
- Daou, C.; Zhang, H. Oat Beta-Glucan: Its Role in Health Promotion and Prevention of Diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Alonso, E.; Gairola, K.; Cocero, M.J. Fixed-Bed Extraction of β-Glucan from Cereals by Means of Pressurized Hot Water. J. Supercrit. Fluids 2013, 82, 122–128. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Dexter, J.E. Barley β-Glucans and Arabinoxylans: Molecular Structure, Physicochemical Properties, and Uses in Food Products—A Review. Food Res. Int. 2008, 41, 850–868. [Google Scholar] [CrossRef]
- Liu, K. Fractionation of Oats into Products Enriched with Protein, Beta-Glucan, Starch, or Other Carbohydrates. J. Cereal Sci. 2014, 60, 317–322. [Google Scholar] [CrossRef]
- Gangopadhyay, N.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Optimisation of Yield and Molecular Weight of β-Glucan from Barley Flour Using Response Surface Methodology. J. Cereal Sci. 2015, 62, 38–44. [Google Scholar] [CrossRef]
- Limberger, V.M.; de Francisco, A.; Borges, M.R.; Oro, T.; Ogliari, P.J.; Scheuer, P.M.; Noronha, C.M. Extração de β-Glucanas de Cevada e Caracterização Parcial Do Amido Residual. Ciênc. Rural 2011, 41, 2217–2223. [Google Scholar] [CrossRef]
- Kao, P.-F.; Wang, S.-H.; Hung, W.-T.; Liao, Y.-H.; Lin, C.-M.; Yang, W.-B. Structural Characterization and Antioxidative Activity of Low-Molecular-Weights Beta-1,3-Glucan from the Residue of Extracted Ganoderma lucidum Fruiting Bodies. J. BioMed Biotech. 2011, 2012, 673764. [Google Scholar] [CrossRef]
- Du, B.; Zhu, F.; Xu, B. Physicochemical and Antioxidant Properties of Dietary Fibers from Qingke (Hull-Less Barley) Flour as Affected by Ultrafine Grinding. Bioact. Carbohydr. Diet. Fibre 2014, 4, 170–175. [Google Scholar] [CrossRef]
- Ookushi, Y.; Sakamoto, M.; Azuma, J. Optimization of Microwave-Assisted Extraction of Polysaccharides from the Fruiting Body of Mushrooms. J. Appl. Glycosci. 2006, 53, 267–272. [Google Scholar] [CrossRef][Green Version]
- Park, H.; Ka, K.-H.; Ryu, S.-R. Enhancement of β-Glucan Content in the Cultivation of Cauliflower Mushroom (Sparassis latifolia) by Elicitation. Mycobiology 2014, 42, 41–45. [Google Scholar] [CrossRef]
- Wood, P.; Paton, D. Extraction of High-Viscosity Gums from Oats. Cereal Chem. 1978, 55, 1038–1049. [Google Scholar]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioproc. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Molecular Aspects of Cereal β-Glucan Functionality: Physical Properties, Technological Applications and Physiological Effects. J. Cereal Sci. 2007, 46, 101–118. [Google Scholar] [CrossRef]
- Comin, L.M.; Temelli, F.; Saldaña, M.D.A. Barley Beta-Glucan Aerogels via Supercritical CO2 Drying. Food Res. Int. 2012, 48, 442–448. [Google Scholar] [CrossRef]
- Yoo, H.-U.; Ko, M.-J.; Chung, M.-S. Hydrolysis of Beta-Glucan in Oat Flour during Subcritical-Water Extraction. Food Chem. 2020, 308, 125670. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Ellis, E.E. A Rapid and Simple Method for the determination of starch and β-glucan in barley and malt. J. Inst. Brew. 1984, 90, 254–259. [Google Scholar] [CrossRef]
- Vasanthan, T.; Temelli, F. Grain Fractionation Technologies for Cereal Beta-Glucan Concentration. Food Res. Int. 2008, 41, 876–881. [Google Scholar] [CrossRef]
- Westerlund, E.; Andersson, R.; Åman, P. Isolation and Chemical Characterization of Water-Soluble Mixed-Linked β-Glucans and Arabinoxylans in Oat Milling Fractions. Carbohydr. Polym. 1993, 20, 115–123. [Google Scholar] [CrossRef]
- Wood, P.; Weisz, J.; Blackwell, B. Molecular Characterization of Cereal β-d-Glucans. Structural Analysis of Oat β-D-Glucan and Rapid Structural Evaluation of β-d-Glucans from Different Sources by High-Performance Liquid Chromatography of Oligosaccharides Released by Lichenase. Cereal Chem. 1991, 68, 31–39. [Google Scholar]
- Irakli, M.; Biliaderis, C.G.; Izydorczyk, M.S.; Papadoyannis, I.N. Isolation, Structural Features and Rheological Properties of Water-Extractable β-Glucans from Different Greek Barley Cultivars. J. Sci. Food Agric. 2004, 84, 1170–1178. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Ahmed, Z. Extraction and Characterization of Beta-D-Glucan from Oat for Industrial Utilization. Int. J. Biol. Macromol. 2010, 46, 304–309. [Google Scholar] [CrossRef]
- Babu, L.R. Green Extraction Techniques, Structural Analysis and Antioxidant Activites of B-Glucan Present in Oats. Int. J. Sci. Res. 2015, 4, 125–135. [Google Scholar]
- Benito-Román, O.; Alonso, E.; Lucas, S. Optimization of the β-Glucan Extraction Conditions from Different Waxy Barley Cultivars. J. Cereal Sci. 2011, 53, 271–276. [Google Scholar] [CrossRef]
- Harris, P.J.; Fincher, G.B. Chapter 4.6—Distribution, Fine Structure and Function of (1,3;1,4)-β-Glucans in the Grasses and Other Taxa. In Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides; Bacic, A., Fincher, G.B., Stone, B.A., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 621–654. [Google Scholar] [CrossRef]
- Zheng, G.H.; Rossnagel, B.G.; Tyler, R.T.; Bhatty, R.S. Distribution of β-Glucan in the Grain of Hull-Less Barley. Cereal Chem. 2000, 77, 140–144. [Google Scholar] [CrossRef]
- Wood, P.J.; Weisz, J.; Fedec, P.; Burrows, V.D. Large-Scale Preparation and Properties of Oat Fractions Enriched in (1-3)(1-4)-β-D-Glucan. Cereal Chem. 1989, 66, 97–103. [Google Scholar]
- Zheng, X.; Li, L.; Wang, X. Molecular Characterization of Arabinoxylans from Hull-Less Barley Milling Fractions. Molecules 2011, 16, 2743–2753. [Google Scholar] [CrossRef]
- De Brier, N.; Hemdane, S.; Dornez, E.; Gomand, S.V.; Delcour, J.A.; Courtin, C.M. Structure, Chemical Composition and Enzymatic Activities of Pearlings and Bran Obtained from Pearled Wheat (Triticum aestivum L.) by Roller Milling. J. Cereal Sci. 2015, 62, 66–72. [Google Scholar] [CrossRef]
- Izydorczyk, M.; Cenkowski, S.; Dexter, J. Optimizing the Bioactive Potential of Oat Bran by Processing. Cereal Foods World 2014, 59, 127–136. [Google Scholar] [CrossRef]
- Cavallero, A.; Empilli, S.; Brighenti, F.; Stanca, A.M. High (1→3,1→4)-β-Glucan Barley Fractions in Bread Making and Their Effects on Human Glycemic Response. J. Cereal Sci. 2002, 36, 59–66. [Google Scholar] [CrossRef]
- Holtekjølen, A.K.; Olsen, H.H.R.; Færgestad, E.M.; Uhlen, A.K.; Knutsen, S.H. Variations in Water Absorption Capacity and Baking Performance of Barley Varieties with Different Polysaccharide Content and Composition. LWT-Food Sci. Technol. 2008, 41, 2085–2091. [Google Scholar] [CrossRef]
- Knuckles, B.; Hudson, C.; Chiu, M.; Sayre, R. Effect of β-Glucan Barley Fractions in High-Fiber Bread and Pasta. Cereal Foods World 1997, 42, 94–99. [Google Scholar]
- Jacobs, M.S.; Izydorczyk, M.S.; Preston, K.R.; Dexter, J.E. Evaluation of Baking Procedures for Incorporation of Barley Roller Milling Fractions Containing High Levels of Dietary Fibre into Bread. J. Sci. Food Agric. 2008, 88, 558–568. [Google Scholar] [CrossRef]
- Kinner, M.; Nitschko, S.; Sommeregger, J.; Petrasch, A.; Linsberger-Martin, G.; Grausgruber, H.; Berghofer, E.; Siebenhandl-Ehn, S. Naked Barley—Optimized Recipe for Pure Barley Bread with Sufficient Beta-Glucan According to the EFSA Health Claims. J. Cereal Sci. 2011, 53, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Fransson, G.; Tietjen, M.; Åman, P. Content and Molecular-Weight Distribution of Dietary Fiber Components in Whole-Grain Rye Flour and Bread. J. Agric. Food Chem. 2009, 57, 2004–2008. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shan, L.; Xie, Y.; Min, F.; Gao, J.; Guo, L.; Ren, C.; Yuan, J.; Gilissen, L.; Chen, H. Effect of Fermentation on Content, Molecule Weight Distribution and Viscosity of β-Glucans in Oat Sourdough. Int. J. Food Sci. Technol. 2019, 54, 62–67. [Google Scholar] [CrossRef]
- Williams, J.G. Polymeric Materials Encyclopedia Edited by Joseph C. Salamone. CRC Press: Boca Raton, FL. 1996. ISBN 0-8493-2470-X. J. Am. Chem. Soc. 1998, 120, 6848–6849. [Google Scholar] [CrossRef]
- Maina, N.H.; Rieder, A.; De Bondt, Y.; Mäkelä-Salmi, N.; Sahlstrøm, S.; Mattila, O.; Lamothe, L.M.; Nyström, L.; Courtin, C.M.; Katina, K.; et al. Process-Induced Changes in the Quantity and Characteristics of Grain Dietary Fiber. Foods 2021, 10, 2566. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, Q.; Liu, H.; Fan, Z.; Shi, J.; Liu, X. Effect of Various Thermal Processing on the Structural and in Vitro Prebiotic Characteristics of β-Glucan from Hulless Barley. Food Hydrocol. 2023, 142, 108818. [Google Scholar] [CrossRef]
- Andersson, A.A.M.; Armö, E.; Grangeon, E.; Fredriksson, H.; Andersson, R.; Åman, P. Molecular Weight and Structure Units of (1→3, 1→4)-β-Glucans in Dough and Bread Made from Hull-Less Barley Milling Fractions. J. Cereal Sci. 2004, 40, 195–204. [Google Scholar] [CrossRef]
- Åman, P.; Rimsten, L.; Andersson, R. Molecular Weight Distribution of β-Glucan in Oat-Based Foods. Cereal Chem. 2004, 81, 356–360. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wu, S.-J.; Shyu, Y.-T. Antioxidant Properties of Certain Cereals as Affected by Food-Grade Bacteria Fermentation. J. Biosci. Bioeng. 2014, 117, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Åman, P. Cereal Arabinoxylan: Occurrence, Structure and Properties. In Advanced Dietary Fibre Technology; John Wiley & Sons: Hoboken, NJ, USA, 2000; pp. 299–314. [Google Scholar] [CrossRef]
- Rieder, A.; Holtekjølen, A.K.; Sahlstrøm, S.; Moldestad, A. Effect of Barley and Oat Flour Types and Sourdoughs on Dough Rheology and Bread Quality of Composite Wheat Bread. J. Cereal Sci. 2012, 55, 44–52. [Google Scholar] [CrossRef]
- Comino, P.; Collins, H.; Lahnstein, J.; Gidley, M.J. Effects of Diverse Food Processing Conditions on the Structure and Solubility of Wheat, Barley and Rye Endosperm Dietary Fibre. J. Food Eng. 2016, 169, 228–237. [Google Scholar] [CrossRef]
- Marklinder, I.; Johansson, L.; Haglund, Å.; Nagel-Held, B.; Seibel, W. Effects of Flour from Different Barley Varieties on Barley Sour Dough Bread. Food Qual. 1996, 7, 275–284. [Google Scholar] [CrossRef]
- Lambo, A.M.; Öste, R.; Nyman, M.E.G.-L. Dietary Fibre in Fermented Oat and Barley β-Glucan Rich Concentrates. Food Chem. 2005, 89, 283–293. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M.; Biliaderis, C.G. Effect of Barley β-Glucan Molecular Size and Level on Wheat Dough Rheological Properties. J. Food Eng. 2009, 91, 594–601. [Google Scholar] [CrossRef]
- Peressini, D.; Pin, M.; Sensidoni, A. Rheology and Breadmaking Performance of Rice-Buckwheat Batters Supplemented with Hydrocolloids. Food Hydrocol. 2011, 25, 340–349. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E.; Sullivan, P.; Flaherty, J.O.; Brunton, N.; Gallagher, E. Probabilistic Methodology for Assessing Changes in the Level and Molecular Weight of Barley β-Glucan during Bread Baking. Food Chem. 2011, 124, 1567–1576. [Google Scholar] [CrossRef]
- Rakha, A.; Åman, P.; Andersson, R. Characterisation of Dietary Fibre Components in Rye Products. Food Chem. 2010, 119, 859–867. [Google Scholar] [CrossRef]
- Delcour, J.A.; Rouau, X.; Courtin, C.M.; Poutanen, K.; Ranieri, R. Technologies for Enhanced Exploitation of the Health-Promoting Potential of Cereals. Trends Food Sci. Technol. 2012, 25, 78–86. [Google Scholar] [CrossRef]
- Djorgbenoo, R.; Hu, J.; Hu, C.; Sang, S. Fermented Oats as a Novel Functional Food. Nutrients 2023, 15, 3521. [Google Scholar] [CrossRef]
- Elliott, H.; Woods, P.; Green, B.D.; Nugent, A.P. Can Sprouting Reduce Phytate and Improve the Nutritional Composition and Nutrient Bioaccessibility in Cereals and Legumes? Nutr. Bull. 2022, 47, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.k.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of Attributes of Cereals by Germination and Fermentation: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmson, A.; Oksman-Caldentey, K.-M.; Laitila, A.; Suortti, T.; Kaukovirta-Norja, A.; Poutanen, K. Development of a Germination Process for Producing High β-Glucan, Whole Grain Food Ingredients from Oat. Cereal Chem. 2001, 78, 715–720. [Google Scholar] [CrossRef]
- Žilić, S.; Basić, Z.; Hadži-Tašković Šukalović, V.; Maksimović, V.; Janković, M.; Filipović, M. Can the Sprouting Process Applied to Wheat Improve the Contents of Vitamins and Phenolic Compounds and Antioxidant Capacity of the Flour? Int. J. Food Sci. Technol. 2014, 49, 1040–1047. [Google Scholar] [CrossRef]
- Ikram, A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Tufail, T.; Hussain, M.; Anjum, F.M. Nutritional and End-Use Perspectives of Sprouted Grains: A Comprehensive Review. Food Sci. Nutr. 2021, 9, 4617–4628. [Google Scholar] [CrossRef]
- Luo, Y.-W.; Xie, W.-H.; Jin, X.-X.; Wang, Q.; He, Y.-J. Effects of Germination on Iron, Zinc, Calcium, Manganese, and Copper Availability from Cereals and Legumes. CyTA-J. Food 2014, 12, 22–26. [Google Scholar] [CrossRef]
- Baranzelli, J.; Kringel, D.H.; Colussi, R.; Paiva, F.F.; Aranha, B.C.; de Miranda, M.Z.; Zavareze, E.d.R.; Dias, A.R.G. Changes in Enzymatic Activity, Technological Quality and Gamma-Aminobutyric Acid (GABA) Content of Wheat Flour as Affected by Germination. LWT-Food Sci. Technol. 2018, 90, 483–490. [Google Scholar] [CrossRef]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.-A.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Comp. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef]
- Edison, L.K.; Reji, S.R.; Pradeep, N.S. Beta-Glucanase in Breweries. In Microbial Beta Glucanases: Molecular Structure, Functions and Applications; Pradeep, N.S., Edison, L.K., Eds.; Springer Nature: Singapore, 2022; pp. 85–98. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the Nutritional, Functional and Technological Effects of the Sourdough Fermentation of Sprouted Flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Hübner, F.; O’Neil, T.; Cashman, K.D.; Arendt, E.K. The Influence of Germination Conditions on Beta-Glucan, Dietary Fibre and Phytate during the Germination of Oats and Barley. Eur. Food Res. Technol. 2010, 231, 27–35. [Google Scholar] [CrossRef]
- Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peñas, E. Sprouted Oat as a Potential Gluten-Free Ingredient with Enhanced Nutritional and Bioactive Properties. Food Chem. 2021, 338, 127972. [Google Scholar] [CrossRef]
- Islam, M.Z.; An, H.-G.; Kang, S.-J.; Lee, Y.-T. Physicochemical and Bioactive Properties of a High β-Glucan Barley Variety ‘Betaone’ Affected by Germination Processing. Int. J. Biol. Macromol. 2021, 177, 129–134. [Google Scholar] [CrossRef]
- Sozer, N.; Poutanen, K. Fibre in Extruded Products. In Fibre-Rich and Wholegrain Foods: Improving Quality; Delcour, J.A., Poutanen, K., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 256–272. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S. Extrusion of Hulled Barley Affecting β-Glucan and Properties of Extrudates. Food Biop. Technol. 2013, 6, 1374–1389. [Google Scholar] [CrossRef]
- Tosh, S.M.; Brummer, Y.; Miller, S.S.; Regand, A.; Defelice, C.; Duss, R.; Wolever, T.M.S.; Wood, P.J. Processing Affects the Physicochemical Properties of β-Glucan in Oat Bran Cereal. J. Agric. Food Chem. 2010, 58, 7723–7730. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef]
- Gajula, H.; Alavi, S.; Adhikari, K.; Herald, T. Precooked Bran-Enriched Wheat Flour Using Extrusion: Dietary Fiber Profile and Sensory Characteristics. J. Food Sci. 2008, 73, S173–S179. [Google Scholar] [CrossRef]
- Sobota, A.; Sykut-Domańska, E.; Rzedzicki, Z. Effect of Extrusion-Cooking Process on the Chemical Composition of Corn-Wheat Extrudates, with Particular Emphasis on Dietary Fibre Fractions. Pol. J. Food Nutr. Sci. 2010, 60, 251–259. [Google Scholar]
- Vasanthan, T.; Gaosong, J.; Yeung, J.; Li, J. Dietary Fiber Profile of Barley Flour as Affected by Extrusion Cooking. Food Chem. 2002, 77, 35–40. [Google Scholar] [CrossRef]
- Sozer, N.; Cicerelli, L.; Heiniö, R.-L.; Poutanen, K. Effect of Wheat Bran Addition on in Vitro Starch Digestibility, Physico-Mechanical and Sensory Properties of Biscuits. J. Cereal Sci. 2014, 60, 105–113. [Google Scholar] [CrossRef]
- Gujral, H.S.; Sharma, P.; Rachna, S. Effect of Sand Roasting on Beta Glucan Extractability, Physicochemical and Antioxidant Properties of Oats. LWT-Food Sci. Technol. 2011, 44, 2223–2230. [Google Scholar] [CrossRef]
- Schmidt, M. Cereal Beta-Glucans: An Underutilized Health Endorsing Food Ingredient. Crit. Rev. Food Sci. Nutr. 2022, 62, 3281–3300. [Google Scholar] [CrossRef]
- Loebnitz, N.; Grunert, K.G. Impact of Self-Health Awareness and Perceived Product Benefits on Purchase Intentions for Hedonic and Utilitarian Foods with Nutrition Claims. Food Qual. 2018, 64, 221–231. [Google Scholar] [CrossRef]
- Wood, P.J. Cereal β-Glucans in Diet and Health. J. Cereal Sci. 2007, 46, 230–238. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta Glucan: A Valuable Functional Ingredient in Foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Zeković, D.B.; Kwiatkowski, S.; Vrvić, M.M.; Jakovljević, D.; Moran, C.A. Natural and Modified (1→3)-β-d-Glucans in Health Promotion and Disease Alleviation. Crit. Rev. Biotech. 2005, 25, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Lyly, M.; Salmenkallio-Marttila, M.; Suortti, T.; Autio, K.; Poutanen, K.; Lähteenmäki, L. Influence of Oat β-Glucan Preparations on the Perception of Mouthfeel and on Rheological Properties in Beverage Prototypes. Cereal Chem. 2003, 80, 536–541. [Google Scholar] [CrossRef]
- Karp, S.; Wyrwisz, J.; Kurek, M.A. Comparative Analysis of the Physical Properties of o/w Emulsions Stabilised by Cereal β-Glucan and Other Stabilisers. Int. J. Biol. Macromol. 2019, 132, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, N.; Xiong, Z.; Wang, G.; Xia, Y.; Lai, P.; Ai, L. Structural Characterization and Rheological Properties of β-D-Glucan from Hull-Less Barley (Hordeum vulgare L. Var. nudum Hook. f.). Phytochemistry 2018, 155, 155–163. [Google Scholar] [CrossRef]
- Chatterjee, B.; Patel, T. Increased Sensory Quality and Consumer Acceptability by Fortification of Chocolate Flavored Milk with Oat Beta Glucan. Int. J. Clin. Biomed. Res. 2016, 2, 25–28. [Google Scholar]
- Kordialik-Bogacka, E.; Bogdan, P.; Diowksz, A. Malted and unmalted oats in brewing. J. Inst. Brew. 2014, 120, 390–398. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Pater, A.; Knapik, A.; Duliński, R. The effect of different oat (Avena sativa L.) malt contents in a top-fermented beer recipe on the brewing process performance and product quality. J. Cereal Sci. 2021, 101, 103301. [Google Scholar] [CrossRef]
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a New Oat-Based Probiotic Drink. Int. J. Food Microbiol. 2006, 112, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Harasym, J.; Suchecka, D.; Gromadzka-Ostrowska, J. Effect of Size Reduction by Freeze-Milling on Processing Properties of Beta-Glucan Oat Bran. J. Cereal Sci. 2015, 61, 119–125. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A Critical Review on the Impacts of β-Glucans on Gut Microbiota and Human Health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Erive, M.; He, F.; Wang, T.; Chen, G. Development of β-Glucan Enriched Wheat Bread Using Soluble Oat Fiber. J. Cereal Sci. 2020, 95, 103051. [Google Scholar] [CrossRef]
- Hager, A.-S.; Ryan, L.A.M.; Schwab, C.; Gänzle, M.G.; O’Doherty, J.V.; Arendt, E.K. Influence of the Soluble Fibres Inulin and Oat β-Glucan on Quality of Dough and Bread. Eur. Food Res. Technol. 2011, 232, 405–413. [Google Scholar] [CrossRef]
- De Paula, R.; Abdel-Aal, E.-S.M.; Messia, M.C.; Rabalski, I.; Marconi, E. Effect of Processing on the Beta-Glucan Physicochemical Properties in Barley and Semolina Pasta. J. Cereal Sci. 2017, 75, 124–131. [Google Scholar] [CrossRef]
- Messia, M.C.; Oriente, M.; Angelicola, M.; De Arcangelis, E.; Marconi, E. Development of Functional Couscous Enriched in Barley β-Glucans. J. Cereal Sci. 2019, 85, 137–142. [Google Scholar] [CrossRef]
- Choo, C.L.; Aziz, N.A.A. Effects of Banana Flour and β-Glucan on the Nutritional and Sensory Evaluation of Noodles. Food Chem. 2010, 119, 34–40. [Google Scholar] [CrossRef]
- Moza, J.; Gujral, H.S. Influence of Barley Non-Starchy Polysaccharides on Selected Quality Attributes of Sponge Cakes. LWT-Food Sci. Technol. 2017, 85, 252–261. [Google Scholar] [CrossRef]
- Vitaglione, P.; Lumaga, R.B.; Montagnese, C.; Messia, M.C.; Marconi, E.; Scalfi, L. Satiating Effect of a Barley Beta-Glucan–Enriched Snack. J. Am. Col. Nutr. 2010, 29, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Zbikowska, A.; Kowalska, M.; Zbikowska, K.; Onacik-Gür, S.; Łempicka, U.; Turek, P. Study on the incorporation of oat and yeast β-glucan into shortbread biscuits as a basis for designing healthier and high-quality food products. Molecules 2022, 27, 1393. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.S.; Tudorica, C.M. Carbohydrate-Based Fat Replacers in the Modification of the Rheological, Textural and Sensory Quality of Yoghurt: Comparative Study of the Utilisation of Barley Beta-Glucan, Guar Gum and Inulin. Int. J. Food Sci. Technol. 2008, 43, 824–833. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Molec. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kim, S.; Liu, S.X. Effect of Purified Oat β-Glucan on Fermentation of Set-Style Yogurt Mix. J. Food Sci. 2012, 77, E195–E201. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Karaca, O.B.; Kaya, A.; Sahan, N.; Yasar, K. Influence of Fat Replacers on Chemical Composition, Proteolysis, Texture Profiles, Meltability and Sensory Properties of Low-Fat Kashar Cheese. J. Dairy Res. 2008, 75, 1–7. [Google Scholar] [CrossRef]
- Volikakis, P.; Biliaderis, C.G.; Vamvakas, C.; Zerfiridis, G.K. Effects of a Commercial Oat-β-Glucan Concentrate on the Chemical, Physico-Chemical and Sensory Attributes of a Low-Fat White-Brined Cheese Product. Food Res. Int. 2004, 37, 83–94. [Google Scholar] [CrossRef]
- Tudorica, C.M.; Jones, T.E.R.; Kuri, V.; Brennan, C.S. The Effects of Refined Barley β-Glucan on the Physico-Structural Properties of Low-Fat Dairy Products: Curd Yield, Microstructure, Texture and Rheology. J. Sci. Food Agric. 2004, 84, 1159–1169. [Google Scholar] [CrossRef]
- Raikos, V.; Grant, S.B.; Hayes, H.; Ranawana, V. Use of β-Glucan from Spent Brewer’s Yeast as a Thickener in Skimmed Yogurt: Physicochemical, Textural, and Structural Properties Related to Sensory Perception. J. Dairy Sci. 2018, 101, 5821–5831. [Google Scholar] [CrossRef]
- Santipanichwong, R.; Suphantharika, M. Carotenoids as Colorants in Reduced-Fat Mayonnaise Containing Spent Brewer’s Yeast β-Glucan as a Fat Replacer. Food Hydrocol. 2007, 21, 565–574. [Google Scholar] [CrossRef]
- Bhaskar, D.; Khatkar, S.K.; Chawla, R.; Panwar, H.; Kapoor, S. Effect of β-Glucan Fortification on Physico-Chemical, Rheological, Textural, Colour and Organoleptic Characteristics of Low Fat Dahi. J. Food Sci. Technol. 2017, 54, 2684–2693. [Google Scholar] [CrossRef] [PubMed]
- Elsanhoty, R.; Zaghlol, A.; Hassanein, A.H. The Manufacture of Low Fat Labneh Containing Barley β-Glucan 1-Chemical Composition, Microbiological Evaluation and Sensory Properties. Curr. Res. Dairy Sci. 2009, 1, 1–12. [Google Scholar] [CrossRef]
- Vithanage, C.J.; Mishra, V.K.; Vasiljevic, T.; Shah, N. Use of β-Glucan in Development of Low-Fat Mozzarella Cheese. Milchwissenschaft 2008, 63, 420–423. [Google Scholar]
- Mykhalevych, A.; Polishchuk, G.; Nassar, K.; Osmak, T.; Buniowska-Olejnik, M. β-Glucan as a Techno-Functional Ingredient in Dairy and Milk-Based Products—A Review. Molecules 2022, 27, 6313. [Google Scholar] [CrossRef]
- Salmerón, I. Fermented Cereal Beverages: From Probiotic, Prebiotic and Synbiotic towards Nanoscience Designed Healthy Drinks. Lett. Appl. Microbiol. 2017, 65, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Lamba, A.; Garg, V. Nanotechnology Approach in Food Science: A Review. Nanotechnology 2018, 3, 183–186. [Google Scholar]
- Huang, J.; Wu, C.; Tang, S.; Zhou, P.; Deng, J.; Zhang, Z.; Wang, Y.; Wang, Z. Chiral Active β-Glucan Nanoparticles for Synergistic Delivery of Doxorubicin and Immune Potentiation. Int. J. Nanomed. 2020, 2020, 5083–5095. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, K.; Gilad, A.A.; Choi, J. Synthesis of Beta-Glucan Nanoparticles for the Delivery of Single Strand DNA. Biotech. Bioproc. Eng. 2018, 23, 144–149. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Physiological effects of different types of β-glucan. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2007, 151, 225–231. [Google Scholar] [CrossRef]
- ul Ashraf, Z.; Shah, A.; Gani, A.; Gani, A.; Masoodi, F.A.; Noor, N. Nanoreduction as a Technology to Exploit β-Glucan from Cereal and Fungal Sources for Enhancing Its Nutraceutical Potential. Carbohydr. Polym. 2021, 258, 117664. [Google Scholar] [CrossRef]
- Bohn, J.A.; BeMiller, J.N. (1→3)-β-d-Glucans as Biological Response Modifiers: A Review of Structure-Functional Activity Relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Peltzer, M.; Delgado, F.J.; Salvay, G.A.; Wagner, R.J. β-Glucan, a Promising Polysaccharide for Bio-Based Films Developments for Food Contact Materials and Medical Applications. Curr. Org. Chem. 2018, 22, 1249–1254. [Google Scholar] [CrossRef]
- Lante, A.; Canazza, E.; Tessari, P. Beta-Glucans of Cereals: Functional and Technological Properties. Nutrients 2023, 15, 2124. [Google Scholar] [CrossRef] [PubMed]
- Pavlek, Z.; Bosnir, J.; Kuharic, Z.; Racz, A.; Jurak, I.; Lasic, D.; Markov, K.; Jakopovic, Z.; Frece, J. The Influence of Binding of Selected Mycotoxin Deactivators and Aflatoxin M1 on the Content of Selected Micronutrients in Milk. Processes 2022, 10, 2431. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, T.; Li, J.; Wu, T.; Li, Q.; Meng, Y.; Cao, Q.; Zhang, M. Physicochemical and Antioxidative Properties of Superfine-Ground Oat Bran Polysaccharides. Food Sci. Technol. Res. 2016, 22, 101–109. [Google Scholar] [CrossRef]
- ul Ashraf, Z.; Shah, A.; Gani, A.; Masoodi, F.A.; Noor, N. Effect of Nano-Reduction on Properties of β-Glucan and Its Use as Encapsulating Agent for Release of α-Tocopherol. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100230. [Google Scholar] [CrossRef]
- Udayangani, R.M.C.; Dananjaya, S.H.S.; Fronte, B.; Kim, C.-H.; Lee, J.; De Zoysa, M. Feeding of Nano Scale Oats β-Glucan Enhances the Host Resistance against Edwardsiella Tarda and Protective Immune Modulation in Zebrafish Larvae. Fish Shellfish Immunol. 2017, 60, 72–77. [Google Scholar] [CrossRef]
| β-Glucan Properties | β-Glucan Sources | ||||
|---|---|---|---|---|---|
| Oat | Barley | Mushrooms | Brewer’s Yeast | Baker’s Yeast | |
| Structure | β-1,3;1,4 glucans | β-1,3;1,4 glucans | β-1,3;1,6 glucans | β-1,3;1,6 glucans | β-1,3;1,6 glucans |
| Reduce serum cholesterol levels | X | ||||
| Attenuate blood glucose level | X | X | X | ||
| Improve/stimulate immune function | X | X | X | ||
| Promotes healthy inflammatory response | X | X | |||
| Topical application/skin treatment/wound healing | X | ||||
| Mycotoxin adsorption | X | X | X | X | |
| Fat replacer in food formulations | X | X | |||
| Food thickener, emulsion stabilizer | X | X | X | ||
| Film-forming biopolymer | X | X | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurkaninová, L.; Dvořáček, V.; Gregusová, V.; Havrlentová, M. Cereal β-d-Glucans in Food Processing Applications and Nanotechnology Research. Foods 2024, 13, 500. https://doi.org/10.3390/foods13030500
Jurkaninová L, Dvořáček V, Gregusová V, Havrlentová M. Cereal β-d-Glucans in Food Processing Applications and Nanotechnology Research. Foods. 2024; 13(3):500. https://doi.org/10.3390/foods13030500
Chicago/Turabian StyleJurkaninová, Lucie, Václav Dvořáček, Veronika Gregusová, and Michaela Havrlentová. 2024. "Cereal β-d-Glucans in Food Processing Applications and Nanotechnology Research" Foods 13, no. 3: 500. https://doi.org/10.3390/foods13030500
APA StyleJurkaninová, L., Dvořáček, V., Gregusová, V., & Havrlentová, M. (2024). Cereal β-d-Glucans in Food Processing Applications and Nanotechnology Research. Foods, 13(3), 500. https://doi.org/10.3390/foods13030500





