Artichoke Industrial Waste in Durum Wheat Bread: Effects of Two Different Preparation and Drying Methods of Flours and Evaluation of Quality Parameters during Short Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Flour from Artichoke
- -
- Fresh sample (A): drying in an oven (Memmert, Milan, Italy) at 40 °C for 48 h. Afterwards, the samples were reduced to smaller fragments using a benchtop blender and then the ‘Cyclotec’ type 120 mill (Falling Number, Huddinge, Sweden) further reduced the flour to diameter of 500 μ.
- -
- Shredded and frozen sample (B): Samples were frozen at −15 °C in a domestic freezer (Beko, Milan, Italy) and then oven-dried (Memmert, Milan, Italy) at 40 °C for 24 h. Then, the samples were cut into smaller fragments using a benchtop blender and reduced to flour with a diameter of 500 μ via the Cyclotec type 120 mill (Falling Number, Huddinge, Sweden). For each drying experiment, the samples reached a final moisture content of between 4 and 6% [24].
2.3. Color Determination
2.4. Polyphenols Content and Antioxidant Activity (DPPH)
2.5. Physical Characteristics of Breads with Different Integration of Artichoke Flour
- -
- Yeast solution: 600 mL distilled water and 80 g yeast (Despar Italia from ZEUS IBA srl, Casalecchio di Reno, Bologna, Italy);
- -
- Salt–sugar solution: 600 g distilled water, 80 g salt (Italkali spa, Palermo, Italy), and 40 g sugar (Conad, Pontelongo, Padua, Italy);
- -
- Ascorbic acid solution: 500 mL distilled water and 0.04 g ascorbic acid (Bontà Infinite srl, Terme Vigliatore, Messina, Italy).
2.6. Determination of Bread Staling Rate
2.7. Statistical Analyses
2.7.1. One-Factor and Two-Factor ANOVA
2.7.2. Cluster Analysis
3. Results and Discussion
3.1. Flours
3.1.1. Polyphenols Content and Antioxidant Activity in Flours
3.1.2. Color Parameters of Flours
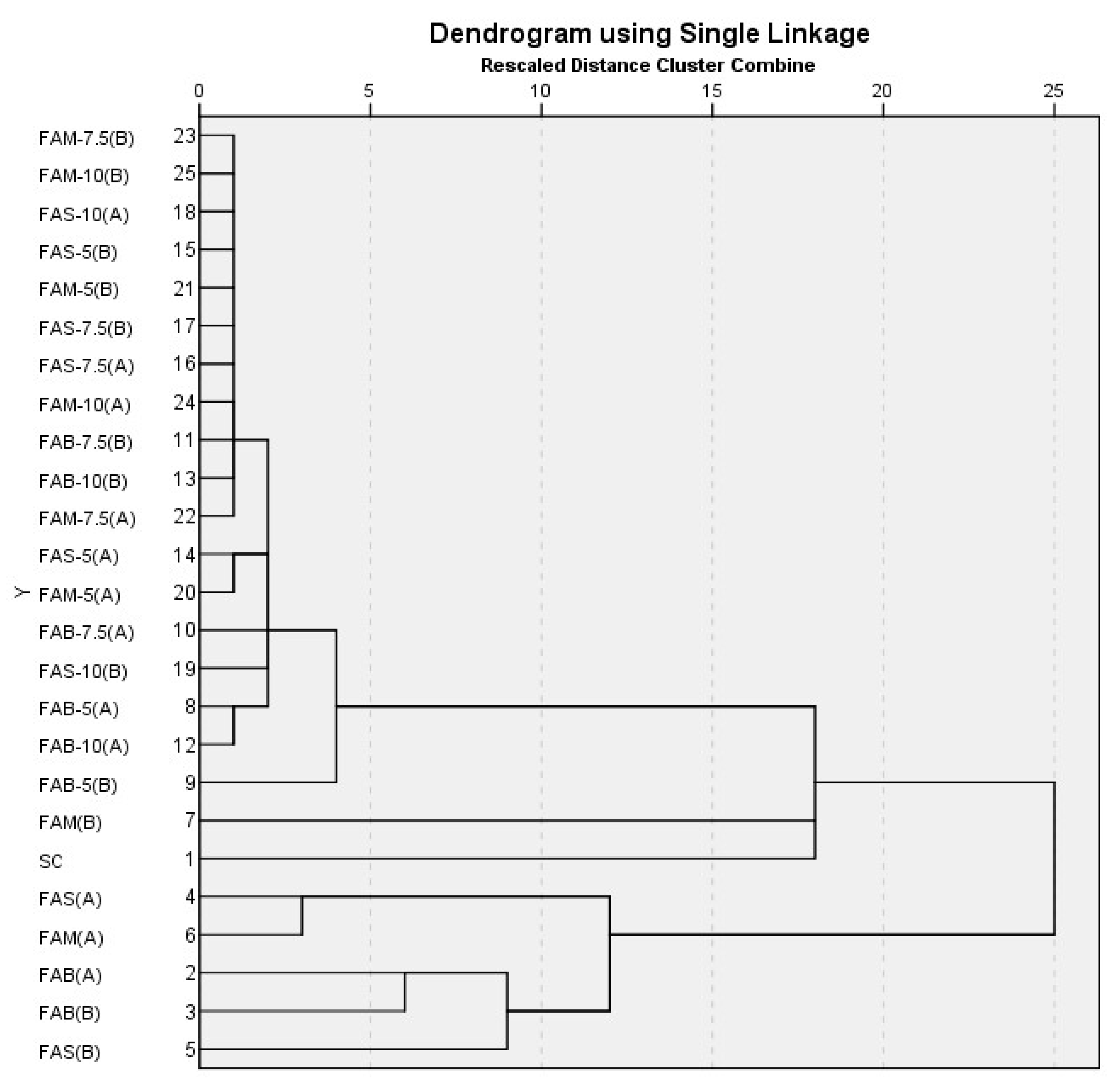
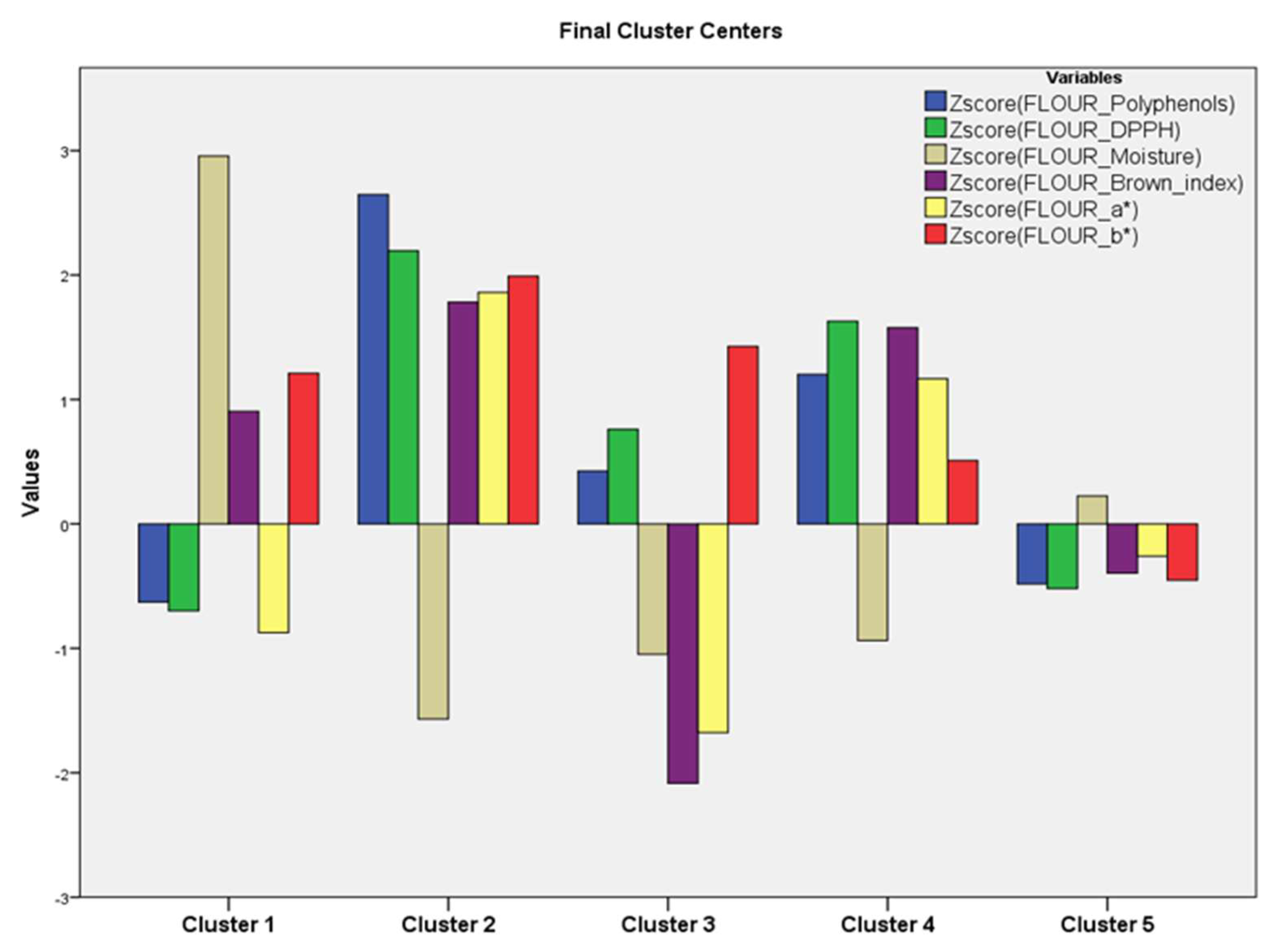
3.1.3. Cluster Analysis (Hierarchical Cluster Analysis and K-Means Cluster Analysis) in Flours
3.2. Breads
3.2.1. Technological Analysis of Breads
3.2.2. TPA (Texture Profile Analysis) of the Breads Supplemented with Different Percentages of Additions
3.2.3. Polyphenols Content, Antioxidant Activity, and Water Activity in Breads with Different Integration Percentages
3.2.4. Color of Bread
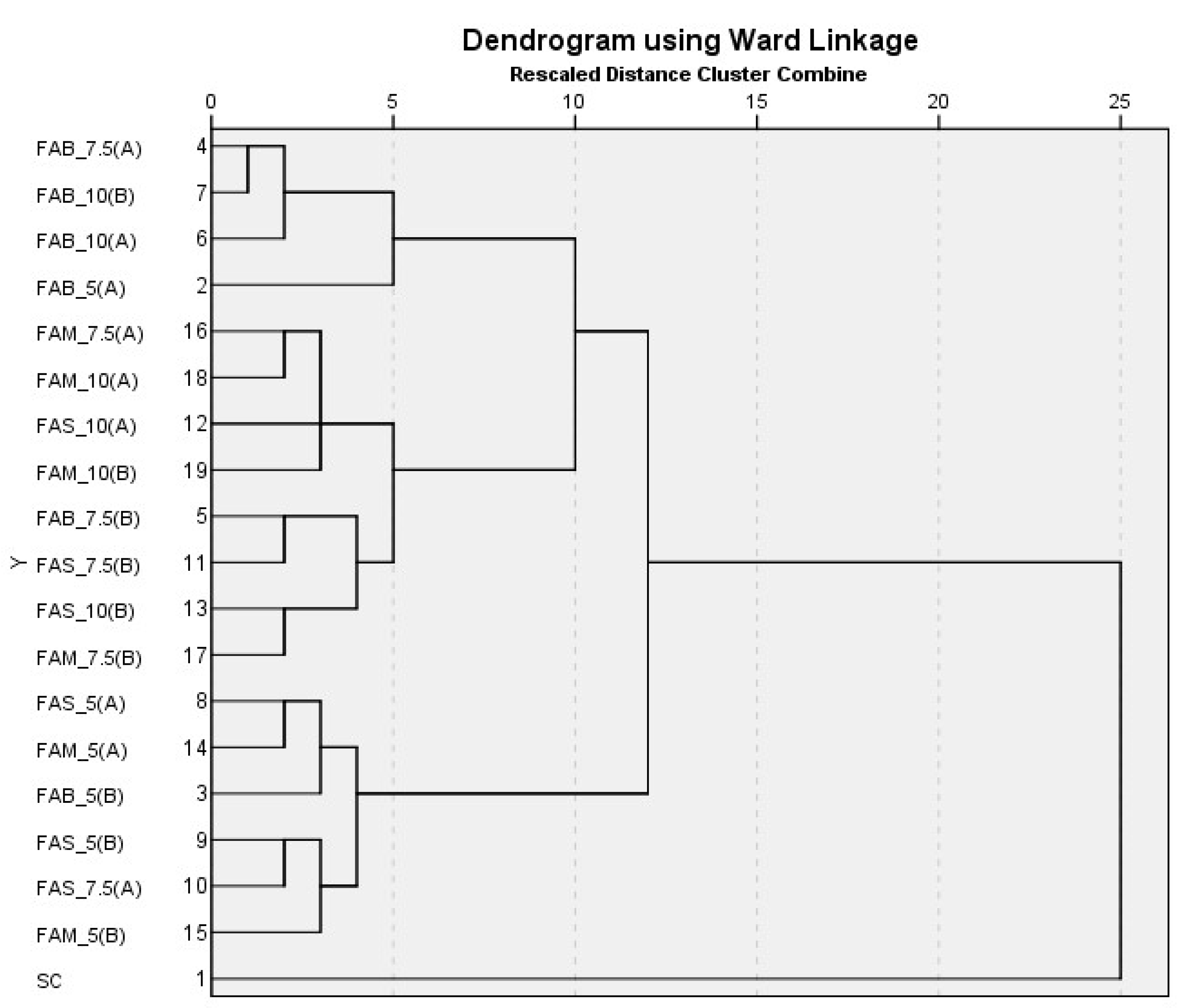
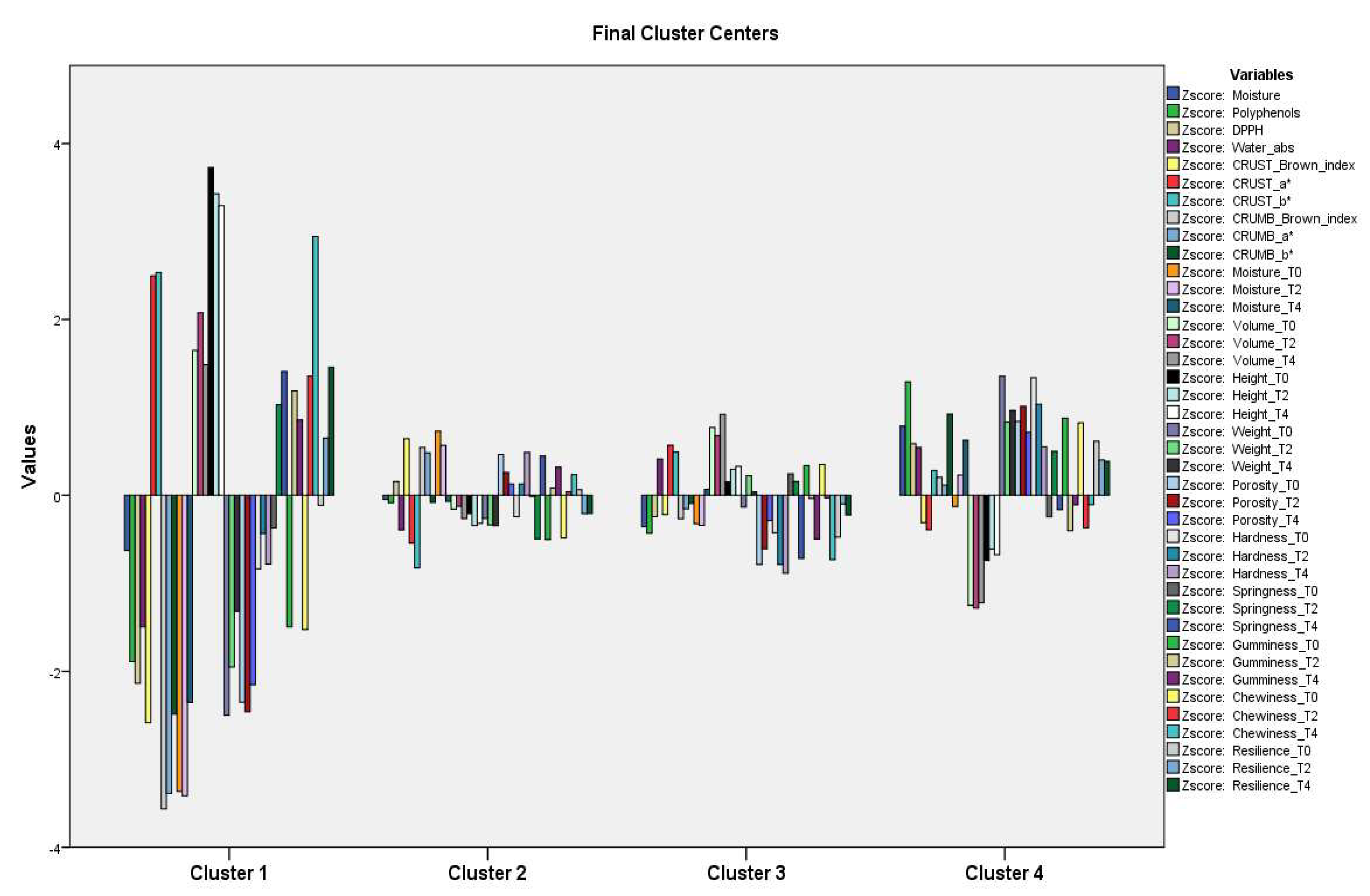
3.2.5. Cluster Analysis (Hierarchical Cluster Analysis and K-Means Cluster Analysis) in Breads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mauromicale, G.; Ierna, A. Effects of gibberellic acid and sowing date on harvest time and yields of seed-grown globe artichoke (Cynara scolymus L.). Agronomie 1995, 15, 527–538. [Google Scholar] [CrossRef]
- Fratianni, F.; Tucci, M.; De Palma, M.; Pepe, R.; Nazzaro, F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Mayor, L.; Calvo-Ramirez, A.; Ruiz-Melero, R.; Rodriguez-Lopez, A.D. Response Surface Optimization of Inulin and Polyphenol Extraction from Artichoke (Cynara scolymus (L.)) Solid Wastes. Appl. Sci. 2022, 12, 7957. [Google Scholar] [CrossRef]
- Meyer, D.; Blaauwhoed, J.P. Inulin. In Handbook of Hydrocolloids, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 829–848. [Google Scholar]
- Long, X.H.; Shao, H.B.; Liu, L.; Liu, L.P.; Liu, Z.P. Jerusalem artichoke: A sustainable biomass feedstock for biorefinery. Renew. Sustain. Energy Rev. 2016, 54, 1382–1388. [Google Scholar] [CrossRef]
- Canale, M.; Spina, A.; Summo, C.; Strano, M.C.; Bizzini, M.; Allegra, M.; Sanfilippo, R.; Amenta, M.; Pasqualone, A. Waste from Artichoke Processing Industry: Reuse in Bread-Making and Evaluation of the Physico-Chemical Characteristics of the Final Product. Plants 2022, 11, 3409. [Google Scholar] [CrossRef]
- Salem, M.B.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Globe artichoke leaves and floral stems as a source of bioactive compounds. Ind. Crops Prod. 2013, 44, 44–49. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Reuse potential of artichoke (Cynara scolimus L.) waste for the recovery of phenolic compounds and bioenergy. J. Clean. Prod. 2016, 111, 279–284. [Google Scholar] [CrossRef]
- Brat, P.; Georgé, S.; Bellamy, A.; Du Chaffaut, L.D.; Scalbert, A.; Mennen, L.; Arnault, N.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 2006, 136, 2368–2373. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D. Functional foods and beverages enhanced with polyphenols in the absence or presence of dietary fiberss. In Polyphenols: Chemistry, Dietary Sources and Health Benefits; Sun, J., Prasad, K.N., Eds.; Nova Science Publishers: New York, NY, USA, 2013; pp. 217–240. [Google Scholar]
- Kalinina, I.; Fatkullin, R.; Naumenko, N.; Popova, N.; Stepanova, D. The Influence of Flavonoid Dihydroquercetin on the Enzymatic Processes of Dough Ripening and the Antioxidant Properties of Bread. Fermentation 2023, 9, 263. [Google Scholar] [CrossRef]
- Dziki, D.; Różyło, R.; Gawlik-Dziki, U.; Świeca, M. Current trends in the enhancement of antioxidant activity of wheat bread by the addition of plant materials rich in phenolic compounds. Trends Food Sci. Technol. 2014, 40, 48–61. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Muccilli, S.; Padalino, L.; Giannone, V.; Lecce, L.; Giovanniello, V.; Del Nobile, M.A.; De Vita, P.; Spina, A. Durum wheat breads ‘high in fibers’ and with reduced in vitro glycaemic response obtained by partial semolina replacement with minor cereals and pulses. J. Food Sci. Technol. 2018, 55, 4458–4467. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- De Falco, E.; Senatore, A.; Roscigno, G.; Pergola, M. The Artichoke “Bianco di Pertosa”: The Enhancement of Crop Residues through Environmentally Friendly Uses. Horticulturae 2022, 8, 900. [Google Scholar] [CrossRef]
- Van Hal, O.; De Boer, I.J.M.; Muller, A.; De Vries, S.; Erb, K.H.; Schader, C.; Gerrits, W.J.J.; Van Zanten, H.H.E. Upcycling food leftovers and grass resources through livestock: Impact of livestock system and productivity. J. Clean. Prod. 2019, 219, 485–496. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Boukid, F.; Angelis, D.D.; Summo, C. Use of almond skins to improve nutritional and functional properties of biscuits: An example of upcycling. Foods 2020, 9, 1705. [Google Scholar] [CrossRef] [PubMed]
- Spratt, O.; Suri, R.; Deutsch, J. Defining upcycled food products. J. Culin. Sci. Technol. 2021, 19, 485–496. [Google Scholar] [CrossRef]
- Dadalı, C. Artichoke bracts as fat and wheat flour replacer in cake: Optimization of reduced fat and reduced wheat flour cake formulation. J. Food Meas. Charact. 2022, 17, 98–107. [Google Scholar] [CrossRef]
- Borsini, A.A.; Llavata, B.; Umaña, M.; Cárcel, J.A. Artichoke by products as a source of antioxidant and fiber: How it can be affected by drying temperature. Foods 2021, 10, 459. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Frutos, M.J.; Hernández-Herrero, J.A.; Pérez-Llamas, F.; Zamora, S. Effect of chlorophyll removal and particle size upon the nutritional and technological properties of powdered by-products from artichoke (Cynara scolymus L.) industrial canning. Int. J. Food Sci. Technol. 2015, 50, 2383–2390. [Google Scholar] [CrossRef]
- Guiné, R.P.; Lima, M.J. Study of the drying kinetics and calculation of mass transfer properties in hot air drying of Cynara cardunculus. Open Agric. 2020, 5, 740–750. [Google Scholar] [CrossRef]
- McCaig, T.N. Extending the use of visible/near-infrared reflectance spectrophotometers to measure color of food and agricultural products. Food Res. Int. 2002, 35, 731–736. [Google Scholar] [CrossRef]
- CIE. Colorimetry, 2nd ed.; Publication CIE No 15.2; Central Bureau of the Commission Internationale de L’Eclairage: Vienna, Austria, 1986. [Google Scholar]
- Spina, A.; Brighina, S.; Muccilli, S.; Mazzaglia, A.; Rapisarda, P.; Fallico, B.; Arena, E. Partial replacement of NaCl in bread from durum wheat (Triticum turgidum L. subsp. durum Desf.) with KCl and yeast extract: Evaluation of quality parameters during long storage. Food Bioprocess Technol. 2015, 8, 1089–1101. [Google Scholar] [CrossRef]
- Kerckhove, E.; Staes, F.; Flour, M.; Stappaerts, K.; Boeckx, W. Reproducibility of repeated measurements on healthy skin with Minolta Chromameter CR-300. Skin Res. Technol. 2001, 7, 56–59. [Google Scholar] [CrossRef]
- Parafati, L.; Restuccia, C.; Palmeri, R.; Fallico, B.; Arena, E. Characterization of Prickly Pear Peel Flour as a Bioactive and Functional Ingredient in Bread Preparation. Foods 2020, 9, 1189. [Google Scholar] [CrossRef]
- Vazquez-Roncero, A.; Janer del Valle, M.I.; Janer del Valle, C. Determination of total phenols in olive oil. Grasas Y Aceites 1973, 24, 350–355. [Google Scholar]
- Brand-Williams, W.; Culiver, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- AACC. Approved Methods of Analysis, 11th ed.; The American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Revision 4; AOAC: Rockville, MD, USA, 2011. [Google Scholar]
- Giannone, V.; Lauro, M.R.; Spina, A.; Pasqualone, A.; Auditore, L.; Puglisi, I.; Puglisi, G. A novel α-amylase-lipase formulation as anti-staling agent in durum wheat bread. LWT-Food Sci. Technol. 2016, 65, 381–389. [Google Scholar] [CrossRef]
- Dallmann, H. Porentabelle, 4th ed.; Verlag Moritz Schäfer: Detmold, Germany, 1981. [Google Scholar]
- Bot, B.; Sánchez, H.; de la Torre, M.; Osella, C. Mother dough in bread making. Food Sci. Nutr. 2014, 2, 24–29. [Google Scholar] [CrossRef][Green Version]
- Różyło, R.; Piekut, J.; Wójcik, M.; Kozłowicz, K.; Smolewska, M.; Krajewska, M.; Szmi-gielski, M.; Bourekoua, H. Black cumin pressing waste material as a functional additive for starch bread. Materials 2021, 14, 4560. [Google Scholar] [CrossRef] [PubMed]
- Duh, P.D.; Yen, G.C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat) varieties in 604 soybean oil emulsion. Food Chem. 1999, 66, 471–476. [Google Scholar] [CrossRef]
- Anagnostopoulou, P.; Kefalas, V.P.; Papageorgiou, A.N.; Assimopoulou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of selected food. Int. J. Food Prop. 2011, 14, 300–308. [Google Scholar] [CrossRef]
- Alén-Ruiz, F.; García-Falcón, M.S.; Pérez-Lamela, M.C.; Martínez-Carballo, E.; Simal-Gándara, J. Influence of major polyphenols on antioxidant activity in Mencía and Brancellao red wines. Food Chem. 2009, 113, 53–60. [Google Scholar] [CrossRef]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. Potential bioaccessibility and functionality of polyphenols and cynaropicrin from breads enriched with artichoke stem. Food Chem. 2018, 245, 838–844. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Effect of solvent type and extraction conditions on the recovery of phenolic compounds from artichoke waste. Chem. Eng. 2014, 39, 463–468. [Google Scholar]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef]
- Todaro, A.; Peluso, O.; Catalano, A.E.; Mauromicale, G.; Spagna, G. Polyphenol oxidase activity from three Sicilian artichoke [Cynara cardunculus L. var. scolymus L. (Fiori)] cultivars: Studies and technological application on minimally processed production. J. Agric. Food Chem. 2010, 58, 1714–1718. [Google Scholar] [CrossRef]
- Cefola, M.; D’Antuono, I.; Pace, B.; Calabrese, N.; Carito, A.; Linsalata, V.; Cardinali, A. Biochemical relationships and browning index for assessing the storage suitability of artichoke genotypes. Food Res. Int. 2012, 48, 397–403. [Google Scholar] [CrossRef]
- Carrubba, A.; Comparato, A.; Labruzzo, A.; Muccilli, S.; Giannone, V.; Spina, A. Quality characteristics of wholemeal flour and bread from durum wheat (Triticum turgidum L. subsp. durum Desf.) after field treatment with plant water extracts. J. Food Sci. 2016, 81, C2158–C2166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fendri, L.B.; Chaari, F.; Maaloul, M.; Kallel, F.; Abdelkafi, L.; Chaabouni, S.E.; Ghribi-Aydi, D. Wheat bread enrichment by pea and broad bean pods fibers: Effect on dough rheology and bread quality. LWT 2016, 73, 584–591. [Google Scholar] [CrossRef]
- Srikiatden, J.; Roberts, J.S. Moisture transfer in solid food materials: A review of mechanisms, models, and measurements. Int. J. Food Prop. 2007, 10, 739–777. [Google Scholar] [CrossRef]
- Legge 4 Luglio 1967, n. 580. Disciplina per la Lavorazione e Commercio dei Cereali, Degli Sfarinati, del Pane e Delle Paste Alimentari, art.16. Available online: https://www.gazzettaufficiale.it/eli/id/1967/07/29/067U0580/sg (accessed on 5 August 2023).
- Decreto del Presidente della Repubblica 30 Novembre 1998, n. 502. Regolamento Recante Norme per la Revisione Della Normativa in Materia di Lavorazione e di Commercio del Pane, a Norma Dell’articolo 50 Della Legge 22 Febbraio 1994, n. 146, art.5. Available online: http://documenti.camera.it/Leg17/dossier/Testi/ag0323.htm (accessed on 5 August 2023).
- Gómez, M.; Ronda, F.; Blanco, C.A.; Caballero, P.A.; Apesteguía, A. Effect of dietary fibers on dough rheology and bread quality. Eur. Food Res. Technol. 2003, 216, 51–56. [Google Scholar] [CrossRef]
- Anil, M. Using of hazelnut testa as a source of dietary fiber in breadmaking. J. Food Eng. 2007, 80, 61–67. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Canale, M.; Dugo, G.; Oliveri, C.; Scarangella, M.; Strano, M.C.; Amenta, M.; Crupi, A.; Spina, A. Effects of Partial Replacement of Durum Wheat Re-Milled Semolina with Bean Flour on Physico-Chemical and Technological Features of Doughs and Breads during Storage. Plants 2023, 12, 1125. [Google Scholar] [CrossRef]
- Tabibloghmany, F.S.; Tehrani, M.M.; Koocheki, A. Effects of substitution level and particle size of extruded soybean hull fractions on physicochemical and sensorial properties of high-fiber pan bread during storage. Food Sci. Nutr. 2022, 10, 4345–4359. [Google Scholar] [CrossRef]
- Abd-El-Khalek, M.H.; Mohamed Amer, T.A.; Ibrahim, M.S. Determination of the staling rate of Egyptian Balady bread by using Texture Profile Analysis (TPA): A new method. Arab Univ. J. Agric. Sci. 2019, 27, 2527–2538. [Google Scholar] [CrossRef]
- Ammar, A.F.; Zhang, H.; Siddeeg, A.; Chamba, M.V.M.; Kimani, B.G.; Hassanin, H.; Obadi, M.; Alhejj, N. Effect of the addition of alhydwan seed flour on the dough rheology, bread quality, texture profile and microstructure of wheat bread. J. Texture Stud. 2016, 47, 484–495. [Google Scholar] [CrossRef]
- Amigo, J.M.; del Olmo Alvarez, A.; Engelsen, M.M.; Lundkvist, H.; Engelsen, S.B. Staling of white wheat bread crumb and effect of maltogenic α-amylases. Part 1: Spatial distribution and kinetic modeling of hardness and resilience. Food Chem. 2016, 208, 318–325. [Google Scholar] [CrossRef]
- Frutos, M.J.; Guilabert-Antón, L.; Tomás-Bellido, A.; Hernández-Herrero, J.A. Effect of artichoke (Cynara scolymus L.) fiber on textural and sensory qualities of wheat bread. Food Sci. Technol. Int. 2008, 14 (Suppl. S5), 49–55. [Google Scholar] [CrossRef]
- Jekle, M.; Fuchs, A.; Becker, T. A normalized texture profile analysis approach to evaluate firming kinetics of bread crumbs independent from its initial texture. J. Cereal Sci. 2018, 81, 147–152. [Google Scholar] [CrossRef]
- Wu, M.Y.; Chang, Y.H.; Shiau, S.Y.; Chen, C.C. Rheology of fiber-enriched steamed bread: Stress relaxation and texture profile analysis. J. Food Drug Anal. 2012, 20, 18. [Google Scholar] [CrossRef]
- Kaack, K.; Pedersen, L.; Laerke, H.N.; Meyer, A. New potato fibers for improvement of texture and color of wheat bread. Eur. Food Res. Technol. 2006, 224, 199–207. [Google Scholar] [CrossRef]
- Crowley, P.; Schober, T.J.; Clarke, C.I.; Arendt, E.K. The effect of storage time on textural and crumb grain characteristics of sourdough wheat bread. Eur. Food Res. Technol. 2002, 214, 489–496. [Google Scholar] [CrossRef]
- Tóth, M.; Kaszab, T.; Meretei, A. Texture profile analysis and sensory evaluation of commercially available gluten-free bread samples. Eur. Food Res. Technol. 2022, 248, 1447–1455. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Relationship between instrumental parameters and sensory characteristics in gluten-free breads. Eur. Food Res. Technol. 2012, 235, 107–117. [Google Scholar] [CrossRef]
- Martínez-Anaya, M.A.; Jiménez, T. Physical properties of enzyme-supplemented doughs and relationship with bread quality parameters. Z. Für Leb. Und-Forsch. A 1998, 206, 134–142. [Google Scholar] [CrossRef]
- Trinh, K.T.; Glasgow, S. On the texture profile analysis test. In Proceedings of the Chemeca 2012, Wellington, New Zealand, 23–26 September 2012; Volume 2012, pp. 23–26. [Google Scholar]
- Sangnark, A.; Noomhorm, A. Chemical, physical and baking properties of dietary fiber prepared from rice straw. Food Res. Int. 2004, 37, 66–74. [Google Scholar] [CrossRef]
- Martinez, O.; Salmeron, J.; Guillen, M.D.; Casas, C. Texture profile analysis of meat products treated with commercial liquid smoke flavourings. Food Control 2004, 15, 457–461. [Google Scholar] [CrossRef]
- Wahyono, A.; Lee, S.B.; Yeo, S.H.; Kang, W.W.; Park, H.D. Effects of concentration of Jerusalem artichoke powder on the quality of artichoke-enriched bread fermented with mixed cultures of Saccharomyces cerevisiae, Torulaspora delbrueckii JK08 and Pichia anomala JK04. Emir. J. Food Agric. 2016, 28, 242–250. [Google Scholar] [CrossRef]
- Onyango, C.; Mutungi, C.; Unbehend, G.; Lindhauer, M.G. Modifcation of gluten-free sorghum batter and bread using maize, potato, cassava or rice starch. LWT-Food Sci. Technol. 2011, 44, 681–686. [Google Scholar] [CrossRef]
- Çetin Babaoğlu, H.; Arslan Tontul, S.; Akin, N. Fiber enrichment of sourdough bread by inulin rich Jerusalem artichoke powder. J. Food Process. Preserv. 2021, 45, e15928. [Google Scholar] [CrossRef]
- Rubel, I.A.; Pérez, E.E.; Manrique, G.D.; Genovese, D.B. Fibers enrichment of wheat bread with Jerusalem artichoke inulin: Effect on dough rheology and bread quality. Food Struct. 2015, 3, 21–29. [Google Scholar] [CrossRef]
- Domínguez-Fernández, M.; Irigoyen, Á.; de los Angeles Vargas-Alvarez, M.; Ludwig, I.A.; De Pena, M.P.; Cid, C. Influence of culinary process on free and bound (poly) phenolic compounds and antioxidant capacity of artichokes. Int. J. Gastron. Food Sci. 2021, 25, 100389. [Google Scholar] [CrossRef]
- Aharon, S.; Hana, B.; Liel, G.; Ran, H.; Yoram, K.; Ilan, S.; Shmuel, G. Total phenolic content and antioxidant activity of chickpea (Cicer arietinum L.) as affected by soaking and cooking conditions. Food Nutr. Sci. 2011, 2, 7233. [Google Scholar]
- Ross, C.F.; Hoye, C., Jr.; Fernandez-Plotka, V.C. Influence of heating on the polyphenolic content and antioxidant activity of grape seed flour. J. Food Sci. 2011, 76, C884–C890. [Google Scholar] [CrossRef]
- Raczyk, M.; Kruszewski, B.; Zachariasz, E. Effect of tomato, beetroot and carrot juice addition on physicochemical, antioxidant and texture properties of wheat bread. Antioxidants 2022, 11, 2178. [Google Scholar] [CrossRef]
| Bread Type | Re-Milled Semolina (g) | Bracts/ Stem/Mix Flour (g) | Yeast Solution (mL) | Salt/Sugar Solution (mL) | Ascorbic Acid Solution (mL) | Distilled Water (mL) |
|---|---|---|---|---|---|---|
| SC (tester) | 200 | 0 | 30 | 30 | 20 | 43.5 |
| FAB-5% (A) | 190 | 10 | 30 | 30 | 20 | 50.1 |
| FAB-5% (B) | 190 | 10 | 30 | 30 | 20 | 50.1 |
| FAB-7.5% (A) | 185 | 15 | 30 | 30 | 20 | 50.1 |
| FAB-7.5% (B) | 185 | 15 | 30 | 30 | 20 | 50.1 |
| FAB-10% (A) | 180 | 20 | 30 | 30 | 20 | 49.9 |
| FAB-10% (B) | 180 | 20 | 30 | 30 | 20 | 49.9 |
| FAS-5% (A) | 190 | 10 | 30 | 30 | 20 | 52.1 |
| FAS-5% (B) | 190 | 10 | 30 | 30 | 20 | 52.1 |
| FAS-7.5% (A) | 185 | 15 | 30 | 30 | 20 | 57.6 |
| FAS-7.5% (B) | 185 | 15 | 30 | 30 | 20 | 57.6 |
| FAS-10% (A) | 180 | 20 | 30 | 30 | 20 | 60.2 |
| FAS-10% (B) | 180 | 20 | 30 | 30 | 20 | 60.2 |
| FAM-5% (A) | 190 | 10 | 30 | 30 | 20 | 52.7 |
| FAM-5% (B) | 190 | 10 | 30 | 30 | 20 | 52.7 |
| FAM-7.5% (A) | 185 | 15 | 30 | 30 | 20 | 57.1 |
| FAM-7.5% (B) | 185 | 15 | 30 | 30 | 20 | 57.1 |
| FAM-10% (A) | 180 | 20 | 30 | 30 | 20 | 58.1 |
| FAM-10% (B) | 180 | 20 | 30 | 30 | 20 | 58.1 |
| Sample | Polyphenols (mg GAE/g d.m.) | DPPH (mg Trolox eq/g d.m.) |
|---|---|---|
| Pure flours | ||
| 1SC | 0.05 ± 0.00 h | 0.05 ± 0.00 d |
| 2FAB (A) | 9.09 ± 0.12 a | 8.59 ± 0.012 a |
| FAB (B) | 6.98 ± 0.04 b | 6.51 ± 0.13 ab |
| 3FAS (A) | 3.40 ± 0.18 d | 6.18 ± 0.25 a–c |
| FAS (B) | 4.93 ± 0.11 c | 5.40 ± 0.97 bc |
| 4FAM (A) | 5.21 ± 0.13 c | 6.66 ± 1.92 ab |
| FAM (B) | 2.62 ± 0.36 e | 3.83 ± 0.09 c |
| Mixes | ||
| FAB-5% (A) | 0.50 ± 0.01 f–h | 0.47 ± 0.01 d |
| FAB-5% (B) | 0.40 ± 0.00 gh | 0.37 ± 0.01 d |
| FAB-7.5% (A) | 0.73 ± 0.01 fg | 0.69 ± 0.01 d |
| FAB-7.5% (B) | 0.57 ± 0.00 f–h | 0.53 ± 0.01 d |
| FAB-10% (A) | 0.96 ± 0.02 f | 0.90 ± 0.02 d |
| FAB-10% (B) | 0.75 ± 0.00 fg | 0.69 ± 0.01 d |
| FAS-5% (A) | 0.22 ± 0.01 gh | 0.42 ± 0.07 d |
| FAS-5% (B) | 0.30 ± 0.00 gh | 0.31 ± 0.04 d |
| FAS-7.5% (A) | 0.13 ± 0.01 h | 0.51 ± 0.02 d |
| FAS-7.5% (B) | 0.17 ± 0.01 h | 0.45 ± 0.07 d |
| FAS-10% (A) | 0.39 ± 0.02 gh | 0.66 ± 0.03 d |
| FAS-10% (B) | 0.54 ± 0.01 fgh | 0.58 ± 0.09 d |
| FAM-5% (A) | 0.31 ± 0.00 gh | 0.38 ± 0.10 d |
| FAM-5% (B) | 0.18 ± 0.02 h | 0.23 ± 0.00 d |
| FAM-7.5% (A) | 0.18 ± 0.02 h | 0.54 ± 0.15 d |
| FAM-7.5% (B) | 0.11 ± 0.01 h | 0.33 ± 0.00 d |
| FAM-10% (A) | 0.57 ± 0.01 f–h | 0.71 ± 0.20 d |
| FAM-10% (B) | 0.31 ± 0.04 gh | 0.42 ± 0.01 d |
| Sample | Brown Index (100-L) | a* | b* |
|---|---|---|---|
| Pure flours | |||
| 1SC | 10.26 ± 0.01 l | −2.38 ± 0.00 q | 17.17 ± 0.00 ab |
| 2FAB (A) | 34.56 ± 0.01 c | −1.21 ± 0.01 p | 16.94 ± 0.01 a–c |
| FAB (B) | 38.70 ± 0.01 b | 2.91 ± 0.00 a | 18.02 ± 0.00 a |
| 3FAS (A) | 44.69 ± 0.01 a | 2.68 ± 0.02 b | 17.53 ± 0.01 a |
| FAS (B) | 41.36 ± 0.00 b | 2.29 ± 0.01 c | 15.74 ± 0.01 d–f |
| 4FAM (A) | 39.31 ± 0.04 b | 0.71 ± 0.02 f | 17.55 ± 0.01 a |
| FAM (B) | 39.40 ± 0.03 b | 2.36 ± 0.01 c | 15.28 ± 0.01 d–g |
| Mixes | |||
| FAB-5% (A) | 22.75 ± 0.01 h–k | −2.01 ± 0.01 p | 15.27 ± 0.01 d–g |
| FAB-5% (B) | 17.75 ± 0.03 i | −0.42 ± 0.01 k | 15.98 ± 0.01 c–e |
| FAB-7.5% (A) | 22.72 ± 0.02 h–k | −1.93 ± 0.01 op | 15.55 ± 0.02 d–f |
| FAB-7.5% (B) | 25.74 ± 0.12 f–h | 0.83 ± 0.01 e | 15.97 ± 0.04 c–e |
| FAB-10% (A) | 23.84 ± 0.01 g–j | −1.87 ± 0.01 o | 15.68 ± 0.01 d–f |
| FAB-10% (B) | 26.31 ± 0.01 d–f | 1.02 ± 0.01 d | 16.21 ± 0.02 b–d |
| FAS-5% (A) | 20.71 ± 0.01 ki | −0.44 ± 0.03 k | 15.09 ± 0.04 d–g |
| FAS-5% (B) | 23.37 ± 0.04 g–k | 0.08 ± 0.01 h | 14.58 ± 0.01 f–h |
| FAS-7.5% (A) | 24.15 ± 0.69 g–j | −0.11 ± 0.01 j | 14.71 ± 0.01 f–h |
| FAS-7.5% (B) | 25.63 ± 0.07 f–h | 0.36 ± 0.00 g | 14.31 ± 0.01 g–h |
| FAS-10% (A) | 27.55 ± 0.02 de | 0.34 ± 0.02 g | 14.93 ± 0.04 e–h |
| FAS-10% (B) | 28.81 ± 2.52 d | 0.66 ± 0.08 f | 13.75 ± 1.02 h |
| FAM-5% (A) | 20.76 ± 0.01 ki | −1.12 ± 0.01 m | 15.09 ± 0.01 d–g |
| FAM-5% (B) | 21.72 ± 0.01 jk | −0.14 ± 0.01 j | 14.89 ± 0.01 e–h |
| FAM-7.5% (A) | 23.59 ± 0.01 g–k | −1.01 ± 0.01 m | 15.35 ± 0.01 d–g |
| FAM-7.5% (B) | 24.32 ± 0.15 g–j | 0.38 ± 0.00 g | 15.14 ± 0.01 d–g |
| FAM-10% (A) | 26.20 ± 0.01 d–f | −0.78 ± 0.01 l | 15.08 ± 0.01 d–g |
| FAM-10% (B) | 26.01 ± 0.00 d–f | 0.65 ± 0.01 f | 15.28 ± 0.01 d–g |
| Time | Sample | Moisture (g/100 g) | Volume (cm3) | Height (cm) | Weight (g) | Porosity * |
|---|---|---|---|---|---|---|
| SC | 33.10 ± 0.19 d | 361.00 ± 5.66 a | 7.65 ± 0.21 a | 132.76 ± 0.54 b | 5.00 ± 0.00 c | |
| T0 | FAB-5% (A) | 37.79 ± 0.19 abc | 300.00 ± 0.00 c–f | 5.15 ± 0.21 bcd | 136.89 ± 0.83 ab | 6.00 ± 0.00 abc |
| FAB-5% (B) | 36.48 ± 0.54 c | 335.00 ± 7.07 a–d | 5.50 ± 0.00 bcd | 136.94 ± 0.04 ab | 5.75 ± 0.35 abc | |
| FAB-7.5% (A) | 37.75 ± 0.04 abc | 262.50 ± 3.54 fg | 4.90 ± 0.00 d | 137.82 ± 0.42 a | 7.00 ± 0.00 a | |
| FAB-7.5% (B) | 37.57 ± 0.25 abc | 297.50 ± 3.54 c–g | 5.28 ± 0.18 bcd | 137.96 ± 0.57 a | 7.00 ± 0.00 a | |
| FAB-10% (A) | 37.81 ± 0.83 abc | 245.00 ± 7.07 g | 4.95 ± 0.21 cd | 137.85 ± 1.07 a | 6.75 ± 0.35 ab | |
| FAB-10% (B) | 37.77 ± 0.18 abc | 267.50 ± 17.68 fg | 5.10 ± 0.14 bcd | 137.73 ± 0.27 a | 6.75 ± 0.00 ab | |
| FAS-5% (A) | 36.92 ± 0.39 bc | 350.00 ± 28.28 abc | 5.68 ± 0.11 bcd | 135.09 ± 0.26 ab | 5.00 ± 0.00 c | |
| FAS-5% (B) | 37.69 ± 0.28 abc | 327.50 ± 3.54 a–e | 5.65 ± 0.07 bcd | 135.45 ± 1.38 ab | 6.00 ± 0.35 abc | |
| FAS-7.5% (A) | 38.18 ± 0.49 abc | 355.00 ± 7.07 ab | 5.70 ± 0.144 bc | 134.40 ± 0.58 ab | 6.25 ± 0.00 abc | |
| FAS-7.5% (B) | 39.66 ± 0.23 a | 325.00 ± 0.00 a–e | 5.43 ± 0.11 bcd | 136.13 ± 1.81 ab | 6.75 ± 0.35 ab | |
| FAS-10% (A) | 38.72 ± 0.50 ab | 325.00 ± 7.07 a–e | 5.43 ± 0.11 bcd | 135.30 ± 0.33 ab | 6.75 ± 0.71 ab | |
| FAS-10% (B) | 39.10 ± 0.37 ab | 302.50 ± 3.54 b–f | 5.33 ± 0.04 bcd | 135.95 ± 0.26 ab | 6.25 ± 0.35 abc | |
| FAM-5% (A) | 37.62 ± 0.01 abc | 330.00 ± 7.07 a–e | 5.78 ± 0.18 b | 136.49 ± 0.46 ab | 6.00 ± 0.00 abc | |
| FAM-5% (B) | 38.15 ± 0.22 abc | 304.00 ± 5.66 b–f | 5.54 ± 0.08 bcd | 135.77 ± 0.86 ab | 7.00 ± 0.00 a | |
| FAM-7.5% (A) | 39.21 ± 0.42 a | 302.50 ± 3.54 b–f | 5.32 ± 0.16 bcd | 135.41 ± 0.12 ab | 6.75 ± 0.00 ab | |
| FAM-7.5% (B) | 39.10 ± 0.08 ab | 306.50 ± 2.12 b–f | 5.51 ± 0.14 bcd | 135.74 ± 0.27 ab | 7.00 ± 0.35 a | |
| FAM-10% (A) | 38.47 ± 0.53 abc | 277.50 ± 3.54 efg | 5.12 ± 0.02 bcd | 135.24 ± 1.12 ab | 6.50 ± 0.00 ab | |
| FAM-10% (B) | 39.18 ± 0.20 a | 282.50 ± 3.54 d–g | 5.27 ± 0.06 bcd | 136.15 ± 0.04 ab | 7.00 ± 0.71 a | |
| T2 | SC | 32.67 ± 0.58 b | 348.75 ± 8.84 a | 7.20 ± 0.28 a | 124.10 ± 0.39 i | 5.50 ± 0.00 c |
| FAB-5% (A) | 36.09 ± 1.69 ab | 277.50 ± 3.54 b–f | 4.90 ± 0.21 bcd | 132.85 ± 0.40 a–d | 6.25 ± 0.00 abc | |
| FAB-5% (B) | 36.54 ± 2.07 ab | 311.25 ± 1.77 a–d | 5.40 ± 0.14 bcd | 134.39 ± 0.15 ab | 6.25 ± 0.00 abc | |
| FAB-7.5% (A) | 38.52 ± 0.50 a | 257.50 ± 3.54 def | 4.88 ± 0.04 bcd | 133.29 ± 0.38 abc | 7.25 ± 0.35 a | |
| FAB-7.5% (B) | 38.14 ± 0.06 a | 258.00 ± 9.90 def | 5.10 ± 0.14 bcd | 129.27 ± 1.07 d–g | 7.25 ± 0.00 a | |
| FAB-10% (A) | 38.00 ± 0.34 a | 240.00 ± 7.07 f | 4.83 ± 0.07 bcd | 131.22 ± 0.93 b–f | 7.00 ± 0.35 ab | |
| FAB-10% (B) | 37.03 ± 0.44 ab | 245.00 ± 21.21 ef | 4.87 ± 0.05 bcd | 135.38 ± 0.14 a | 7.00 ± 0.00 ab | |
| FAS-5% (A) | 37.10 ± 0.22 ab | 330.00 ± 28.28 ab | 5.60 ± 0.07 b | 129.39 ± 0.85 d–g | 6.00 ± 0.00 bc | |
| FAS-5% (B) | 37.46 ± 0.00 a | 298.00 ± 2.83 a–e | 5.55 ± 0.07 bc | 129.11 ± 0.03 efg | 6.00 ± 0.00 bc | |
| FAS-7.5% (A) | 37.60 ± 1.60 a | 319.50 ± 7.78 abc | 5.53 ± 0.32 bc | 125.35 ± 0.16 hi | 6.25 ± 0.00 abc | |
| FAS-7.5% (B) | 37.43 ± 1.06 a | 302.50 ± 3.54 a–d | 5.35 ± 0.14 bcd | 128.61 ± 0.25 e–h | 6.25 ± 0.35 abc | |
| FAS-10% (A) | 38.22 ± 1.60 a | 302.50 ± 3.54 a–d | 5.38 ± 0.04 bcd | 125.77 ± 0.17 ghi | 6.75 ± 0.71 abc | |
| FAS-10% (B) | 38.71 ± 1.63 a | 272.50 ± 3.54 c–f | 5.20 ± 0.14 bcd | 127.91 ± 0.12 fgh | 6.75 ± 0.71 abc | |
| FAM-5% (A) | 37.77 ± 0.59 a | 310.00 ± 2.83 a–d | 5.61 ± 0.06 b | 131.82 ± 0.11 a–e | 6.75 ± 0.00 abc | |
| FAM-5% (B) | 37.68 ± 0.21 a | 277.50 ± 3.54 b–f | 5.20 ± 0.14 bcd | 129.63 ± 0.43 def | 6.00 ± 0.00 bc | |
| FAM-7.5% (A) | 39.19 ± 0.46 a | 292.50 ± 3.54 a–f | 5.18 ± 0.11 bcd | 130.67 ± 0.16 c–f | 7.00 ± 0.00 ab | |
| FAM-7.5% (B) | 38.57 ± 0.70 a | 294.00 ± 1.41 a–f | 5.00 ± 0.00 bcd | 128.40 ± 0.01 e–h | 7.00 ± 0.71 ab | |
| FAM-10% (A) | 38.91 ± 0.19 a | 269.00 ± 1.41 c–f | 4.70 ± 0.14 cd | 129.98 ± 1.82 c–f | 7.00 ± 0.00 ab | |
| FAM-10% (B) | 38.58 ± 0.26 a | 262.50 ± 3.54 def | 4.55 ± 0.14 d | 129.68 ± 0.14 c–f | 7.00 ± 0.00 ab | |
| T4 | SC | 29.45 ± 0.73 c | 316.50 ± 4.95 ab | 6.86 ± 0.23 a | 118.81 ± 0.11 g | 6.00 ± 0.00 a |
| FAB-5% (A) | 35.69 ± 0.78 ab | 272.50 ± 3.54 a–e | 4.78 ± 0.04 bcd | 127.31 ± 0.42 b | 7.00 ± 0.00 a | |
| FAB-5% (B) | 37.38 ± 0.16 a | 301.00 ± 1.41 abc | 5.34 ± 0.20 bc | 131.20 ± 0.03 a | 7.00 ± 0.35 a | |
| FAB-7.5% (A) | 36.65 ± 0.93 ab | 252.50 ± 3.54 cde | 4.65 ± 0.10 cd | 130.28 ± 0.23 a | 7.50 ± 0.71 a | |
| FAB-7.5% (B) | 35.37 ± 0.59 abc | 254.00 ± 8.49 cde | 4.95 ± 0.21 bcd | 127.15 ± 0.55 b | 7.50 ± 0.00 a | |
| FAB-10% (A) | 34.77 ± 1.97 abc | 235.00 ± 7.07 e | 4.67 ± 0.05 cd | 127.10 ± 0.45 b | 7.63 ± 0.35 a | |
| FAB-10% (B) | 36.66 ± 0.71 ab | 241.00 ± 19.80 de | 4.86 ± 0.06 bcd | 131.45 ± 0.32 a | 7.25 ± 0.00 a | |
| FAS-5% (A) | 32.33 ± 1.25 abc | 326.50 ± 30.41 a | 5.54 ± 0.08 b | 126.85 ± 0.76 b | 6.75 ± 0.35 a | |
| FAS-5% (B) | 36.08 ± 0.00 ab | 295.00 ± 2.83 a–d | 5.39 ± 0.01 bc | 127.53 ± 0.25 b | 6.75 ± 0.35 a | |
| FAS-7.5% (A) | 32.71 ± 1.58 abc | 316.50 ± 6.36 ab | 5.40 ± 0.14 bc | 122.19 ± 0.26 ef | 7.50 ± 0.35 a | |
| FAS-7.5% (B) | 34.59 ± 2.45 abc | 252.50 ± 3.54 cde | 5.31 ± 0.13 bcd | 122.19 ± 0.08 ef | 7.00 ± 0.35 a | |
| FAS-10% (A) | 30.74 ± 0.13 bc | 282.50 ± 3.54 a–e | 5.23 ± 0.11 bcd | 118.83 ± 0.05 g | 7.50 ± 1.41 a | |
| FAS-10% (B) | 36.52 ± 0.36 ab | 272.50 ± 3.54 a–e | 5.10 ± 0.14 bcd | 124.21 ± 0.25 d | 7.50 ± 0.35 a | |
| FAM-5% (A) | 33.74 ± 1.41 abc | 299.00 ± 1.41 abc | 5.40 ± 0.14 bc | 127.40 ± 0.23 b | 7.25 ± 0.35 a | |
| FAM-5% (B) | 35.33 ± 1.26 abc | 278.50 ± 2.12 a–e | 5.03 ± 0.11 bcd | 126.26 ± 0.04 bc | 6.25 ± 0.35 a | |
| FAM-7.5% (A) | 32.41 ± 2.76 abc | 276.25 ± 1.77 a–e | 5.14 ± 0.08 bcd | 123.72 ± 0.37 de | 7.25 ± 0.35 a | |
| FAM-7.5% (B) | 33.94 ± 0.28 abc | 281.00 ± 1.41 a–e | 4.88 ± 0.18 bcd | 121.11 ± 0.09 f | 7.25 ± 0.71 a | |
| FAM-10% (A) | 36.25 ± 1.59 ab | 260.00 ± 2.83 cde | 4.59 ± 0.16 cd | 124.28 ± 0.05 d | 7.25 ± 0.35 a | |
| FAM-10% (B) | 35.11 ± 0.73 abc | 262.50 ± 3.54 b–e | 4.48 ± 0.22 d | 124.55 ± 0.45 cd | 7.75 ± 0.35 a |
| Time | Sample | Hardness (N) | Springiness | Cohesiveness | Gumminess (N) | Chewiness (N × mm) |
|---|---|---|---|---|---|---|
| SC-100% | 6.44 ± 0.93 e–h | 0.95 ± 0.07 ab | 0.71 ± 0.02 bcd | 6.50 ± 0.68 h | 5.64 ± 0.34 m | |
| T0 | FAB-5% (A) | 14.15 ± 0.35 abc | 1.00 ± 0.01 a | 0.56 ± 0.01 e | 24.68 ± 0.47 cd | 24.29 ± 0.03 e |
| FAB-5% (B) | 9.01 ± 0.53 c–g | 0.96 ± 0.01 ab | 0.77 ± 0.06 abc | 44.83 ± 0.85 a | 43.81 ± 0.02 a | |
| FAB-7.5% (A) | 15.25 ± 0.07 ab | 0.99 ± 0.01 ab | 0.70 ± 0.01 bcd | 44.91 ± 0.16 a | 44.07 ± 0.08 a | |
| FAB-7.5% (B) | 11.75 ± 0.92 b–e | 0.94 ± 0.01 ab | 0.81 ± 0.01 ab | 22.80 ± 0.04 d | 21.45 ± 0.13 f | |
| FAB-10% (A) | 19.35 ± 2.47 a | 0.96 ± 0.00 ab | 0.82 ± 0.02 ab | 30.63 ± 0.02 bc | 29.23 ± 0.08 d | |
| FAB-10% (B) | 13.55 ± 0.64 bcd | 0.94 ± 0.01 ab | 0.70 ± 0.02 bcd | 45.92 ± 0.40 a | 42.95 ± 0.07 a | |
| FAS-5% (A) | 3.42 ± 0.27 h | 0.96 ± 0.01 ab | 0.63 ± 0.01 de | 42.47 ± 0.74 a | 41.25 ± 0.02 b | |
| FAS-5% (B) | 10.27 ± 0.47 b–f | 1.00 ± 0.01 a | 0.72 ± 0.01 a–d | 19.52 ± 0.39 def | 19.59 ± 0.12 gh | |
| FAS-7.5% (A) | 7.80 ± 0.37 e–h | 1.00 ± 0.01 a | 0.66 ± 0.01 cde | 30.48 ± 0.42 bc | 30.30 ± 0.44 d | |
| FAS-7.5% (B) | 8.38 ± 0.87 d–h | 0.98 ± 0.01 ab | 0.83 ± 0.01 a | 14.11 ± 0.12 fg | 13.74 ± 0.03 ij | |
| FAS-10% (A) | 9.21 ± 1.02 c–f | 0.96 ± 0.00 ab | 0.68 ± 0.01 cde | 11.80 ± 0.28 gh | 11.22 ± 0.07 k | |
| FAS-10% (B) | 9.10 ± 0.37 c–g | 0.94 ± 0.01 ab | 0.72 ± 0.01 a–d | 33.43 ± 4.41 b | 34.16 ± 0.10 c | |
| FAM-5% (A) | 3.71 ± 0.82 gh | 0.91 ± 0.02 b | 0.76 ± 0.03 abc | 14.17 ± 0.16 fg | 12.61 ± 0.11 j | |
| FAM-5% (B) | 8.01 ± 0.73 e–h | 0.97 ± 0.01 ab | 0.67 ± 0.01 cde | 10.10 ± 0.18 gh | 9.80 ± 0.31 l | |
| FAM-7.5% (A) | 11.30 ± 1.56 b–f | 1.00 ± 0.01 a | 0.70 ± 0.01 bcd | 20.80 ± 0.18 de | 20.72 ± 0.22 fg | |
| FAM-7.5% (B) | 10.25 ± 0.35 b–f | 0.95 ± 0.01 ab | 0.72 ± 0.01 a–d | 15.77 ± 0.11 efg | 14.90 ± 0.04 i | |
| FAM-10% (A) | 7.61 ± 0.60 e–h | 0.91 ± 0.01 b | 0.67 ± 0.03 cde | 20.11 ± 0.05 def | 18.65 ± 0.49 h | |
| FAM-10% (B) | 6.31 ± 0.01 fgh | 0.99 ± 0.01 ab | 0.75 ± 0.01 a–d | 24.53 ± 0.03 cd | 23.97 ± 0.01 e | |
| T2 | SC-100% | 20.40 ± 0.57 c–f | 0.99 ± 0.01 ab | 0.81 ± 0.04 ab | 64.68 ± 0.62 b | 62.93 ± 0.24 abc |
| FAB-5% (A) | 16.85 ± 0.07 def | 1.00 ± 0.00 a | 0.90 ± 0.05 a | 26.34 ± 0.62 e–h | 26.29 ± 0.55 fgh | |
| FAB-5% (B) | 13.05 ± 0.21 f | 0.93 ± 0.01 ab | 0.63 ± 0.08 b | 51.04 ± 0.23 c | 47.06 ± 0.20 de | |
| FAB-7.5% (A) | 22.85 ± 1.34 c–f | 0.97 ± 0.01 ab | 0.79 ± 0.05 ab | 29.57 ± 0.80 ef | 29.49 ± 0.56 fgh | |
| FAB-7.5% (B) | 37.15 ± 4.31 ab | 0.97 ± 0.01 ab | 0.80 ± 0.05 ab | 29.75 ± 1.07 ef | 29.82 ± 0.41 fgh | |
| FAB-10% (A) | 47.80 ± 0.00 a | 0.95 ± 0.01 ab | 0.79 ± 0.03 ab | 41.08 ± 0.66 cd | 39.57 ± 0.68 ef | |
| FAB-10% (B) | 24.90 ± 2.26 b–f | 0.98 ± 0.02 ab | 0.78 ± 0.01 ab | 18.34 ± 5.25 g–j | 17.80 ± 4.77 ghi | |
| FAS-5% (A) | 18.50 ± 3.96 c–f | 0.94 ± 0.01 ab | 0.77 ± 0.04 ab | 9.01 ± 0.92 j | 8.40 ± 0.88 i | |
| FAS-5% (B) | 15.15 ± 1.63 ef | 0.94 ± 0.01 ab | 0.70 ± 0.03 ab | 49.13 ± 0.23 c | 46.93 ± 0.18 de | |
| FAS-7.5% (A) | 23.25 ± 1.63 b–f | 0.98 ± 0.03 ab | 0.72 ± 0.04 ab | 62.84 ± 0.23 b | 59.50 ± 0.74 bcd | |
| FAS-7.5% (B) | 27.15 ± 0.21 b–f | 0.94 ± 0.08 ab | 0.64 ± 0.01 ab | 34.76 ± 0.35 de | 34.88 ± 0.55 ef | |
| FAS-10% (A) | 30.30 ± 0.57 bcd | 0.85 ± 0.08 ab | 0.80 ± 0.01 ab | 12.06 ± 0.60 ij | 11.23 ± 0.05 i | |
| FAS-10% (B) | 31.85 ± 2.90 bc | 0.81 ± 0.06 b | 0.61 ± 0.10 b | 92.81 ± 4.33 a | 75.44 ± 8.97 a | |
| FAM-5% (A) | 17.25 ± 1.63 def | 0.90 ± 0.10 ab | 0.78 ± 0.06 ab | 28.06 ± 0.35 efg | 26.95 ± 0.12 fgh | |
| FAM-5% (B) | 27.10 ± 0.14 b–f | 0.88 ± 0.09 ab | 0.74 ± 0.04 ab | 19.77 ± 0.63 f–i | 15.93 ± 0.62 hi | |
| FAM-7.5% (A) | 15.55 ± 0.07 ef | 0.96 ± 0.00 ab | 0.87 ± 0.02 ab | 49.89 ± 0.48 c | 48.68 ± 0.80 cde | |
| FAM-7.5% (B) | 29.25 ± 3.04 b–e | 0.97 ± 0.01 ab | 0.70 ± 0.03 ab | 66.45 ± 0.81 b | 64.77 ± 0.47 ab | |
| FAM-10% (A) | 16.55 ± 2.62 def | 0.95 ± 0.03 ab | 0.79 ± 0.02 ab | 30.72 ± 0.43 e | 30.73 ± 0.78 fg | |
| FAM-10% (B) | 24.50 ± 5.52 b–f | 0.97 ± 0.04 ab | 0.80 ± 0.02 ab | 16.36 ± 0.65 hij | 15.91 ± 0.13 hi | |
| T4 | SC-100% | 23.35 ± 2.33 c–f | 0.95 ± 0.01 a | 0.81 ± 0.05 a | 277.90 ± 0.95 | 263.70 ± 2.73 a |
| FAB-5% (A) | 28.50 ± 1.56 b–f | 0.74 ± 0.02 c | 0.42 ± 0.05 c | 60.96 ± 2.45 | 43.15 ± 5.64 c | |
| FAB-5% (B) | 18.95 ± 1.06 ef | 0.92 ± 0.01 ab | 0.74 ± 0.02 ab | 103.35 ± 1.37 | 80.80 ± 23.30 bc | |
| FAB-7.5% (A) | 26.70 ± 3.11 c–f | 0.84 ± 0.01 abc | 0.71 ± 0.01 abc | 82.64 ± 5.20 | 65.76 ± 9.39 bc | |
| FAB-7.5% (B) | 41.60 ± 0.00 abc | 0.87 ± 0.02 abc | 0.74 ± 0.02 ab | 172.41 ± 10.15 | 130.55 ± 39.48 abc | |
| FAB-10% (A) | 50.45 ± 1.63 a | 0.88 ± 0.03 abc | 0.72 ± 0.01 abc | 118.97 ± 8.63 | 104.46 ± 11.63 bc | |
| FAB-10% (B) | 31.90 ± 2.40 a–f | 0.87 ± 0.04 abc | 0.69 ± 0.01 abc | 149.76 ± 4.86 | 74.18 ± 87.27 bc | |
| FAS-5% (A) | 16.95 ± 0.78 f | 0.78 ± 0.03 bc | 0.72 ± 0.01 abc | 42.91 ± 3.26 | 30.82 ± 2.59 c | |
| FAS-5% (B) | 23.70 ± 3.82 c–f | 0.87 ± 0.01 abc | 0.72 ± 0.00 abc | 81.69 ± 1.29 | 71.02 ± 2.65 bc | |
| FAS-7.5% (A) | 28.85 ± 2.19 b–f | 0.84 ± 0.02 abc | 0.70 ± 0.01 abc | 62.63 ± 4.81 | 53.74 ± 3.42 c | |
| FAS-7.5% (B) | 46.65 ± 5.59 ab | 0.86 ± 0.01 abc | 0.68 ± 0.04 abc | 100.94 ± 3.85 | 82.70 ± 9.31 bc | |
| FAS-10% (A) | 36.95 ± 1.06 a–e | 0.86 ± 0.03 abc | 0.57 ± 0.04 cd | 100.25 ± 5.52 | 90.00 ± 2.04 bc | |
| FAS-10% (B) | 28.55 ± 7.28 b–f | 0.95 ± 0.02 a | 0.74 ± 0.01 ab | 127.29 ± 35.09 | 146.91 ± 0.45 abc | |
| FAM-5% (A) | 16.40 ± 0.99 f | 0.86 ± 0.01 abc | 0.69 ± 0.01 abc | 80.98 ± 3.42 | 73.62 ± 2.39 bc | |
| FAM-5% (B) | 40.90 ± 2.83 abc | 0.91 ± 0.01 ab | 0.72 ± 0.00 abc | 71.38 ± 0.15 | 66.82 ± 3.75 bc | |
| FAM-7.5% (A) | 21.25 ± 0.35 def | 0.87 ± 0.04 abc | 0.64 ± 0.02 bc | 103.33 ± 0.40 | 90.87 ± 0.93 bc | |
| FAM-7.5% (B) | 48.95 ± 5.59 a | 0.93 ± 0.07 ab | 0.74 ± 0.05 ab | 214.21 ± 16.08 | 200.54 ± 30.52 ab | |
| FAM-10% (A) | 31.95 ± 3.04 a–f | 0.86 ± 0.00 abc | 0.59 ± 0.01 bc | 125.59 ± 1.81 | 111.11 ± 2.06 bc | |
| FAM-10% (B) | 40.60 ± 4.10 a–d | 0.93 ± 0.01 ab | 0.65 ± 0.02 bc | 131.65 ± 1.51 | 114.18 ± 8.07 bc |
| Sample | Polyphenols (mg GAE/g d.m.) | DPPH (mg Trolox eq/g d.m.) | Aw |
|---|---|---|---|
| SC | 0.01 ± 0.01 i | n.d. * | 0.64 ± 0.01 f |
| FAB-5% (A) | 0.29 ± 0.01 d | 0.32 ± 0.00 c–f | 0.86 ± 0.03 ab |
| FAB-5% (B) | 0.22 ± 0.01 ef | 0.24 ± 0.03 fgh | 0.90 ± 0.02 a |
| FAB-7.5% (A) | 0.35 ± 0.00 b | 0.44 ± 0.00 abc | 0.87 ± 0.01 ab |
| FAB-7.5% (B) | 0.29 ± 0.00 d | 0.23 ± 0.05 fgh | 0.86 ± 0.01 ab |
| FAB-10% (A) | 0.57 ± 0.01 a | 0.55 ± 0.00 a | 0.77 ± 0.02 b–e |
| FAB-10% (B) | 0.37 ± 0.00 b | 0.42 ± 0.00 a–d | 0.78 ± 0.03 bcd |
| FAS-5% (A) | 0.14 ± 0.00 h | 0.27 ± 0.01 e–h | 0.78 ± 0.02 abc |
| FAS-5% (B) | 0.14 ± 0.00 h | 0.17 ± 0.03 gh | 0.74 ± 0.01 c–f |
| FAS-7.5% (A) | 0.18 ± 0.00 gh | 0.41 ± 0.00 bcd | 0.76 ± 0.05 b–e |
| FAS-7.5% (B) | 0.24 ± 0.01 ef | 0.28 ± 0.00 efg | 0.86 ± 0.02 ab |
| FAS-10% (A) | 0.23 ± 0.00 ef | 0.54 ± 0.00 a | 0.85 ± 0.03 ab |
| FAS-10% (B) | 0.30 ± 0.02 cd | 0.45 ± 0.04 abc | 0.82 ± 0.01 abc |
| FAM-5% (A) | 0.17 ± 0.00 gh | 0.30 ± 0.04 d–g | 0.78 ± 0.02 bcd |
| FAM-5% (B) | 0.13 ± 0.01 h | 0.14 ± 0.02 h | 0.63 ± 0.02 f |
| FAM-7.5% (A) | 0.26 ± 0.00 de | 0.39 ± 0.02 cde | 0.66 ± 0.01 ef |
| FAM-7.5% (B) | 0.15 ± 0.01 gh | 0.17 ± 0.01 gh | 0.67 ± 0.01 def |
| FAM-10% (A) | 0.34 ± 0.01 bc | 0.54 ± 0.00 ab | 0.65 ± 0.01 f |
| FAM-10% (B) | 0.20 ± 0.01 fg | 0.25 ± 0.01 fgh | 0.73 ± 0.02 c–f |
| Sample | Crust | Crumb | ||||
|---|---|---|---|---|---|---|
| Brown Index (100-L) | a* | b* | Brown Index (100-L) | a* | b* | |
| SC | 58.56 ± 3.03 abcd | 16.38 ± 0.33 a | 22.43 ± 0.81 bc | 25.88 ± 0.01 l | −2.20 ± 0.03 h | 24.11 ± 0.06 a |
| FAB-5% (A) | 48.09 ± 3.25 d | 8.29 ± 0.10 bc | 28.30 ± 1.14 a | 42.95 ± 0.39 i | 1.27 ± 0.28 e | 20.41 ± 0.06 bcd |
| FAB-5% (B) | 49.59 ± 0.89 cd | 9.19 ± 0.16 bc | 28.55 ± 1.03 ab | 49.48 ± 0.12 defghi | 2.78 ± 0.04 bcdefg | 20.15 ± 0.04 bcdefg |
| FAB-7.5% (A) | 48.37 ± 2.78 d | 8.45 ± 0.65 bc | 27.91 ± 0.47 ab | 47.20 ± 0.35 fghi | 2.02 ± 0.04 ef | 19.46 ± 0.45 defgh |
| FAB-7.5% (B) | 53.30 ± 3.13 abcd | 8.94 ± 0.35 bc | 24.50 ± 2.84 abc | 51.45 ± 0.78 cdef | 3.92 ± 0.11 abc | 20.46 ± 0.78 bcd |
| FAB-10% (A) | 56.28 ± 2.40 abcd | 8.03 ± 1.34 bc | 23.47 ± 1.16 abc | 55.07 ± 0.12 abcd | 4.71 ± 0.11 a | 20.32 ± 0.31 bcd |
| FAB-10% (B) FAS-5% (A) | 56.93 ± 1.67 abcd 51.89 ± 1.94 bcd | 6.97 ± 0.74 c 8.55 ± 5.10 bc | 21.93 ± 0.08 bc 24.88 ± 2.28 abc | 55.67 ± 0.30 abc 48.51 ± 0.54 efghi | 2.46 ± 0.28 def 2.47 ± 0.40 def | 18.91 ± 0.16 gh 19.73 ± 0.20 cdefgh |
| FAS-5% (A) FAS-5% (B) | 51.89 ± 1.94 bcd 51.90 ± 1.39 bcd | 8.55 ± 5.10 bc 9.84 ± 0.25 bc | 24.88 ± 2.28 abc 25.56 ± 0.74 abc | 48.51 ± 0.54 efghi 46.54 ± 0.45 ghi | 2.47 ± 0.40 def 2.50 ± 0.06 def | 19.73 ± 0.20 cdefgh 20.14 ± 0.14 bcdef |
| FAS-7.5% (A) FAS-7.5% (B) | 57.50 ± 0.21 abc 59.93 ± 0.54 ab | 11.37 ± 1.39 abc 12.07 ± 0.49 | 22.20 ± 2.97 bc 21.93 ± 1.90 bc | 51.28 ± 0.18 cdefg 50.43 ± 0.13 defgh | 3.47 ± 0.39 abcd 3.51 ± 0.15 abcd | 20.62 ± 0.48 bc 19.59 ± 0.02 defgh |
| FAS-10% (A) FAS-10% (B) | 61.35 ± 1.64 a 54.60 ± 5.25 abcd | 10.14 ± 0.62 abc 8.66 ± 0.30 bc | 19.83 ± 3.59 c 23.33 ± 1.33 abc | 58.49 ± 0.06 a 54.61 ± 0.06 abcd | 3.95 ± 0.01 abc 3.79 ± 0.04 abc | 18.83 ± 0.08 g 19.15 ± 0.05 fgh |
| FAM-5% (A) FAM-5% (B) | 54.21 ± 2.33 abcd 52.98 ± 2.74 abcd | 11.22 ± 0.11 bc 12.49 ± 0.92 abc | 26.02 ± 0.23 ab 27.12 ± 0.85 ab | 46.20 ± 0.07 hi 46.76 ± 0.29 fghi | 2.00 ± 0.02 ef 2.70 ± 0.11 cdef | 21.00 ± 0.03 b 20.45 ± 0.13 bcd |
| FAM-7.5% (A) FAM-7.5% (B) | 54.03 ± 0.98 abcd 56.70 ± 0.03 abcd | 10.60 ± 0.03 ab 11.29 ± 0.22 bc | 25.60 ± 0.07 abc 22.93 ± 0.08 bc | 52.13 ± 0.08 bcde 54.68 ± 0.13 abcd | 2.78 ± 0.14 cdef 2.15 ± 0.04 ef | 20.17 ± 0.09 bcde 19.86 ± 0.03 cdefg |
| FAM-10% (A) FAM-10% (B) | 57.85 ± 0.33 abc 55.83 ± 1.05 abcd | 10.07 ± 0.47 abc 10.27 ± 0.66 bc | 22.21 ± 0.89 bc 24.70 ± 0.88 abc | 56.63 ± 0.11 ab 54.17 ± 0.06 abcd | 3.14 ± 0.05 bcde 4.07 ± 0.06 ab | 19.30 ± 0.03 efgh 20.04 ± 0.02 bcdef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canale, M.; Sanfilippo, R.; Strano, M.C.; Amenta, M.; Allegra, M.; Proetto, I.; Papa, M.; Palmeri, R.; Todaro, A.; Spina, A. Artichoke Industrial Waste in Durum Wheat Bread: Effects of Two Different Preparation and Drying Methods of Flours and Evaluation of Quality Parameters during Short Storage. Foods 2023, 12, 3419. https://doi.org/10.3390/foods12183419
Canale M, Sanfilippo R, Strano MC, Amenta M, Allegra M, Proetto I, Papa M, Palmeri R, Todaro A, Spina A. Artichoke Industrial Waste in Durum Wheat Bread: Effects of Two Different Preparation and Drying Methods of Flours and Evaluation of Quality Parameters during Short Storage. Foods. 2023; 12(18):3419. https://doi.org/10.3390/foods12183419
Chicago/Turabian StyleCanale, Michele, Rosalia Sanfilippo, Maria Concetta Strano, Margherita Amenta, Maria Allegra, Ilaria Proetto, Martina Papa, Rosa Palmeri, Aldo Todaro, and Alfio Spina. 2023. "Artichoke Industrial Waste in Durum Wheat Bread: Effects of Two Different Preparation and Drying Methods of Flours and Evaluation of Quality Parameters during Short Storage" Foods 12, no. 18: 3419. https://doi.org/10.3390/foods12183419
APA StyleCanale, M., Sanfilippo, R., Strano, M. C., Amenta, M., Allegra, M., Proetto, I., Papa, M., Palmeri, R., Todaro, A., & Spina, A. (2023). Artichoke Industrial Waste in Durum Wheat Bread: Effects of Two Different Preparation and Drying Methods of Flours and Evaluation of Quality Parameters during Short Storage. Foods, 12(18), 3419. https://doi.org/10.3390/foods12183419










