Comprehensive Analysis of Phenolic Constituents, Biological Activities, and Derived Aroma Differences of Penthorum chinense Pursh Leaves after Processing into Green and Black Tea
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Tea Samples
2.3. Analysis of Volatile Components
2.4. Sample Extraction
2.5. Total Phenolic Content (TPC)
2.6. Total Flavonoid Content (TFC)
2.7. Total Proanthocyanidin Content (TPAC)
2.8. Phenolic Composition
2.9. Antioxidant Activity
2.10. ADH and ALDH Activities
2.11. Statistical Analysis
3. Results and Discussion
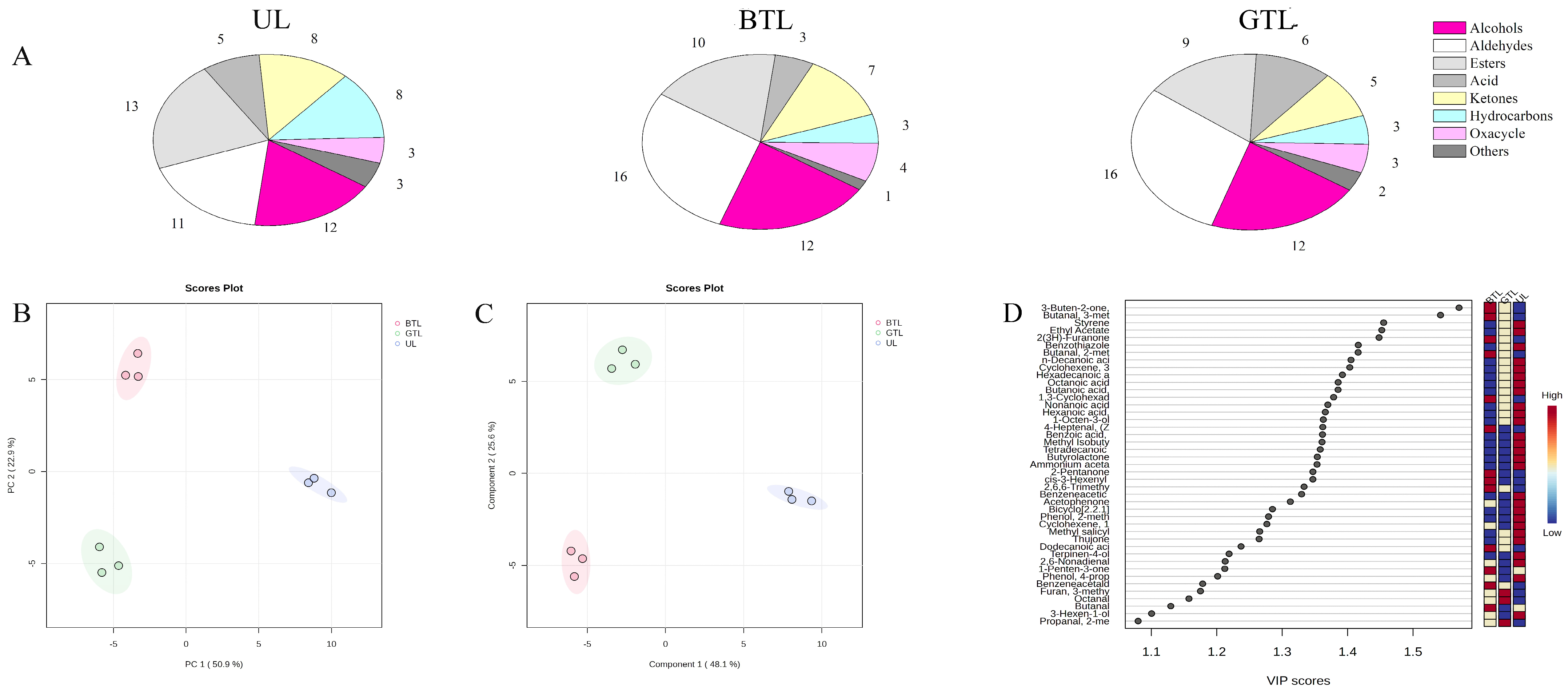
3.1. Volatile Flavor Compounds
3.2. Differences in TPC, TFC, and TPAC
3.3. Differences in Individual Phenolic Compounds
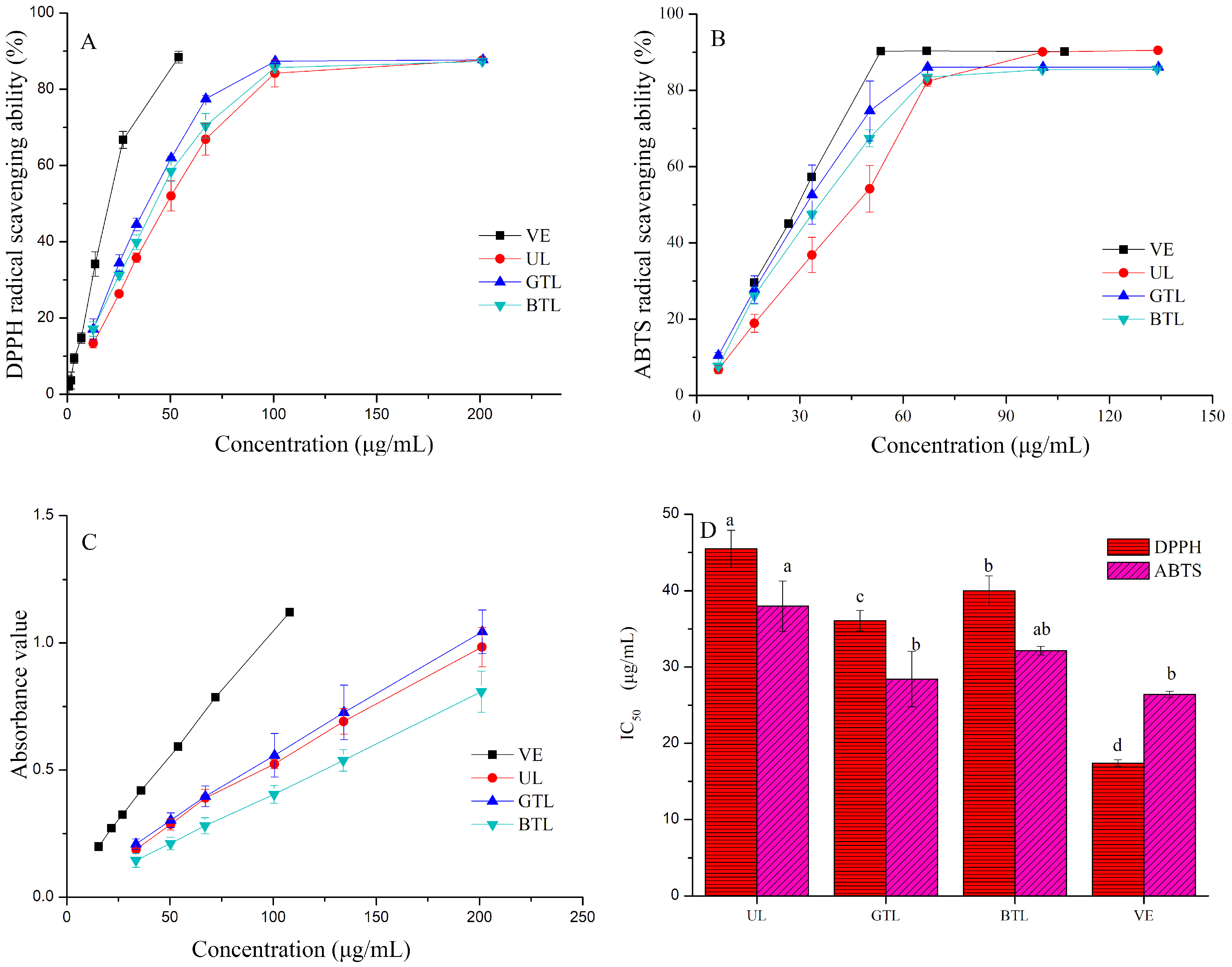
3.4. Antioxidant Activity
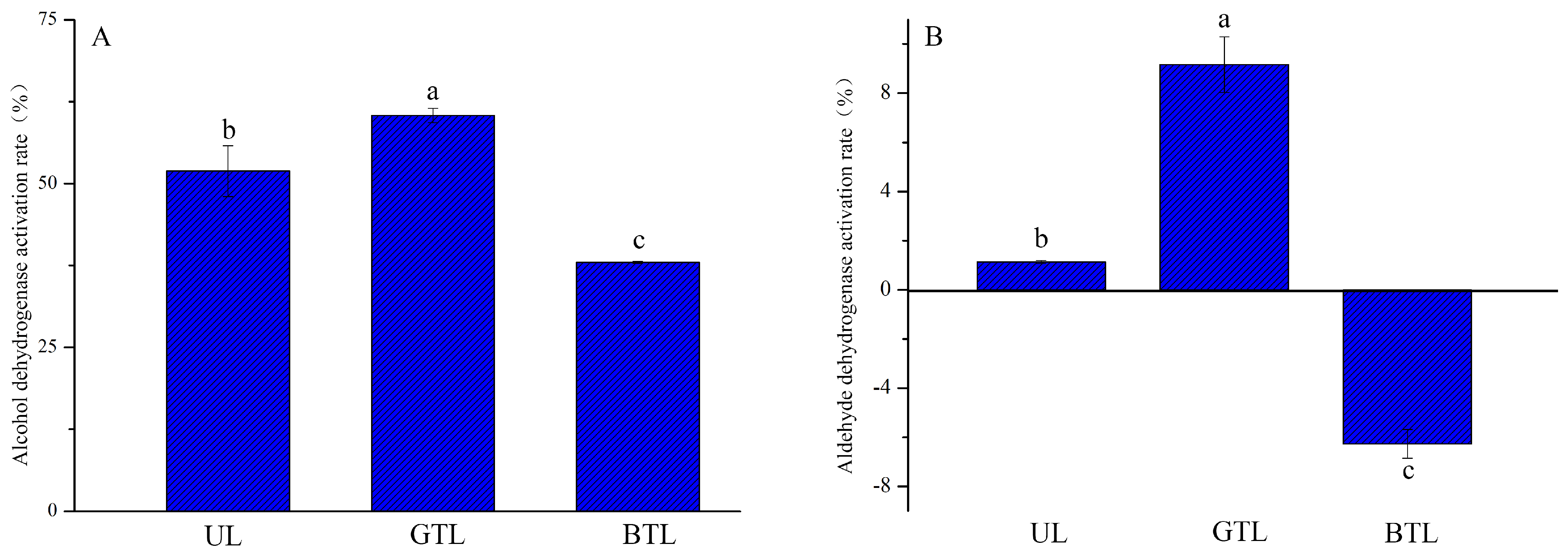
3.5. ADH and ALDH Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, L.B.; Zhang, S.Y.; Luo, C.M.; Sun, Y.R.; Lu, Q.X.; Huang, L.; Chen, F.; Tang, L. Functional teas from the stems of Penthorum chinense Pursh.: Phenolic constituents, antioxidant and hepatoprotective activity. Plant Foods Hum. Nutr. 2019, 74, 83–90. [Google Scholar] [CrossRef]
- Lu, Q.; Jiang, M.H.; Jiang, J.G.; Zhang, R.F.; Zhang, M.W. Isolation and identification of compounds from Penthorum chinense Pursh with antioxidant and antihepatocarcinoma properties. J. Agric. Food Chem. 2012, 60, 11097–11103. [Google Scholar] [CrossRef]
- Wang, A.Q.; Lin, L.G.; Wang, Y.T. Traditional Chinese Herbal Medicine Penthorum chinense Pursh: A Phytochemical and Pharmacological Review. Am. J. Chin. Med. 2015, 43, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, M.; Huang, H.; Xiao, Z.; Shen, J.; Zhao, Y.; Yin, J.; Kaboli, P.J.; Cao, J.; Cho, C.H.; et al. A review of Penthorum chinense Pursh for hepatoprotection: Traditional use, phytochemistry, pharmacology, toxicology and clinical trials. J. Ethnopharmacol. 2020, 251, 112569. [Google Scholar] [CrossRef]
- Cao, Y.W.; Jiang, Y.; Zhang, D.Y.; Wang, M.; Chen, W.S.; Su, H.; Wang, Y.T.; Wan, J.B. Protective effects of Penthorum chinense pursh against chronic ethanol-in-duced liver injury in mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef]
- Fu, M.; Wei, L.; Yu, J.; Hu, Z. Chemical constituents of Penthorum chinense Pursh. Chin. Pharmaceut. J. 2013, 22, 1911–1914. [Google Scholar]
- Sun, Z.L.; Zhang, Y.Z.; Zhang, F.; Zhang, J.W.; Zheng, G.C.; Tan, L.; Wang, C.Z.; Zhou, L.D.; Zhang, Q.H.; Yuan, C.S. Quality assessment of Penthorum chinense Pursh through multicomponent qualification and fingerprint, chemometric, and antihepatocarcinoma analyses. Food Funct. 2018, 9, 3807–3814. [Google Scholar] [CrossRef]
- Yang, S.Q.; Fan, L.J.; Tan, P.; Lei, W.Z.; Liang, J.J.; Gao, Z.P. Effects of Eurotium cristatum on chemical constituents and α-glucosidase activity of mulberry leaf tea. Food Biosci. 2023, 53, 102557. [Google Scholar] [CrossRef]
- Yang, D.D.; Chen, Y.; Guo, F.X.; Huang, B.T.; Okyereb, S.A.; Wang, H. Comparative analysis of chemical composition, antioxidant and antimicrobial activities of leaves, leaf tea and root from Codonopsis pilosula. Ind. Crops Prod. 2019, 142, 111844. [Google Scholar] [CrossRef]
- Medina, R.N.; de Queiróz, J.H.; Machado Rocha Ribeiro, S.; Lopes Toledo, R.C.; Castro Moreira, M.E.; Mafra, C.L.; Benjamin, L.A.; Coelho, C.M.; Veloso, M.P.; Martino, H.S.D. Mango leaf tea promotes hepatoprotective effects in obese rats. J. Funct. Foods 2018, 49, 437–446. [Google Scholar] [CrossRef]
- Du, Y.; Yang, W.; Yang, C.; Yang, X. A comprehensive review on microbiome, aromas and flavors, chemical composition, nutrition and future prospects of Fuzhuan brick tea. Trends Food Sci. Technol. 2022, 119, 452–466. [Google Scholar] [CrossRef]
- Xiang, Z.Y.; Xia, C.; Feng, S.L.; Chen, T.; Zhou, L.J.; Liu, L.; Kong, Q.B.; Yang, H.Y.; Ding, C.B. Assessment of free and bound phenolics in the flowers and floral organs of two Camellia species flower and their antioxidant activities. Food Biosci. 2022, 49, 101905. [Google Scholar] [CrossRef]
- Vallee, B.L.; Hoch, F.L. Zinc: A component of yeast alcohol dehydrogenase. Proc. Natl. Acad. Sci. USA 1955, 41, 3327–3328. [Google Scholar] [CrossRef]
- Blair, A.H.; Bodley, F.H. Human liver aldehyde dehydrogenase: Partial purication and properties. Can. J. Biochem. 1969, 47, 265–272. [Google Scholar] [CrossRef]
- Wang, X.T.; Wang, D.H.; Wang, H.M.; Jiao, S.Y.; Wu, J.P.; Hou, Y.X.; Sun, J.R.; Yuan, J.F. Chemical Profile and Antioxidant Capacity of Kombucha Tea by the Pure Cultured Kombucha. Food Sci. Technol. 2022, 168, 113931. [Google Scholar] [CrossRef]
- Lachenmeir, D.W.; Uebelacker, M. Risk assessment of thujone in foods and medicines containing safe and wormwood evidence for a need of regulatory change? Regul. Toxicol. Pharmacol. 2010, 58, 437–443. [Google Scholar] [CrossRef]
- Kang, D.; Su, M.; Duan, Y.; Huang, Y. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 2019, 10, 5032–5045. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Machan, T. Fingerprint of volatile flavour constituents and antioxidant activities of teas from Thailand. Food Chem. 2011, 125, 797–802. [Google Scholar] [CrossRef]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemometr. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Zhang, J.; Van Mullem, J.; Dias, D.R.; Schwan, R.F. The chemistry and sensory characteristics of new herbal tea-based Kombuchas. J. Food Sci. 2021, 86, 740–748. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Han, Z.S.; Zhu, M.T.; Wan, X.C.; Zhang, L. Effects of thermal processing on transformation of polyphenols and flavor quality. Curr. Opin. Food Sci. 2023, 51, 101014. [Google Scholar] [CrossRef]
- Yin, J.H.; Ren, W.; Wei, B.; Huang, H.M.; Lia, M.X.; Wu, X.X.; Wang, A.Q.; Xiao, Z.G.; Shen, J.; Zhao, Y.S.; et al. Characterization of chemical composition and prebiotic effect of a dietary medicinal plant Penthorum chinense Pursh. Food Chem. 2020, 319, 126568. [Google Scholar] [CrossRef]
- Wang, A.Q.; Wang, S.P.; Jiang, Y.; Chen, M.W.; Wang, Y.T.; Lin, L.G. Bio-assay guided identification of hepatoprotective polyphenols from Penthorum chinense Pursh on t-BHP induced oxidative stress injured L02 cells. Food Funct. 2016, 7, 2074–2083. [Google Scholar] [CrossRef]
- Wang, W.; Shang, H.; Li, J.Z.; Ma, Y.; Xu, C.; Ma, J.G.; Hou, J.C.; Jiang, Z.M. Four Different Structural Dietary Polyphenols, Especially Dihydromyricetin, Possess Superior Protective Effect on Ethanol-Induced ICE-6 and AML-12 Cytotoxicity: The Role of CYP2E1 and Keap1-Nrf2 Pathways. J. Agric. Food Chem. 2023, 71, 1518–1530. [Google Scholar] [CrossRef]
- Jin, L.; Li, X.B.; Tian, D.Q.; Fang, X.P.; Yu, Y.M.; Zhu, H.Q.; Ge, Y.Y.; Ma, G.Y.; Wang, W.Y.; Xiao, W.F.; et al. Antioxidant properties and color parameters of herbal teas in China. Ind. Crops Prod. 2016, 87, 198–209. [Google Scholar] [CrossRef]
- Abram, V.; Čeh, B.; Vidmar, M.; Hercezi, M.; Lazić, N.; Bucik, V.; Možina, S.S.; Košir, I.J.; Kač, M.; Demšar, M. A comparison of antioxidant and antimicrobial activity between hop leaves and hop cones. Ind. Crops Prod. 2015, 64, 124–134. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Baldermann, S.; Watanabe, N. Formation of damascenone and its related compounds from carotenoids in tea. Tea Health Dis. Prev. 2013, 31, 375–386. [Google Scholar]
- McCarty, M.F. Nutraceutical strategies for ameliorating the toxic effects of alcohol. Med. Hypotheses 2013, 80, 456–462. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Bankar, S.B.; Granström, T.; Ojamo, H.; Singhal, R.S.; Survase, S.A. Enhanced stability of alcohol dehydrogenase by non-covalent interaction with polysaccharides. Appl. Microbiol. Biot. 2014, 98, 6307–6316. [Google Scholar] [CrossRef]
- Paulíková, H.; Valusová, E.; Antalík, M. Interactions between heparinoids and alcohol dehydrogenase. Biochem. Mol. Biol. Int. 1997, 42, 667–676. [Google Scholar] [CrossRef]
- Jiang, Y.B.; Zhong, M.; Zhan, H.P.; Tao, X.B.; Zhang, Y.F.; Mao, J.X.; Geng, Z.; Gao, B.X. Integrated strategy of network pharmacology, molecular docking, HPLC-DAD and mice model for exploring active ingredients and pharmacological mechanisms of Penthorum chinense Pursh against alcoholic liver injury. J. Ethnopharmacol. 2022, 298, 115589. [Google Scholar] [CrossRef] [PubMed]

| UL | GTL | BTL | |
|---|---|---|---|
| TPC (mg GAE/g DW) | 179.34 ± 12.53 a | 138.90 ± 6.98 b | 97.13 ± 7.32 c |
| TFC (mg RE/g DW) | 71.93 ± 0.52 a | 59.88 ± 0.83 b | 40.07 ± 2.53 c |
| TPAC (mg CE/g DW) | 65.91 ± 0.99 a | 57.74 ± 0.42 b | 54.13 ± 2.14 c |
| Compounds | UL | GTL | BTL |
|---|---|---|---|
| gallic acid | 0.44 ± 0.01 a | 0.22 ± 0.01 c | 0.32 ± 0.01 b |
| (+)-catechin | 5.91 ± 0.39 ab | 6.68 ± 0.48 a | 5.21 ± 0.11 b |
| (−)-epicatechin | 0.26 ± 0.03 a | 0.27 ± 0.03 a | - |
| rutin | 0.06 ± 0.01 a | 0.07 ± 0.01 a | 0.06 ± 0.00 a |
| isoquercitrin | 0.22 ± 0.02 a | 0.23 ± 0.02 a | 0.21 ± 0.01 a |
| kaempferol-3-O-rutinoside | 0.18 ± 0.01 a | 0.18 ± 0.01 a | 0.20 ± 0.00 a |
| astragaline | 1.61 ± 0.18 a | 1.78 ± 0.17 a | 1.83 ± 0.05 a |
| quercetin | 4.88 ± 0.53 a | 5.36 ± 0.52 a | 5.48 ± 0.14 a |
| afzelin | 0.88 ± 0.01 a | 0.91 ± 0.00 a | 0.82 ± 0.01 b |
| pinocembrin 7-O-glucoside | 2.70 ± 0.02 a | 0.23 ± 0.02 b | 0.28 ± 0.01 b |
| kaempferol | 10.46 ± 0.55 a | 10.68 ± 0.00 a | 9.87 ± 0.60 a |
| pinocembrin 7-O-(3″-O-galloy-4″,6″-hexahydroxydiphenoyl)-glucoside | 7.08 ± 0.81 b | 7.69 ± 0.86 b | 9.73 ± 0.41 a |
| pinocembrin | 0.76 ± 0.12 b | 1.20 ± 0.09 a | 0.93 ± 0.05 b |
| thonningianin A | 86.32 ± 6.33 a | 99.38 ± 9.04 a | 96.30 ± 0.26 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Z.; Zhu, B.; Yang, X.; Deng, J.; Zhu, Y.; Gan, L.; Yu, M.; Chen, J.; Xia, C.; Chen, S. Comprehensive Analysis of Phenolic Constituents, Biological Activities, and Derived Aroma Differences of Penthorum chinense Pursh Leaves after Processing into Green and Black Tea. Foods 2024, 13, 399. https://doi.org/10.3390/foods13030399
Xiang Z, Zhu B, Yang X, Deng J, Zhu Y, Gan L, Yu M, Chen J, Xia C, Chen S. Comprehensive Analysis of Phenolic Constituents, Biological Activities, and Derived Aroma Differences of Penthorum chinense Pursh Leaves after Processing into Green and Black Tea. Foods. 2024; 13(3):399. https://doi.org/10.3390/foods13030399
Chicago/Turabian StyleXiang, Zhuoya, Boyu Zhu, Xing Yang, Junlin Deng, Yongqing Zhu, Lu Gan, Manyou Yu, Jian Chen, Chen Xia, and Song Chen. 2024. "Comprehensive Analysis of Phenolic Constituents, Biological Activities, and Derived Aroma Differences of Penthorum chinense Pursh Leaves after Processing into Green and Black Tea" Foods 13, no. 3: 399. https://doi.org/10.3390/foods13030399
APA StyleXiang, Z., Zhu, B., Yang, X., Deng, J., Zhu, Y., Gan, L., Yu, M., Chen, J., Xia, C., & Chen, S. (2024). Comprehensive Analysis of Phenolic Constituents, Biological Activities, and Derived Aroma Differences of Penthorum chinense Pursh Leaves after Processing into Green and Black Tea. Foods, 13(3), 399. https://doi.org/10.3390/foods13030399






