Contamination Status and Acute Dietary Exposure Assessment of Paralytic Shellfish Toxins in Shellfish in the Dalian Area of the Yellow-Bohai Sea, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Standard Solution Preparation
2.3. Sample Collection
2.4. Sample Preparation
2.5. LC–MS Analysis
2.6. Analytical Validation
2.7. Acute Dietary Exposure Risk Assessment for PSTs from Shellfish for Chinese General Consumers
2.7.1. Shellfish Consumption Data of Chinese General Consumers
2.7.2. The Equivalent Concentrations of STX in Shellfish Samples
2.7.3. Probabilistic Model of Acute Dietary Exposure to PSTs from Shellfish among Chinese General Consumers
2.8. Estimation of Acute Dietary Exposure to PSTs from Shellfish by Consumers in Coastal Areas of China
2.8.1. Shellfish Consumption Data of Special Consumers in the Bohai Rim Region
2.8.2. Probabilistic Model of Acute Dietary Exposure to PSTs from Shellfish Consumed by Typical Consumers
2.9. Statistical Analysis
3. Results and Discussion
3.1. PST Toxin Content in Shellfish in the Yellow-Bohai Sea in Dalian
3.2. Seasonal Variation in the PSTs in Bivalves and Gastropods in the Yellow-Bohai Sea in Dalian
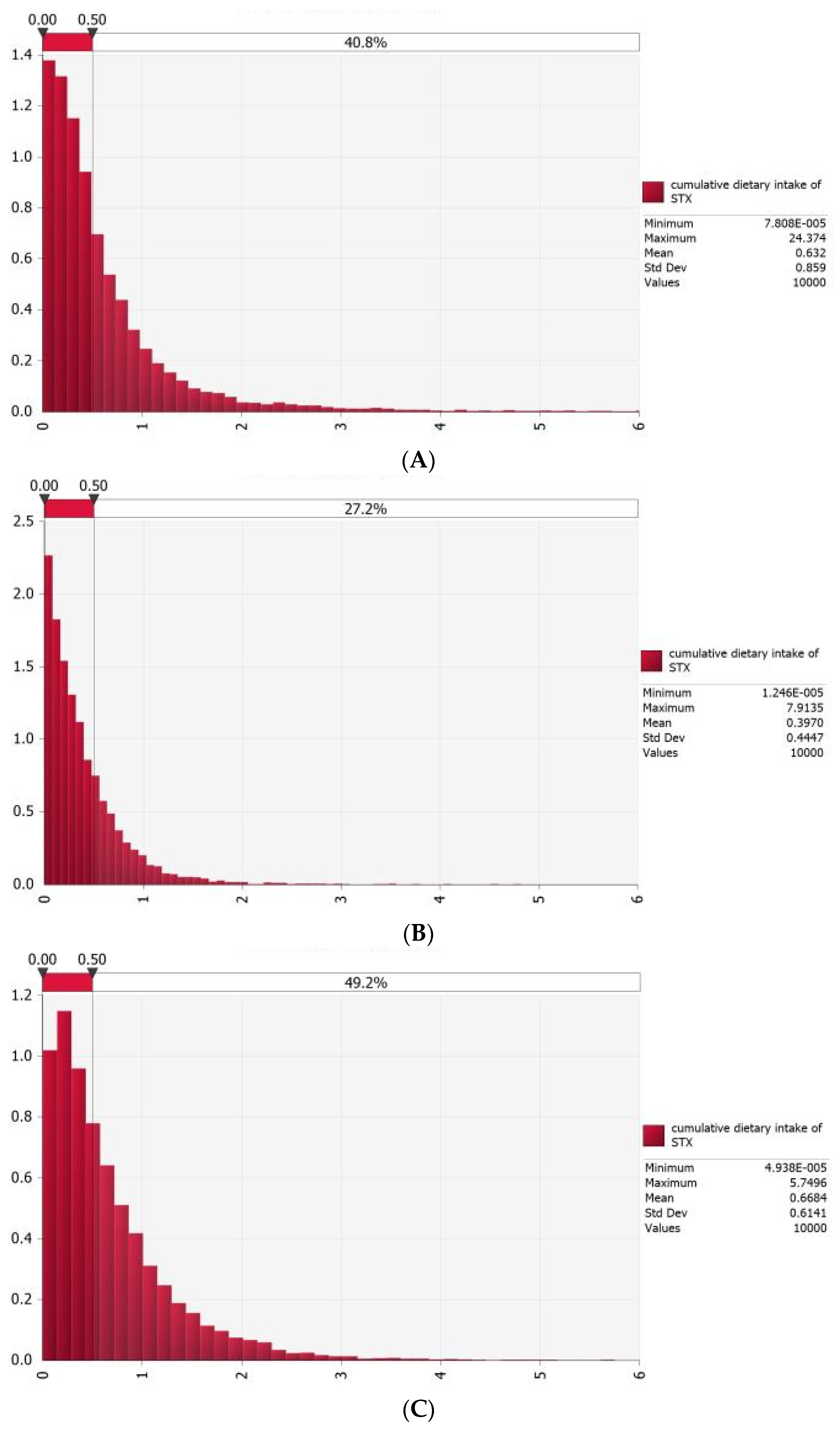
3.3. Probabilistic Estimation of Acute Dietary Exposure to PSTs for Chinese General Consumers
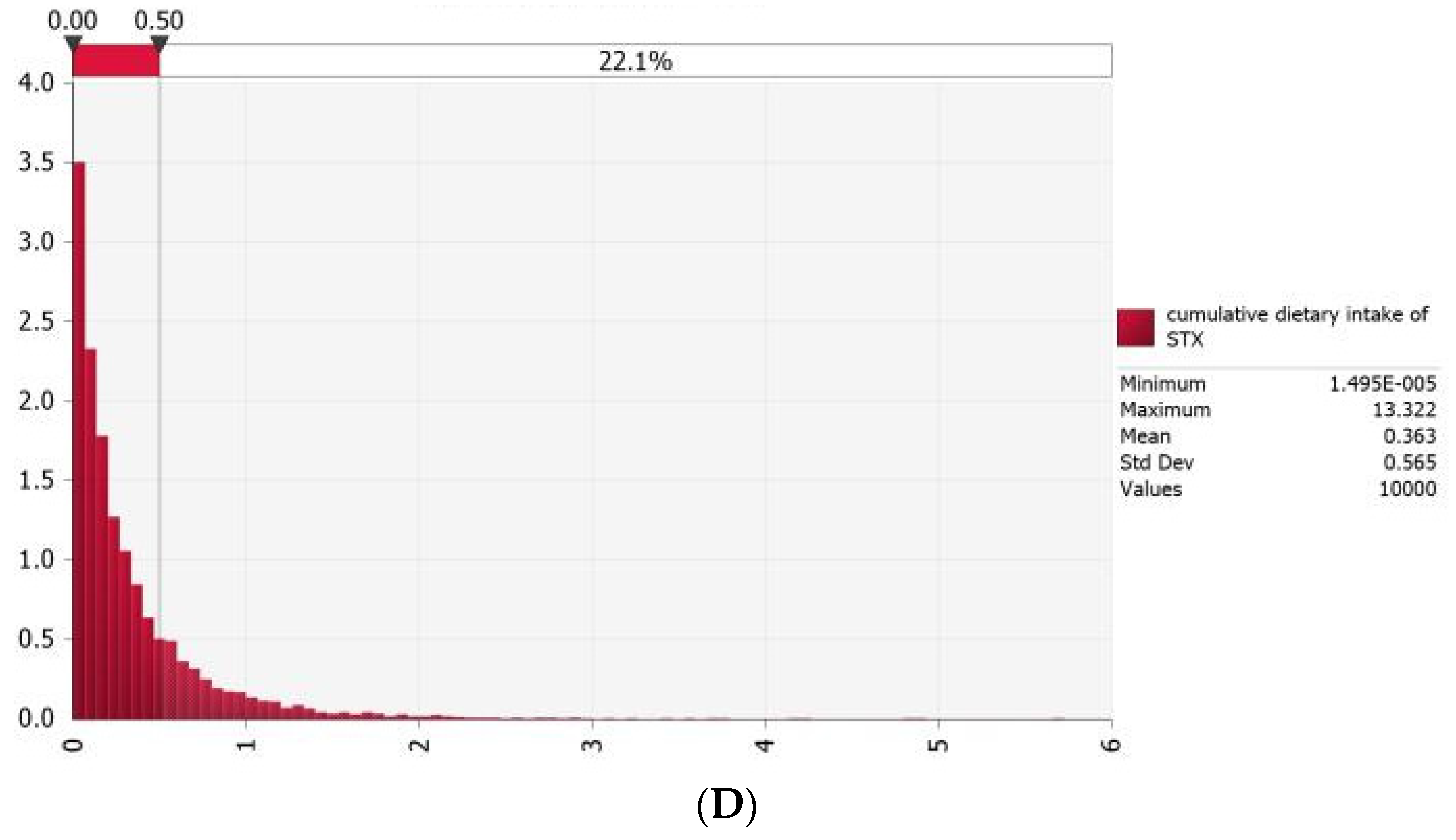
3.4. Probabilistic Estimation of Acute Dietary Exposure to PSTs for Typical Consumers in Coastal Areas of China
3.5. Uncertainty Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Dai, L.; Chen, Z.F.; Geng, H.X.; Lin, Z.R.; Zhao, Y.; Zhou, Z.X.; Kong, F.Z.; Yu, R.C.; Zhou, M.J. Spatiotemporal variation of paralytic shellfish toxins in the sea area adjacent to the Changjiang River estuary. Environ. Pollut. 2020, 259, 113730. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, J. Occurrence and potential risks of harmful algal blooms in the East China Sea. Sci. Total Environ. 2009, 407, 4012–4021. [Google Scholar] [CrossRef]
- Leal, J.F.; Cristiano, M.L.S. Marine paralytic shellfish toxins: Chemical properties, mode of action, newer analogues, and structure-toxicity relationship. Nat. Prod. Rep. 2022, 39, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.I.C.; Gomes, M.T.S.R.; Botelho, M.J.; Rudnitskaya, A. Paralytic Shellfish Toxins (PST)-Transforming Enzymes: A Review. Toxins 2020, 22, 344. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.T.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.T. Ultrahigh-Performance Hydrophilic Interaction Liquid Chromatography with Tandem Mass Spectrometry Method for the Determination of Paralytic Shellfish Toxins and Tetrodotoxin in Mussels, Oysters, Clams, Cockles, and Scallops: Collaborative Study. J. AOAC Int. 2020, 103, 533–562. [Google Scholar] [CrossRef]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography-mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef]
- Cao, Y.; Qiu, J.; Li, A.; Zhang, L.; Yan, G.; Ji, Y.; Zhang, J.; Zhao, P.; Wu, X. Occurrence and spatial distribution of paralytic shellfish toxins in seawater and marine organisms in the coastal waters of Qinhuangdao, China. Chemosphere 2023, 315, 137746. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Zhang, J.; Zhang, J.; Wang, Z.; Pan, L.; Huang, B.; Huang, K.; Chen, X.; Zhao, Q.; et al. Dietary exposure assessment of paralytic shellfish toxins through shellfish consumption in Shenzhen population, China. Environ. Sci. Pollut. Res. Int. 2022, 29, 10222–10234. [Google Scholar] [CrossRef]
- Shin, C.; Jo, H.; Kim, S.H.; Kang, G.J. Exposure assessment to paralytic shellfish toxins through the shellfish consumption in Korea. Food Res. Int. 2018, 108, 274–279. [Google Scholar] [CrossRef]
- Gerssen, A.; Pol-Hofstad, I.E.; Poelman, M.; Mulder, P.P.; van den Top, H.J.; de Boer, J. Marine toxins: Chemistry, toxicity, occurrence and detection, with special reference to the Dutch situation. Toxins 2010, 2, 878–904. [Google Scholar] [CrossRef]
- Ching, P.K.; Ramos, R.A.; de los Reyes, V.C.; Sucaldito, M.N.; Tayag, E. Lethal paralytic shellfish poisoning from consumption of green mussel broth, Western Samar, Philippines, August 2013. West. Pac. Surveill. Response J. WPSAR 2015, 6, 22–26. [Google Scholar] [CrossRef]
- Chen, J.Z.; Hong, S.P.; Cai, M.R.; Chen, L.I.; Zhang, T.L. An outbreak of foodborne disease caused by paralytic shellfish toxin. Chin. J. Food Hyg. 2018, 30, 445–448. [Google Scholar]
- Zhou, J.; Zhou, Z.F.; Deng, K.J.; Jiang, L.X. A food poisoning caused by shellfish toxins. Occup. Health 2013, 29, 2025–2026. [Google Scholar]
- Du, K.M.; Jiang, T.J.; Wu, N. The pattern of paralytic shellfish poisoning in shellfish cultured in the coast of Yellow Sea, China. Mar. Environ. Sci. 2013, 32, 182–184. [Google Scholar]
- Weng, Q.; Zhou, B. Paralytic shellfish poisoning contamination status and dietary exposure assessment in coastal cities of China. Prev. Med. 2023, 35, 501–505. [Google Scholar]
- GB 2733-2015; National Food Safety Standard for Fresh and Frozen Animal Aquatic Products. National Health Commission of the People’s Republic of China: Beijing, China, 2015.
- European Food Safety Authority (EFSA). Marine biotoxins in shellfish-Saxitoxin group-Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA J. 2009, 1019, 3. [Google Scholar]
- Joint Expert Committee on Food Additives (JECFA). Report of the Joint FAO/IOC/WHO Adhoc Expert Consultation on Biotoxins in Bivalve Molluscs; Joint Expert Committee on Food Additives (JECFA): Oslo, Norway, 2004. [Google Scholar]
- Joint Expert Committee on Food Additives (JECFA). Toxicity Equivalence Factors for Marine Biotoxins Associated with Bivalve Molluscs; Joint Expert Committee on Food Additives (JECFA): Rome, Italy, 2016. [Google Scholar]
- Weng, Q.; Zhang, R.; Wu, P.; Chen, J.; Pan, X.; Zhao, D.; Wang, J.; Zhang, H.; Qi, X.; Wu, X.; et al. An Occurrence and Exposure Assessment of Paralytic Shellfish Toxins from Shellfish in Zhejiang Province, China. Toxins 2023, 15, 624. [Google Scholar] [CrossRef]
- Pan, F.; Luan, D.C.; Zhang, T.W.; Mao, W.F.; Liang, D.; Liu, A.D.; Li, J.W. Assessment of sugar-sweetened beverages consumption and free sugar intake among urban residents aged 3 and above in China. Chin. J. Food Hyg. 2022, 34, 126–130. [Google Scholar]
- Sun, J.F.; Wu, Y.N. Evaluation of dietary exposure to deoxynivalenol (DON) and its derivatives from cereals in China. Food Control 2016, 69, 90–99. [Google Scholar] [CrossRef]
- Cao, P.; Yang, D.J.; Zhu, J.H.; Liu, Z.P.; Jiang, D.G.; Xu, H.B. Estimated assessment of cumulative dietary exposure to organophosphorus residues from tea infusion in China. Environ. Health Prev. Med. 2018, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Joint FAO/WHO Food Standards Programme; Codex classification of foods and animal feeds; Codex Alimentarius Commission: Rome, Italy, 1993. [Google Scholar]
- Xue, J.; Zartarian, V.; Wang, S.W.; Liu, S.V.; Georgopoulos, P. Probabilistic Modeling of Dietary Arsenic Exposure and Dose and Evaluation with 2003-2004 NHANES Data. Environ. Health Perspect. 2010, 118, 345–350. [Google Scholar] [CrossRef]
- World Health Organization (WHO). GEMS/Food-EURO Workshop on Reliable Evaluation of Low-Level Contamination of Food: Report on a Workshop in the Frame of GEMS/Food-Euro, Kulmbach; World Health Organization (WHO): Geneva, Switzerland, 1995. [Google Scholar]
- Cui, J.J.; Cao, P.; Sun, J.Y.; Sun, Y.Y.; Yu, J.R.; Wang, X.D. Investigation on Consumption Status of Shellfish in Areas Around Bohai Sea. Food Nutr. China 2021, 27, 40–44. [Google Scholar]
- Samsur, M.; Yamaguchi, Y.; Sagara, T.; Takatani, T.; Arakawa, O.; Noguchi, T. Accumulation and depuration profiles of PSP toxins in the short-necked clam Tapes japonica fed with the toxic dinoflagellate Alexandrium catenella. Toxicon 2006, 48, 323–330. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Shumway, S.E. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation. Rev. Fish Sci. Aquac. 2010, 6, 315–383. [Google Scholar] [CrossRef]
- Wong, C.K.; Hung, P.; Lee, K.L.H.; Mok, T.; Kam, K.M. Effect of steam cooking on distribution of paralytic shellfish toxins in different tissue compartments of scallops Patinopecten yessoensis. Food Chem. 2009, 114, 72–80. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Li, Z.X.; Sun, X.; Xing, L.H.; Zhang, M.T.; Zhu, P.P.; Wang, J.Y.; Su, W.Q. Surveillance and Risk Assessment of Diarrhetic and Paralytic Shellfish Toxins in the Tangshan Shellfish Culture Areas of Bohai Sea, China. Prog. Fish. Sci. 2023, 5, 231–240. [Google Scholar]
- Liu, Y.; Yu, R.C.; Kong, F.Z.; Chen, Z.F.; Dai, L.; Gao, Y.; Zhang, Q.C.; Wang, Y.F.; Yan, T.; Zhou, M.J. Paralytic shellfish toxins in phytoplankton and shellfish samples collected from the Bohai Sea, China. Mar. Pollut. Bull. 2017, 115, 324–331. [Google Scholar] [CrossRef]
- Finnis, S.; Krstic, N.; McIntyre, L.; Nelson, T.A.; Henderson, S.B. Spatiotemporal patterns of paralytic shellfish toxins and their relationships with environmental variables in British Columbia, Canada from 2002 to 2012. Environ. Res. 2017, 156, 190–200. [Google Scholar] [CrossRef]
- David, C.P.C.; Maria, Y.Y.S.; Siringan, F.P.; Reotita, J.M.; Zamora, P.B.; Villanoy, C.L.; Sombrito, E.Z.; Azanza, R.V. Coastal pollution due to increasing nutrient flux in aquaculture sites. Environ. Geol. 2009, 58, 447–454. [Google Scholar] [CrossRef]
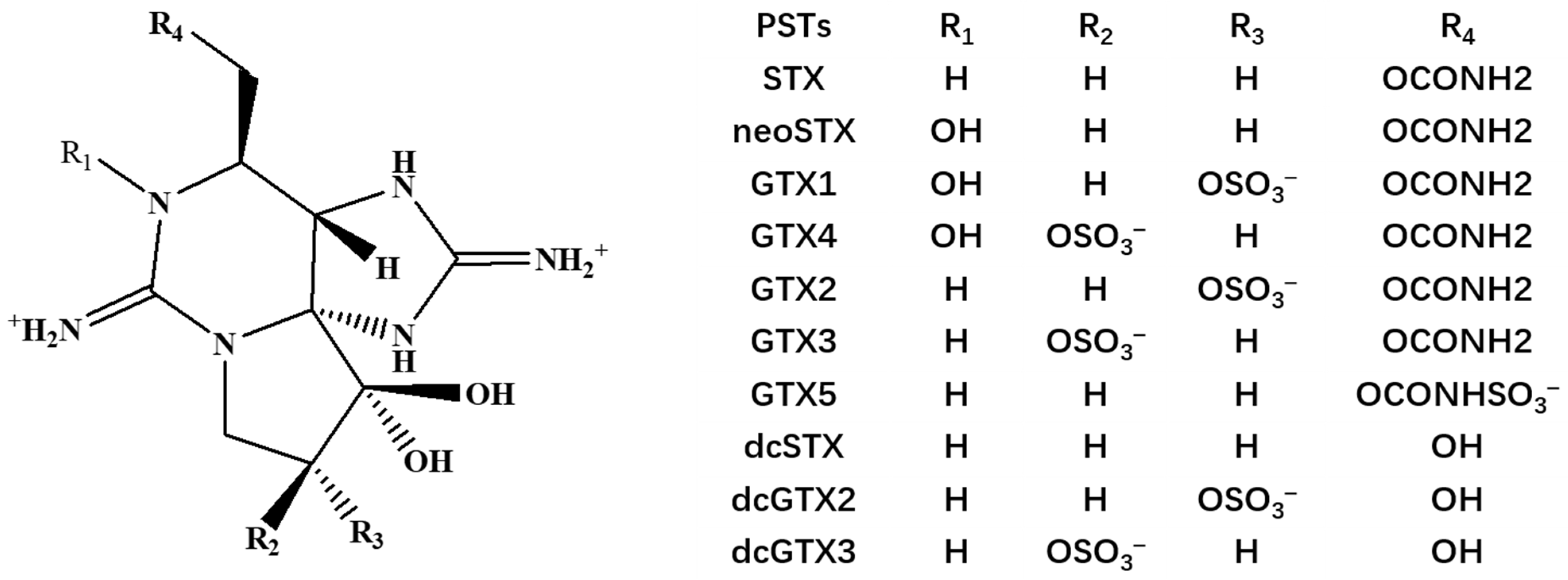
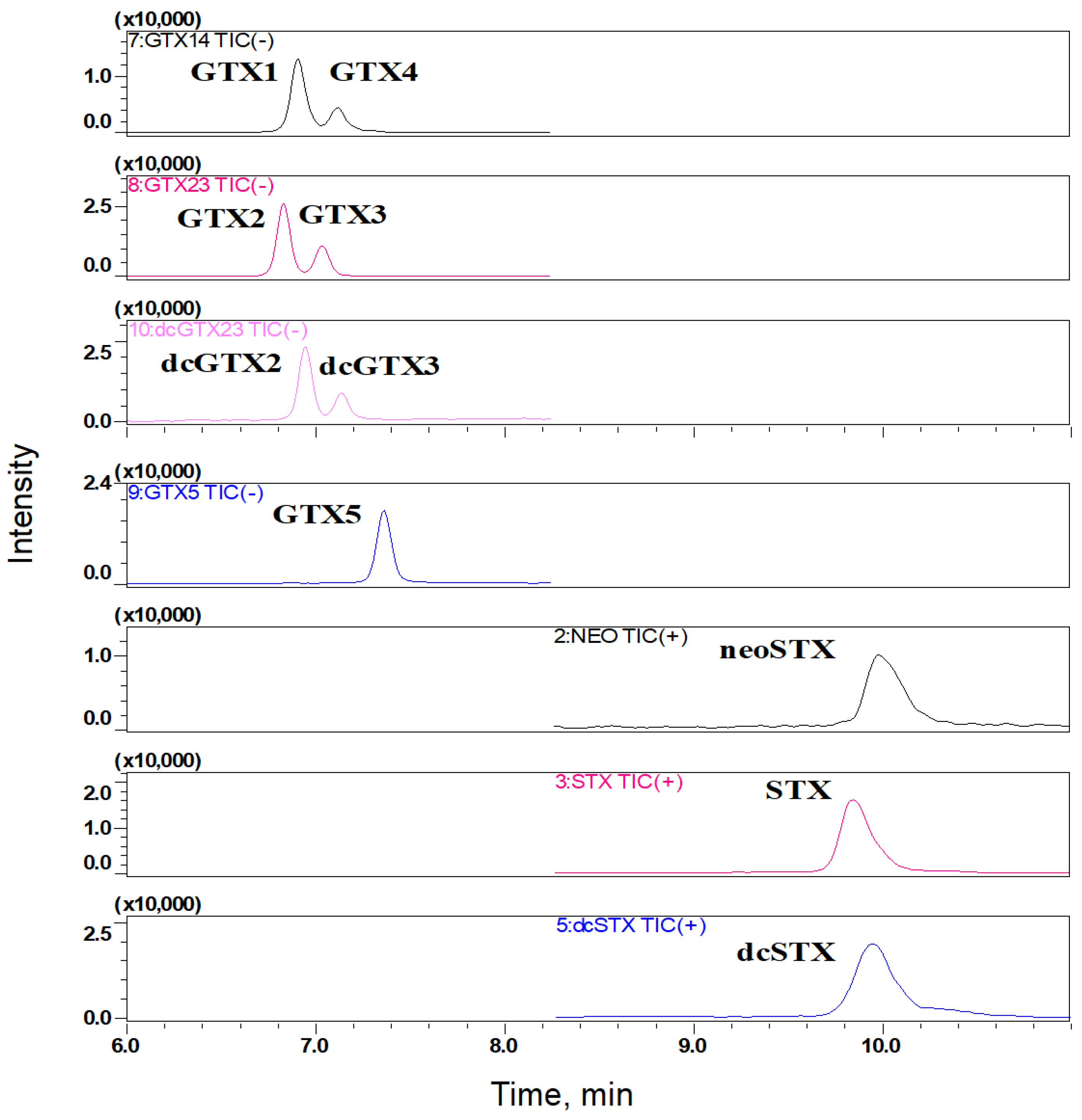
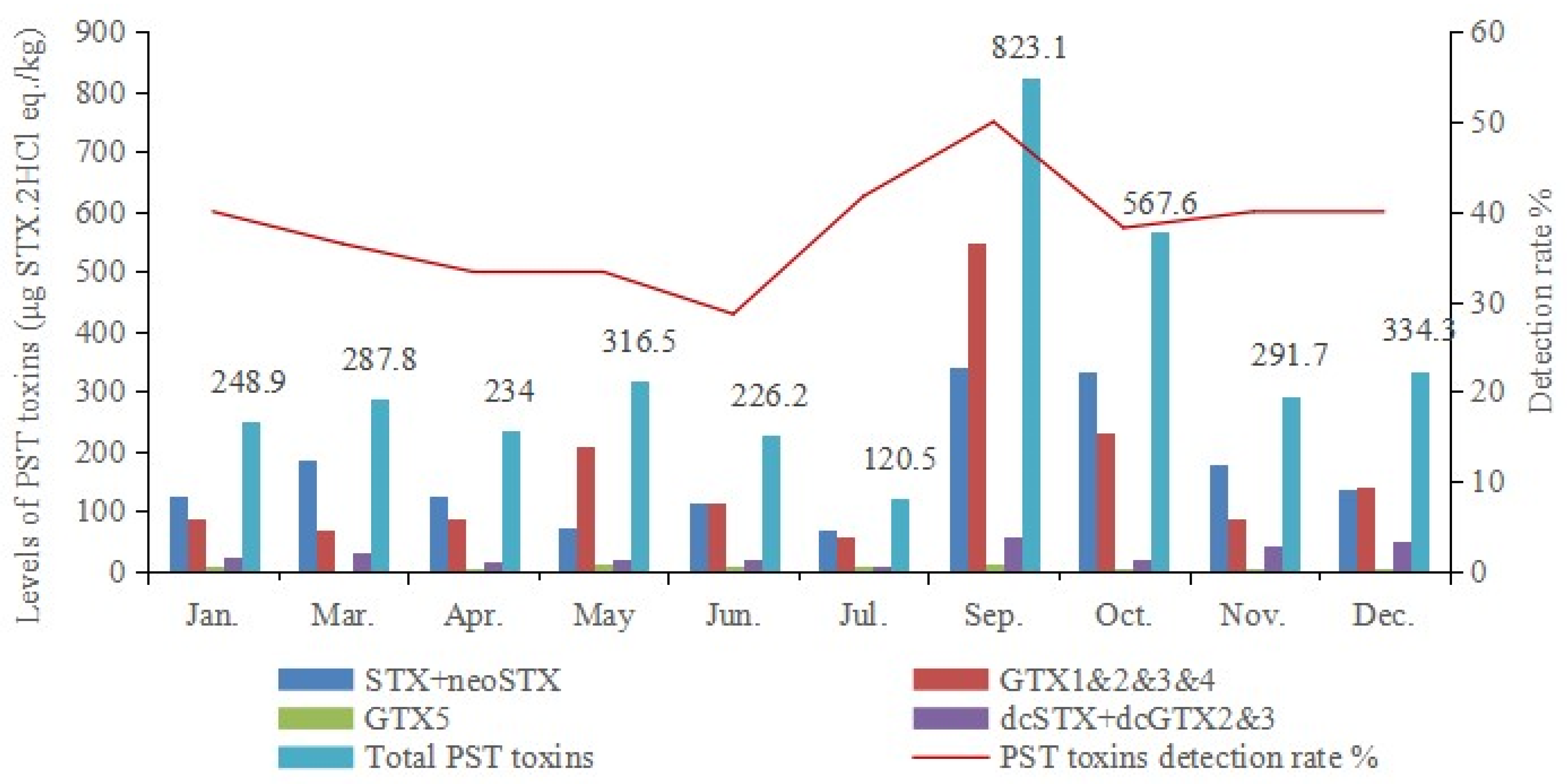
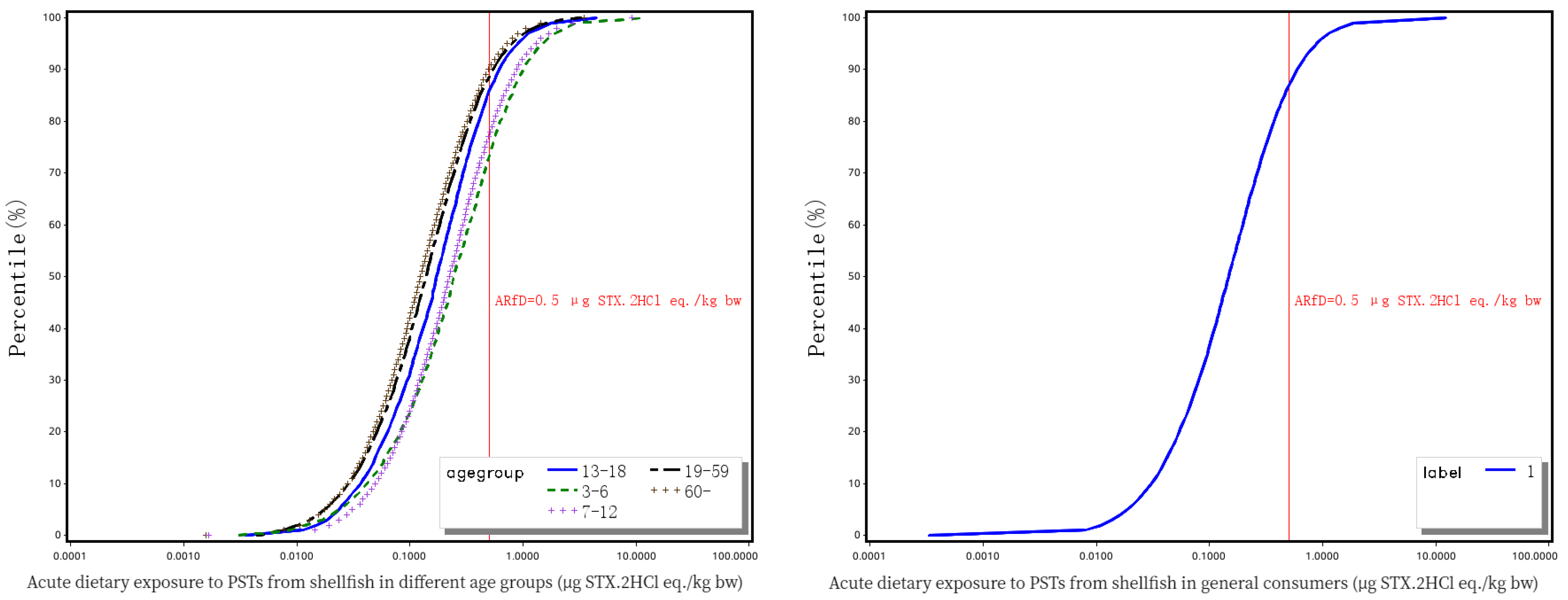
| Toxins | Molar Concentration (μmol/L) | Mass Concentration (μg/mL) | Standard Solution Volume (μL) | Blank Matrix Volume (μL) | Total Volume (μL) | The Concentrations of Mixed Standard Intermediate Solutions (ng/mL) |
|---|---|---|---|---|---|---|
| STX | 66.3 | 24.68 | 20 | 1840 | 2000 | 247 |
| neoSTX | 65.6 | 25.47 | 20 | 255 | ||
| dcSTX | 65 | 21.40 | 20 | 214 | ||
| GTX5 | 55.7 | 25.19 | 40 | 504 | ||
| GTX1 | 60.4 | 29.25 | 25 | 366 | ||
| GTX4 | 19.7 | 9.54 | 119 | |||
| GTX2 | 114.2 | 53.48 | 15 | 401 | ||
| GTX3 | 43.4 | 20.32 | 152 | |||
| dcGTX2 | 116 | 49.33 | 20 | 493 | ||
| dcGTX3 | 26.1 | 11.10 | 111 |
| Toxin | ESI Mode | Precursor Ion (m/z) | Collision Energy (eV) | Product Ion (m/z) | Collision Energy (eV) | LOD (µg/kg) |
|---|---|---|---|---|---|---|
| STX | ESI+ | 300.2/204.0 | 30 | 300.2/138.1 | 35 | 5 |
| dcSTX | ESI+ | 257.1/126.1 | 30 | 257.1/221.1 | 22 | 5 |
| neoSTX | ESI+ | 316.1/298.1 | 34 | 316.1/220.1 | 34 | 20 |
| GTX2 | ESI- | 394.0/351.1 | 16 | 394.0/333.1 | 22 | 10 |
| GTX3 | ESI- | 394.0/333.1 | 22 | 394.0/351.1 | 16 | 10 |
| GTX1 | ESI- | 410.1/367.1 | 15 | 410.1/349.1 | 22 | 10 |
| GTX4 | ESI- | 410.1/349.1 | 22 | 410.1/367.1 | 15 | 10 |
| GTX5 | ESI- | 378.1/122.1 | 25 | 378.1/360.1 | 20 | 10 |
| dcGTX2 | ESI- | 351.1/164.0 | 30 | 351.1/333.1 | 17 | 20 |
| dcGTX3 | ESI- | 351.1/333.1 | 17 | 351.1/164.0 | 30 | 20 |
| Shellfish Species | Toxins | Standard Solution Concentration (μg/kg) | Recovery (%) | RSDr (%) |
|---|---|---|---|---|
| Mussel | STX | 98.8 | 101.0 | 11.6 |
| neoSTX | 102.0 | 111.0 | 8.9 | |
| dcSTX | 85.6 | 92.1 | 9.0 | |
| GTX5 | 202.0 | 109.0 | 10.4 | |
| GTX1 | 146.0 | 86.3 | 8.1 | |
| GTX4 | 47.6 | 82.0 | 13.9 | |
| GTX2 | 160.0 | 82.9 | 8.3 | |
| GTX3 | 60.8 | 86.3 | 12.8 | |
| dcGTX2 | 197.0 | 98.5 | 7.0 | |
| dcGTX3 | 44.4 | 86.3 | 10.4 | |
| Scallop | STX | 198.0 | 94.8 | 6.8 |
| neoSTX | 204.0 | 102.0 | 9.0 | |
| dcSTX | 171.0 | 91.6 | 3.0 | |
| GTX5 | 403.0 | 111.0 | 5.8 | |
| GTX1 | 293.0 | 102.0 | 5.9 | |
| GTX4 | 95.2 | 106.0 | 9.8 | |
| GTX2 | 321.0 | 118.0 | 10.6 | |
| GTX3 | 122.0 | 109.0 | 14.0 | |
| dcGTX2 | 394.0 | 114.0 | 10.8 | |
| dcGTX3 | 88.8 | 112.0 | 11.3 | |
| Oyster | STX | 494.0 | 101.0 | 8.0 |
| neoSTX | 510.0 | 107.3 | 7.3 | |
| dcSTX | 428.0 | 97.1 | 4.7 | |
| GTX5 | 1008.0 | 101.1 | 9.6 | |
| GTX1 | 732.0 | 98.5 | 8.0 | |
| GTX4 | 238.0 | 88.5 | 8.0 | |
| GTX2 | 802.0 | 101.3 | 9.0 | |
| GTX3 | 304.0 | 99.4 | 9.8 | |
| dcGTX2 | 986.0 | 101.9 | 6.8 | |
| dcGTX3 | 44.4 | 104.2 | 5.0 |
| Shellfish Species | Consumption Days | Consumption of Shellfish (g/d) | |||||
|---|---|---|---|---|---|---|---|
| Mean | P50 | P90 | P97.5 | P99 | Max | ||
| Manila clam | 2153 | 88.4 | 75 | 180 | 250 | 300 | 500 |
| Scallop | 471 | 42.3 | 20 | 100 | 250 | 500 | 650 |
| Mussel | 112 | 78.4 | 75 | 150 | 250 | 500 | 500 |
| Oyster | 1154 | 61.1 | 50 | 110 | 200 | 260 | 650 |
| Blood clam | 9 | 115.6 | 110 | 200 | 200 | 200 | 200 |
| STX and Analogues | TEFs |
|---|---|
| STX | 1.0 |
| neoSTX | 2.0 |
| GTX1 | 1.0 |
| GTX2 | 0.4 |
| GTX3 | 0.6 |
| GTX4 | 0.7 |
| GTX5 | 0.1 |
| dcSTX | 0.5 |
| dcGTX2 | 0.2 |
| dcGTX3 | 0.4 |
| Shellfish Species | Number of Consumers | Proportion of Consumers (%) | Average Monthly Consumption Frequency | Consumption of Shellfish (g/d/Person) | |||
|---|---|---|---|---|---|---|---|
| Mean | P50 | P97.5 | Range | ||||
| Scallop | 737 | 84.2 | 4 | 183.8 | 175.0 | 525.0 | 10.2~875.0 |
| Mussel | 432 | 49.4 | 2 | 302.0 | 245.0 | 980.0 | 24.5~1225.0 |
| Oyster | 672 | 76.8 | 3 | 191.1 | 125.0 | 625.0 | 20.0~1875.0 |
| Blood clam | 184 | 21.0 | 1 | 114.0 | 87.5 | 375.0 | 8.8~500.0 |
| Variables | Definition | Assumption/Distribution/Formula | Source |
|---|---|---|---|
| ConcSTX * | Equivalent concentrations of PSTs in detected samples | Lognorm (120.67, 223.77) a | Monitoring data |
| Lognorm (326.75, 143.34) b | |||
| Lognorm (285.39, 136.24) c | |||
| ConcSTX ** | Equivalent concentrations of PSTs in undetected samples | Uniform (0, 118.5) | Monitoring data |
| Pp | Rate of shellfish sample with detectable concentration | 94.4% a | Calculated |
| 94.4% b | |||
| 12.5% c | |||
| Pn | Rate of shellfish sample with undetectable concentration | 5.6% a | Calculated |
| 5.6% b | |||
| 87.5% c | |||
| ConcSTXs | Equivalent concentrations of PSTs in shellfish samples | Discrete (ConcSTX *:ConcSTX **, Pn:Pp) | Calculated |
| Cons | Consumption of shellfish in Bohai Rim region in China | Pert (0, 61.4, 979.3) a | Specific survey of shellfish consumption in Bohai Rim region |
| Pert (8.5, 29.5, 588.7) b | |||
| Pert (20, 20, 1891.7) c | |||
| bw | Body weight for shellfish consumers in Bohai Rim region in China | Normal (70.3, 13.1) | Specific survey of shellfish consumption in Bohai Rim region |
| Shellfish Species | Samples | Detection Rate of PSTs (%) | PSTs in Detected Samples (μg STX.2HCl eq./kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STX | neoSTX | GTX1 | GTX4 | GTX2 | GTX3 | GTX5 | dcSTX | dcGTX2 | dcGTX3 | Total Content of PSTs | |||
| Pacific oyster | 34 | 5.9 | 11.3 | ND | 36.2~44.7 | 10.7~14.0 | 38.9~43.3 | 23.9~25.7 | 1.8~1.9 | ND | 11.6~12.8 | 6.2~8.9 | 132.6~159.3 |
| Mediterranean blue mussels | 25 | 20.0 | 15.2~235.8 | ND | 118.8~149.9 | 25.9~32.1 | 5.4~19.1 | 13.3~14.2 | ND | ND | ND | ND | 5.4~235.8 |
| Manila clam | 34 | 0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Scallop | 36 | 94.4 | 5.9~928.3 | 23.2~893.6 | 12.9~1409.3 | 7.0~259.6 | 4.8~558.6 | 6.1~359.4 | 1.0~39.4 | 5.7~95.9 | 4.9~77.6 | 5.2~34.0 | 43.0~3953.5 |
| Veined rapa whelks | 34 | 0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Blood clam | 36 | 94.4 | 12.2~235.8 | 20.6~309.0 | 10.1~238.5 | 5.0~69.4 | 8.9~179.4 | 6.5~84.4 | 2.2~10.3 | 6.5~29.9 | 3.8~43.7 | 4.1~18.9 | 94.4~993.4 |
| Total | 199 | 37.3 | 5.9~928.3 | 20.6~893.6 | 10.1~1409.3 | 5.0~259.6 | 4.8~558.6 | 6.1~359.4 | 1.0~39.4 | 5.7~95.9 | 3.8~77.6 | 4.1~34.0 | 5.4~3953.5 |
| Scallops | Detection Rate of PSTs (%) | PSTs in Detected Samples (μg STX.2HCl eq./kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| STX | neoSTX | GTX1 | GTX4 | GTX2 | GTX3 | GTX5 | dcSTX | dcGTX2 | dcGTX3 | Total Content of PSTs | ||
| Scallop adductor muscle | 29.4 | 7.6~96.8 | 42.8~79.0 | 24.9~67.3 | 8.3 | 4.4~49.9 | 6.7~18.8 | 1.3~7.4 | ND | 8.3 | ND | 4.4~332.1 |
| Scallop viscera | 94.4 | 5.9~397.1 | 23.2~893.6 | 12.9~1145.0 | 7.0~182.6 | 4.8~374.6 | 3.1~239.2 | 1.0~24.8 | 6.6~57.0 | 2.9~49.8 | 5.2~33.8 | 10.0~2605.5 |
| Scallop skirt | 79.4 | 6.9~434.4 | 32.0~58.2 | 14.7~197.0 | 7.3~68.7 | 4.4~134.1 | 6.2~101.3 | 1.3~14.0 | 5.7~38.9 | 3.7~19.5 | 6.9~9.2 | 10.0~1015.9 |
| Group | Acute Dietary Exposure to PSTs from Shellfish (μg STX.2HCl eq./kg bw) | QR | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | P25 | P50 | P75 | P90 | P97.5 | P99 | P99.9 | |||
| 3~6 | 0.46 | 0.10 | 0.25 | 0.55 | 1.07 | 2.37 | 3.16 | 6.73 | 0.10 | 2.01~2.37 |
| 7~12 | 0.40 | 0.11 | 0.23 | 0.47 | 0.88 | 1.86 | 2.69 | 5.77 | 0.07 | 1.64~1.92 |
| 13~18 | 0.29 | 0.08 | 0.17 | 0.35 | 0.61 | 1.28 | 1.92 | 4.38 | 0.08 | 1.19~1.38 |
| 19~59 | 0.24 | 0.06 | 0.14 | 0.29 | 0.54 | 1.10 | 1.60 | 3.28 | 0.01 | 1.09~1.14 |
| ≥60 | 0.22 | 0.06 | 0.13 | 0.26 | 0.48 | 0.94 | 1.49 | 3.38 | 0.03 | 0.90~0.99 |
| Total | 0.27 | 0.07 | 0.15 | 0.31 | 0.60 | 1.24 | 1.85 | 3.84 | 0.02 | 1.22~1.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, P.; Zhang, L.; Huang, Y.; Li, S.; Wang, X.; Pan, F.; Yu, X.; Sun, J.; Liang, J.; Zhou, P.; et al. Contamination Status and Acute Dietary Exposure Assessment of Paralytic Shellfish Toxins in Shellfish in the Dalian Area of the Yellow-Bohai Sea, China. Foods 2024, 13, 361. https://doi.org/10.3390/foods13030361
Cao P, Zhang L, Huang Y, Li S, Wang X, Pan F, Yu X, Sun J, Liang J, Zhou P, et al. Contamination Status and Acute Dietary Exposure Assessment of Paralytic Shellfish Toxins in Shellfish in the Dalian Area of the Yellow-Bohai Sea, China. Foods. 2024; 13(3):361. https://doi.org/10.3390/foods13030361
Chicago/Turabian StyleCao, Pei, Lei Zhang, Yaling Huang, Shuwen Li, Xiaodan Wang, Feng Pan, Xiaojin Yu, Jinfang Sun, Jiang Liang, Pingping Zhou, and et al. 2024. "Contamination Status and Acute Dietary Exposure Assessment of Paralytic Shellfish Toxins in Shellfish in the Dalian Area of the Yellow-Bohai Sea, China" Foods 13, no. 3: 361. https://doi.org/10.3390/foods13030361
APA StyleCao, P., Zhang, L., Huang, Y., Li, S., Wang, X., Pan, F., Yu, X., Sun, J., Liang, J., Zhou, P., & Xu, X. (2024). Contamination Status and Acute Dietary Exposure Assessment of Paralytic Shellfish Toxins in Shellfish in the Dalian Area of the Yellow-Bohai Sea, China. Foods, 13(3), 361. https://doi.org/10.3390/foods13030361








