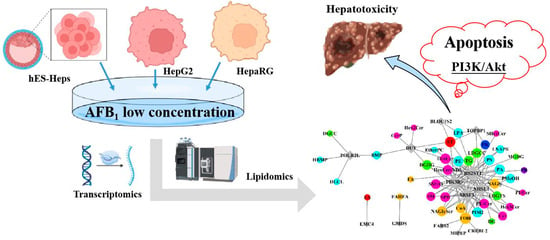
AFB1 Triggers Lipid Metabolism Disorders through the PI3K/Akt Pathway and Mediates Apoptosis Leading to Hepatotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cell Viability Assay
2.3. Cell Apoptosis Analysis
2.4. Transcriptome Data Mining and Gene Expression Analysis
2.5. Identification of Core Genes and Pathway
2.6. Lipidomics Analysis
2.7. Data Analysis
3. Results
3.1. Effects of AFB1 Exposure on Transcriptomic Levels of Human Hepatocytes
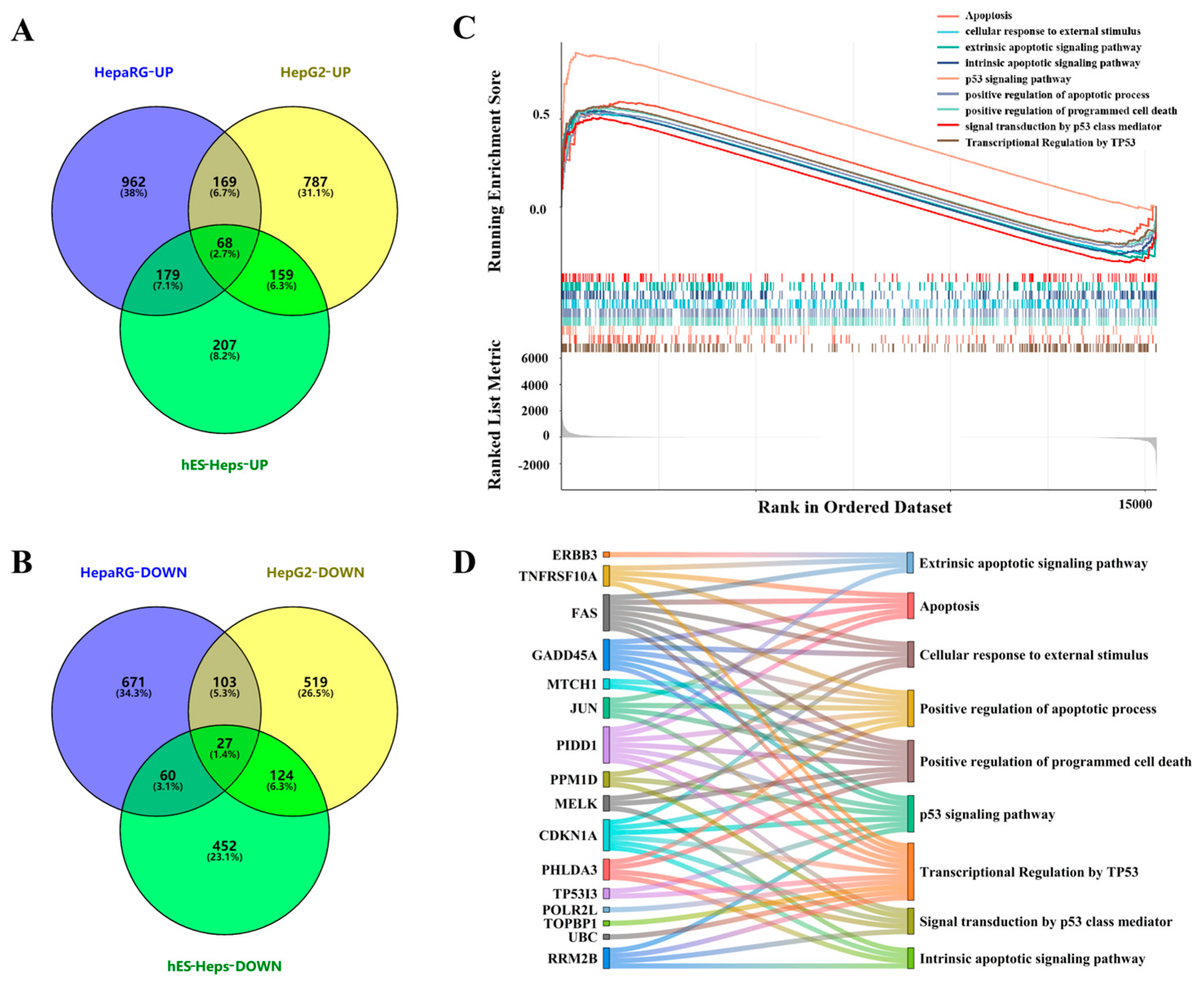
3.1.1. DEGs and Transcriptome Analysis of Three Types of Human Hepatocytes Treated with AFB1
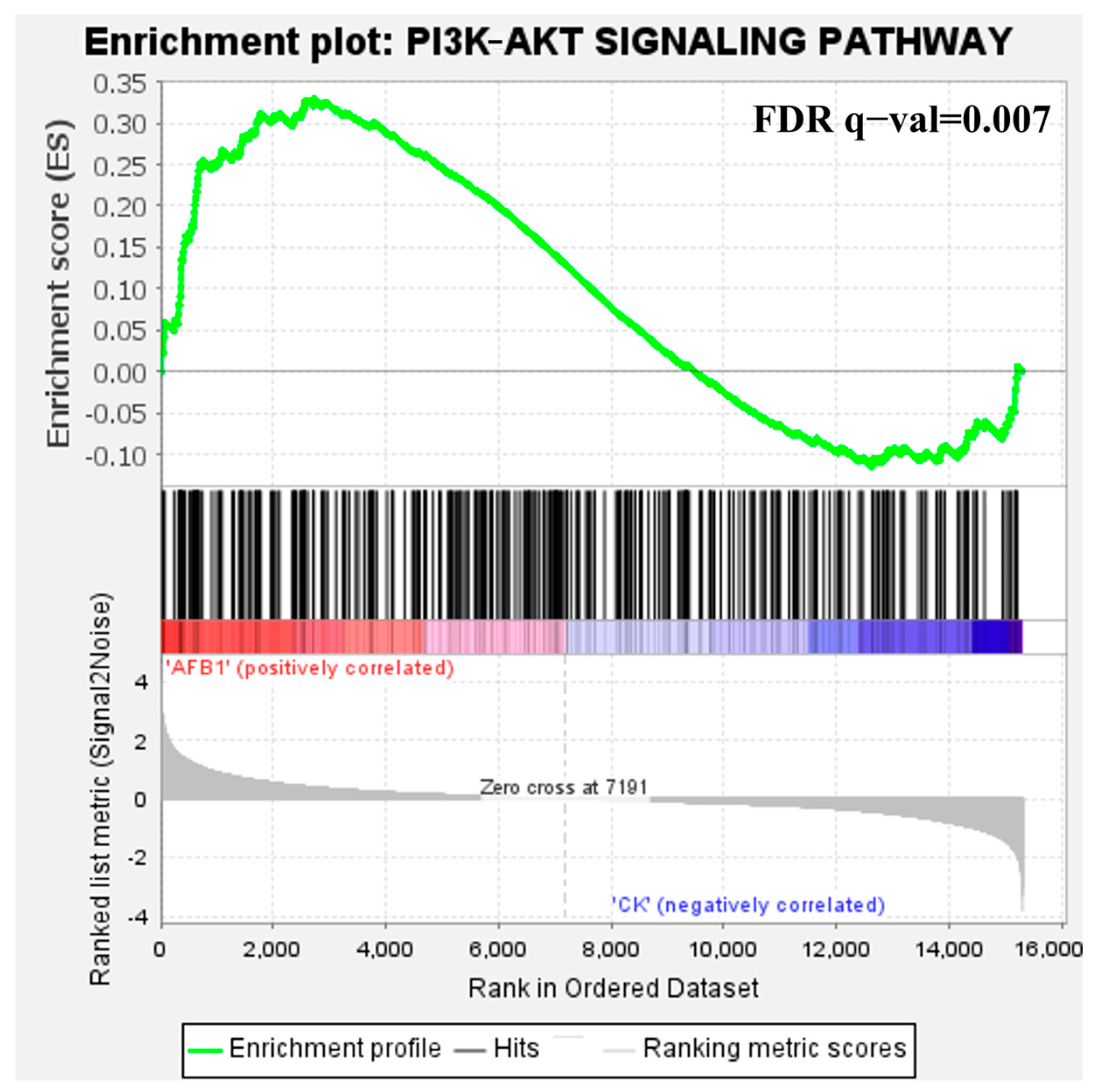
3.1.2. Identification and Analysis of Core Pathways
3.2. Functional Analysis of Core Genes and Verification of Apoptosis-Related Core Pathways
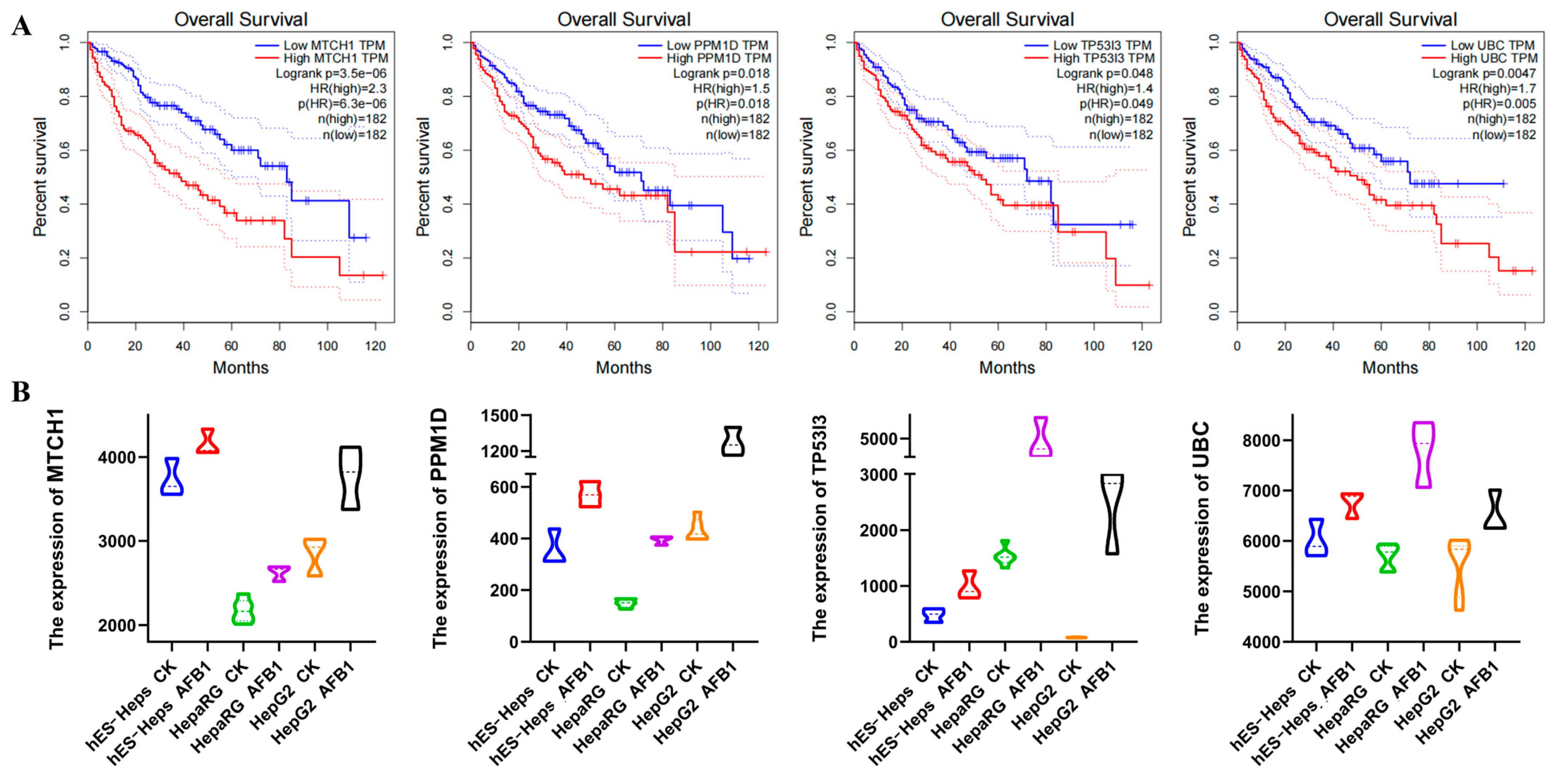
3.2.1. Functional Analysis of Core Gene
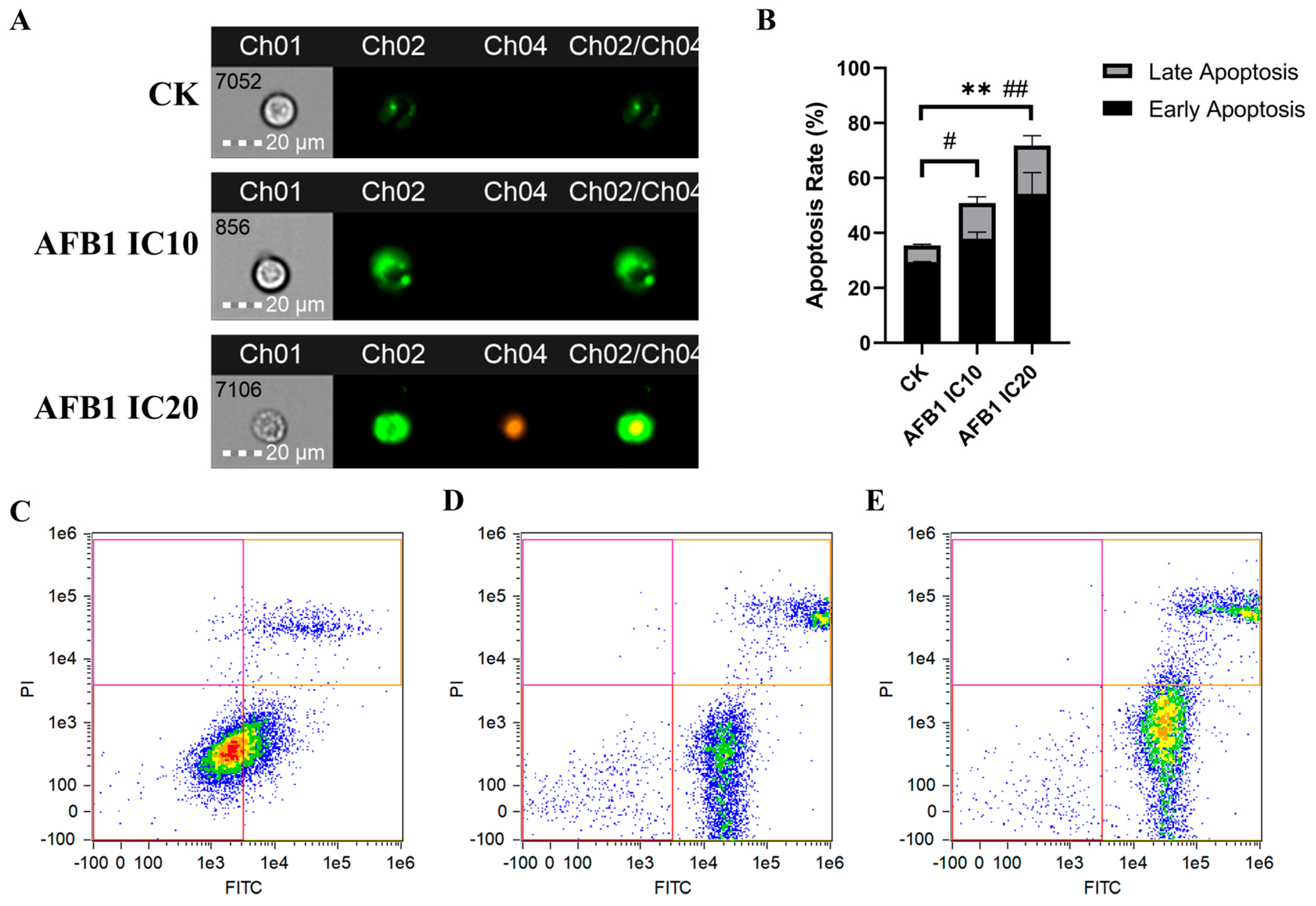
3.2.2. Induction of Apoptosis of HepG2 Cells by AFB1
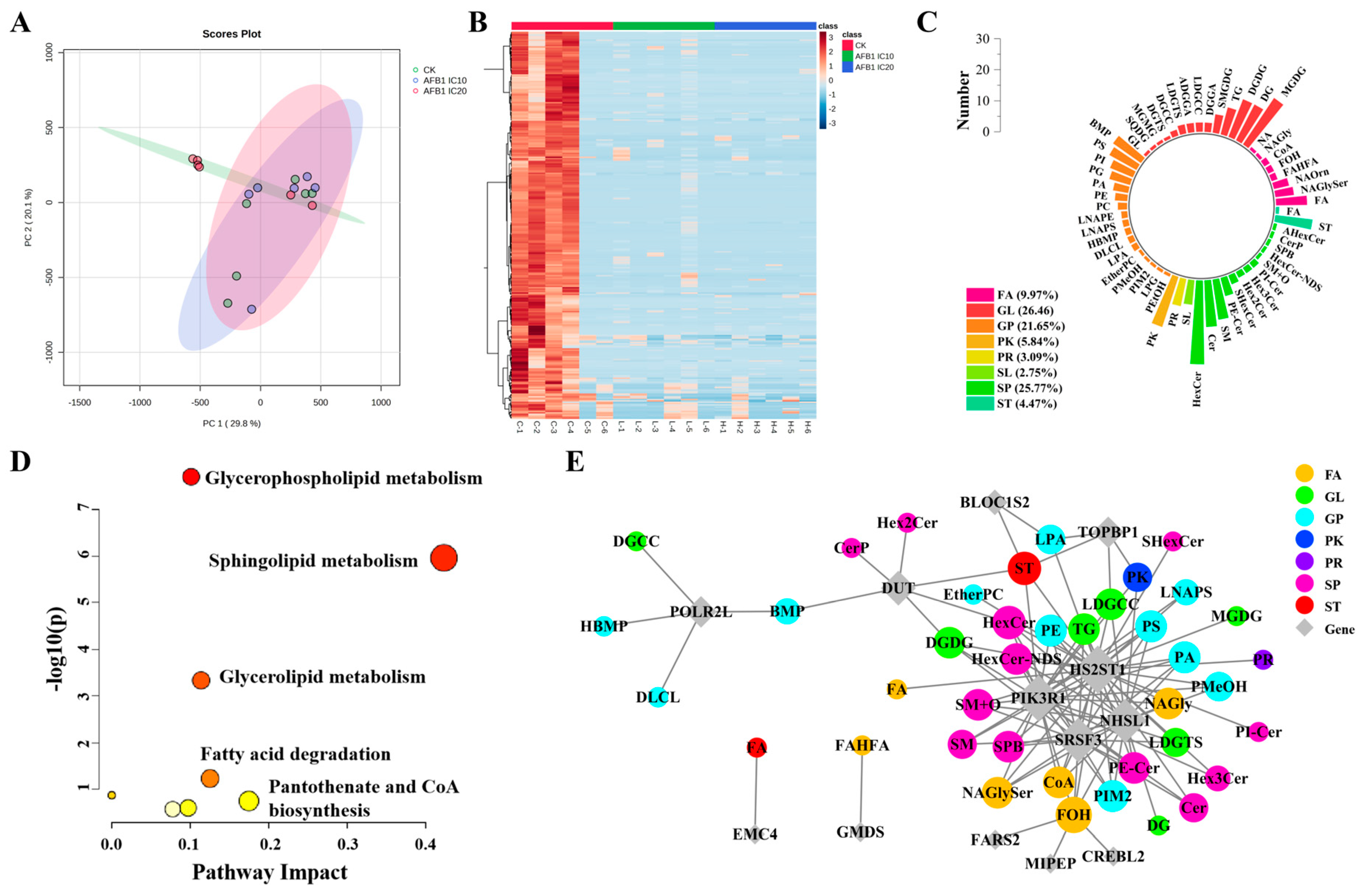
3.3. AFB1 Treatment Altered the Lipid Profile of HepG2 Cells
3.4. Correlation Analysis of DEGs and DELs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Gong, Y.; Holmes, M.; Pan, X.; Xu, Y.; Zou, X.; Fernandes, A.R. Geospatial visualisation of food contaminant distribu-tions: Polychlorinated naphthalenes (PCNs), potentially toxic elements (PTEs) and aflatoxins. Chemosphere 2019, 230, 559–566. [Google Scholar]
- Parihar, A.; Choudhary, N.K.; Sharma, P.; Khan, R. MXene-based aptasensor for the detection of aflatoxin in food and agricultural products. Environ. Pollut. 2023, 316, 120695. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.; Wang, W.; Yang, H.; Yang, J.; Hallisey, V.M.; Deng, J.; Verheul SM, L.; Hwang, S.H.; Gartung, A.; Wang, Y.; et al. Resolution of eicosanoid/cytokine storm prevents carcinogen and inflammation-initiated hepatocellular cancer progression. Proc. Natl. Acad. Sci. USA 2020, 117, 21576–21587. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Qian, Y.; Chen, N.; Ling, T.; Wang, J.; Jiang, H.; Wang, X.; Qi, K.; Zhou, Y. Detoxification of aflatoxin B1 by Stenotrophomonas sp. CW117 and characterization the thermophilic degradation process. Environ. Pollut. 2020, 261, 114178. [Google Scholar] [CrossRef]
- Braun, D.; Abia, W.A.; Šarkanj, B.; Sulyok, M.; Waldhoer, T.; Erber, A.C.; Krska, R.; Turner, P.C.; Marko, D.; Ezekiel, C.N.; et al. Mycotoxin-mixture assessment in mother-infant pairs in Nigeria: From mothers’ meal to infants’ urine. Chemosphere 2022, 287, 132226. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 Toxicity and Protective Effects of Curcumin: Molecular Mechanisms and Clinical Implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Ma, H.-H.; Dong, P.-Y.; Yuan, Z.-N.; Zhang, S.-E.; Zhao, A.-H.; Liu, H.-Q.; De Felici, M.; Shen, W.; Zhang, X.-F. Aflatoxin B1 disrupts testicular development via the cell cycle-related Ras/PI3K/Akt signaling in mice and pig. Environ. Pollut. 2023, 329, 121729. [Google Scholar] [CrossRef]
- Hojnik, N.; Modic, M.; Walsh, J.L.; Žigon, D.; Javornik, U.; Plavec, J.; Žegura, B.; Filipič, M.; Cvelbar, U. Unravelling the pathways of air plasma induced aflatoxin B1 degradation and detoxification. J. Hazard. Mater. 2021, 403, 123593. [Google Scholar] [CrossRef]
- Wang, L.; He, L.; Zeng, H.; Fu, W.; Wang, J.; Tan, Y.; Zheng, C.; Qiu, Z.; Luo, J.; Lv, C.; et al. Low-dose microcystin-LR antagonizes aflatoxin B1 induced hepatocarcinogenesis through decreasing cytochrome P450 1A2 expression and aflatoxin B1-DNA adduct generation. Chemosphere 2020, 248, 126936. [Google Scholar] [CrossRef]
- Lin, J.-X.; Xu, C.-Y.; Wu, X.-M.; Che, L.; Li, T.-Y.; Mo, S.-M.; Guo, D.-B.; Lin, Z.-N.; Lin, Y.-C. Rab7a-mTORC1 signaling-mediated cho-lesterol trafficking from the lysosome to mitochondria ameliorates hepatic lipotoxicity induced by aflatoxin B1 exposure. Chemosphere 2023, 320, 138071. [Google Scholar] [CrossRef] [PubMed]
- Vornoli, A.; Tibaldi, E.; Gnudi, F.; Sgargi, D.; Manservisi, F.; Belpoggi, F.; Tovoli, F.; Mandrioli, D. Evaluation of Toxicant-Associated Fatty Liver Disease and Liver Neoplastic Progress in Sprague-Dawley Rats Treated with Low Doses of Aflatoxin B1 Alone or in Combination with Extremely Low Frequency Electromagnetic Fields. Toxins 2022, 14, 325. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.W. Morphological observations of aflatoxin-induced liposis in rat hepatocytes. Chemosphere 1978, 7, 625–632. [Google Scholar] [CrossRef]
- Ren, X.-L.; Han, P.; Meng, Y. Aflatoxin B1-Induced COX-2 Expression Promotes Mitophagy and Contributes to Lipid Accu-mulation in Hepatocytes In Vitro and In Vivo. Int. J. Toxicol. 2020, 39, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Kövesi, B.; Cserháti, M.; Erdélyi, M.; Zándoki, E.; Mézes, M.; Balogh, K. Lack of Dose- and Time-Dependent Effects of Aflatoxin B1 on Gene Expression and Enzymes Associated with Lipid Peroxidation and the Glutathione Redox System in Chicken. Toxins 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mishra, S.K.; Wang, T.; Xu, Z.; Zhao, X.; Wang, Y.; Yin, H.; Fan, X.; Zeng, B.; Yang, M.; et al. AFB1 Induced Transcrip-tional Regulation Related to Apoptosis and Lipid Metabolism in Liver of Chicken. Toxins 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Bai, H.; Chang, X.; Wu, Z.; Dong, W.; Ma, Q.; Yang, J. Aflatoxin B1-induced early developmental hepatotoxicity in larvae zebrafish. Chemosphere 2023, 340, 139940. [Google Scholar] [CrossRef]
- Chen, L.; Chen, F.; Liu, T.; Feng, F.; Guo, W.; Zhang, Y.; Feng, X.; Lin, J.-M.; Zhang, F. Lipidomics Profiling of HepG2 Cells and Interference by Mycotoxins Based on UPLC-TOF-IMS. Anal. Chem. 2022, 94, 6719–6727. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; Narduzzi, L.; Ouzia, S.; Soetart, N.; Jaillardon, L.; Guitton, Y.; Le Bizec, B.; Dervilly, G. Metabolomics and lipidomics to identify biomarkers of effect related to exposure to non-dioxin-like polychlorinated biphenyls in pigs. Chemosphere 2022, 296, 133957. [Google Scholar] [CrossRef]
- Tan SL, W.; Israeli, E.; Ericksen, R.E.; Chow PK, H.; Han, W. The altered lipidome of hepatocellular carcinoma. Semin. Cancer Biol. 2022, 86, 445–456. [Google Scholar] [CrossRef]
- Yang, H.; Deng, Q.; Ni, T.; Liu, Y.; Lu, L.; Dai, H.; Wang, H.; Yang, W. Targeted Inhibition of LPL/FABP4/CPT1 fatty acid met-abolic axis can effectively prevent the progression of nonalcoholic steatohepatitis to liver cancer. Int. J. Biol. Sci. 2021, 17, 4207–4222. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, G.; Long, C.; Xu, J.; Cen, J.; Yang, X. The antioxidant activity and genotoxicity of isogarcinol. Food Chem. 2018, 253, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, M.; Chen, C.; Yang, X.; Qian, Y. Three widely used pesticides and their mixtures induced cytotoxicity and apoptosis through the ROS-related caspase pathway in HepG2 cells. Food Chem. Toxicol. 2021, 152, 112162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liang, Z. Comparison between annexin V-FITC/PI and Hoechst33342/PI double stainings in the detection of apoptosis by flow cytometry. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chin. J. Cell. Mol. Immunol. 2014, 30, 1209–1212. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, J.; Xu, Y.; Liao, G.; Jia, Q.; Pan, Y.; Wang, T.; Qian, Y. Integrated non-targeted lipidomics and metabolomics analyses for fluctuations of neonicotinoids imidacloprid and acetamiprid on Neuro-2a cells. Environ. Pollut. 2021, 284, 117327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Wang, T.; Liao, G.; Gu, J.; Hou, R.; Qiu, J. Lipidomic profiling study on neurobehavior toxicity in zebrafish treated with aflatoxin B1. Sci. Total. Environ. 2023, 898, 165553. [Google Scholar] [CrossRef]
- Doktorova, T.Y.; Yildirimman, R.; Vinken, M.; Vilardell, M.; Vanhaecke, T.; Gmuender, H.; Bort, R.; Brolen, G.; Holmgren, G.; Li, R.; et al. Transcriptomic responses generated by hepatocarcinogens in a battery of liver-based in vitro models. Carcinog 2013, 34, 1393–1402. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, M.; Liang, X.; Su, M.; Li, R. Clinical features of aflatoxin B1-exposed patients with liver cancer and the molecular mechanism of aflatoxin B1 on liver cancer cells. Environ. Toxicol. Pharmacol. 2019, 71, 103225. [Google Scholar] [CrossRef]
- Fulda, S. Modulation of mitochondrial apoptosis by PI3K inhibitors. Mitochondrion 2013, 13, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Y.; Qi, X.; Zhao, T.; Wang, X.; Ma, F.; Zhang, L.; Zhang, Q.; Li, P. Quantitative analysis of metabolites in the aflatoxin biosynthesis pathway for early warning of aflatoxin contamination by UHPLC-HRMS combined with QAMS. J. Hazard. Mater. 2022, 431, 128531. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspec-tives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, C.; Chen, Y.; Ni, C.; Chen, X.; Zhang, L.; Xu, X.; Chen, M.; Ma, X.; Zhan, H.; et al. Aflatoxin B1 impairs leydig cells through inhibiting AMPK/mTOR-mediated autophagy flux pathway. Chemosphere 2019, 233, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Qin, C.; Han, X. Lipid Metabolism and Lipidomics Applications in Cancer Research. In Lipid Metabolism in Tumor Immunity; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–24. [Google Scholar]
- Li, D.; Miao, J.; Pan, L.; Zhou, Y.; Gao, Z.; Bi, Y.; Tang, J. Integrated lipidomics and transcriptomics analysis reveal lipid metab-olism disturbance in scallop (Chlamys farreri) exposure to benzo a pyrene. Chemosphere 2023, 331, 138787. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guan, M.; Lin, Y.; Cui, X.; Zhang, Y.; Zhao, Z.; Zhu, J. Aberrant Lipid Metabolism in Hepatocellular Carcinoma Revealed by Liver Lipidomics. Int. J. Mol. Sci. 2017, 18, 2550. [Google Scholar] [CrossRef]
- Hall, Z.; Chiarugi, D.; Charidemou, E.; Leslie, J.; Scott, E.; Pellegrinet, L.; Allison, M.; Mocciaro, G.; Anstee, Q.M.; Evan, G.I.; et al. Lipid Remodeling in Hepatocyte Proliferation and Hepatocellular Carcinoma. Hepatology 2021, 73, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Passos-Castilho, A.M.; Turco, E.L.; Ferraz, M.L.; Matos, C.; Silva, I.; Parise, E.; Pilau, E.; Gozzo, F.; Granato, C. Plasma Lipidomic Fingerprinting to Distinguish among Hepatitis C-related Hepatocellular Carcinoma, Liver Cirrhosis, and Chronic Hepatitis C using MALDI-TOF Mass Spectrometry: A Pilot Study. J. Gastrointest. Liver Dis. 2015, 24, 43–49. [Google Scholar] [CrossRef]
- Ismail, I.T.; Elfert, A.; Helal, M.; Salama, I.; El-Said, H.; Fiehn, O. Remodeling Lipids in the Transition from Chronic Liver Disease to Hepatocellular Carcinoma. Cancers 2020, 13, 88. [Google Scholar] [CrossRef]
- Eraslan, G.; Sarica, Z.S.; Bayram, L.C.; Tekeli, M.Y.; Kanbur, M.; Karabacak, M. The effects of diosmin on aflatox-in-induced liver and kidney damage. Environ. Sci. Pollut. Res. 2017, 24, 27931–27941. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, Y.; An, Y.; Tian, Y.; Wang, Y.; Tang, H. Systems Responses of Rats to Aflatoxin B1 Exposure Revealed with Metabonomic Changes in Multiple Biological Matrices. J. Proteome Res. 2011, 10, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hu, B.; Shao, L.; Tian, Y.; Jin, T.; Jin, Y.; Ji, S.; Fan, X. Integrated analysis of transcriptomics and metabonomics profiles in aflatoxin B1-induced hepatotoxicity in rat. Food Chem. Toxicol. 2013, 55, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, S.; Fan, C.; Zheng, N.; Zhang, Y.; Li, S.; Wang, J. Metabolomic analysis of alterations in lipid oxidation, carbohydrate and amino acid metabolism in dairy goats caused by exposure to Aflotoxin B1. J. Dairy Res. 2017, 84, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Verheij, M.; Haimovitz-Friedman, A.; Scotto, K.; Fuks, Z.; Kolesnick, R. Ceramide synthase mediates daunorubicin-induced apoptosis: An alternative mechanism for generating death signals. Cell 1995, 82, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; de Cathelineau, A.; Daleke, D.L.; Henson, P.M.; Bratton, D.L. Loss of Phospholipid Asymmetry and Surface Exposure of Phosphatidylserine Is Required for Phagocytosis of Apoptotic Cells by Macrophages and Fibroblasts. J. Biol. Chem. 2001, 276, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barros, M.; Coant, N.; Truman, J.-P.; Snider, A.J.; Hannun, Y.A. Sphingolipids in colon cancer. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2014, 1841, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Barclay, L.; Walensky, L.D.; Saghatelian, A. Regulation of mitochondrial ceramide distribution by members of the BCL-2 family. J. Lipid Res. 2015, 56, 1501–1510. [Google Scholar] [CrossRef]
- Frangiamone, M.; Cimbalo, A.; Alonso-Garrido, M.; Vila-Donat, P.; Manyes, L. In vitro and in vivo evaluation of AFB1 and OTA-toxicity through immunofluorescence and flow cytometry techniques: A systematic review. Food Chem. Toxicol. 2022, 160, 112798. [Google Scholar] [CrossRef]
- Li, N.; Sancak, Y.; Frasor, J.; Atilla-Gokcumen, G.E. A Protective Role for Triacylglycerols during Apoptosis. Biochemistry 2018, 57, 72–80. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, H.; Wang, T.; Xie, Y.; Liu, L.; He, X.; Liu, C.; Zhao, Q.; Song, X.; Zheng, L.; et al. Pro-inflammation and pro-atherosclerotic responses to short-term air pollution exposure associated with alterations in sphingolipid ceramides and neutrophil extracellular traps. Environ. Pollut. 2023, 335, 122301. [Google Scholar] [CrossRef]
- Cakir, Z.; Saydam, G.; Sahin, F.; Baran, Y. The roles of bioactive sphingolipids in resveratrol-induced apoptosis in HL60 acute myeloid leukemia cells. J. Cancer Res. Clin. Oncol. 2011, 137, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Young, M.M.; Kester, M.; Wang, H.-G. Sphingolipids: Regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013, 54, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C. Mechanisms of apoptosis. Am. J. Pathol. 2000, 157, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Mo, Y.; Zhang, Y.; Zhang, Y.; Zhang, Q. Nickel nanoparticles induce autophagy and apoptosis via HIF-1 al-pha/mTOR signaling in human bronchial epithelial cells. Environ. Pollut. 2023, 329, 121670. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Praharaj, P.P.; Naik, P.P.; Panigrahi, D.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Sethi, G.; Bhutia, S.K. Intricate role of mitochondrial lipid in mitophagy and mitochondrial apoptosis: Its implication in cancer therapeutics. Cell. Mol. Life Sci. 2018, 76, 1641–1652. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, J.; Shi, M.; Guo, L.; Liu, L.; Tang, H.; Liu, X. Triphenyl phosphate disturbs the lipidome and induces endoplasmic reticulum stress and apoptosis in JEG-3 cells. Chemosphere 2021, 275, 129978. [Google Scholar] [CrossRef]
- Symonds, H.; Krall, L.; Remington, L.; Saenz-Robles, M.; Lowe, S.; Jacks, T.; Van Dyke, T. p53-Dependent apoptosis suppresses tumor growth and progression in vivo. Cell 1994, 78, 703–711. [Google Scholar] [CrossRef]
- Messina, A.; Luce, E.; Hussein, M.; Dubart-Kupperschmitt, A. Pluripotent-Stem-Cell-Derived Hepatic Cells: Hepato-cytes and Organoids for Liver Therapy and Regeneration. Cells 2020, 9, 420. [Google Scholar] [CrossRef]
- Ceelen, L.; Lieveld, M.; Forsyth, R.; Vinken, M. The HepaRG cell line: A valuable in vitro tool for hepatitis virus infection studies. Hepatol. Int. 2013, 7, 394–399. [Google Scholar] [CrossRef]
- Sun, D.; Nassal, M. Stable HepG2- and Huh7-based human hepatoma cell lines for efficient regulated expression of infectious hepatitis B virus. J. Hepatol. 2006, 45, 636–645. [Google Scholar] [CrossRef]
- Meex, S.J.; Andreo, U.; Sparks, J.D.; Fisher, E.A. Huh-7 or HepG2 cells: Which is the better model for studying human apolipoprotein-B100 assembly and secretion? J. Lipid Res. 2011, 52, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Gunn, P.J.; Green, C.J.; Pramfalk, C.; Hodson, L. In vitro cellular models of human hepatic fatty acid metabolism: Differences between Huh7 and HepG2 cell lines in human and fetal bovine culturing serum. Physiol. Rep. 2017, 5, e13532. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bao, Z.; Huang, Y.; Wang, Z.; Zhao, Y. Selection of Suitable Reference Genes for qPCR Gene Expression Analysis of HepG2 and L02 in Four Different Liver Cell Injured Models. BioMed Res. Int. 2020, 2020, 8926120. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Huang, J.; Lin, J.-X.; Xu, C.-Y.; Wu, X.-M.; Du, Z.-B.; Wu, J.-S.; Lin, Z.-N.; Lin, Y.-C. Aflatoxin B1 exposure triggers hepatic lipotoxicity via p53 and perilipin 2 interaction-mediated mitochondria-lipid droplet contacts: An in vitro and in vivo assessment. J. Hazard. Mater. 2023, 445, 130584. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Biogenesis of mitochondrial carrier proteins: Molecular mechanisms of import into mitochondria. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2013, 1833, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Mo, S.; Yuan, D. Upregulation Mitochondrial Carrier 1 (MTCH1) Is Associated with Cell Proliferation, Invasion, and Migration of Liver Hepatocellular Carcinoma. BioMed Res. Int. 2021, 2021, 9911784. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Nguyen, T.-A.; Moon, S.-H.; Darlington, Y.; Sommer, M.; Donehower, L.A. The type 2C phosphatase Wip1: An oncogenic regulator of tumor suppressor and DNA damage response pathways. Cancer Metastasis Rev. 2008, 27, 123–135. [Google Scholar] [CrossRef]
- Koom, W.S.; Park, S.-Y.; Kim, W.; Kim, M.; Kim, J.-S.; Kim, H.; Choi, I.-K.; Yun, C.-O.; Seong, J. Combination of Radiotherapy and Adenovirus-Mediated p53 Gene Therapy for MDM2-Overexpressing Hepatocellular Carcinoma. J. Radiat. Res. 2012, 53, 202–210. [Google Scholar] [CrossRef]
- Ali, A.Y.; Abedini, M.R.; Tsang, B.K. The oncogenic phosphatase PPM1D confers cisplatin resistance in ovarian carcinoma cells by attenuating checkpoint kinase 1 and p53 activation. Oncogene 2011, 31, 2175–2186. [Google Scholar] [CrossRef]
- Yu, Z.; Song, Y.; Cai, M.; Jiang, B.; Zhang, Z.; Wang, L.; Jiang, Y.; Zou, L.; Liu, X.; Yu, N.; et al. PPM1D is a potential prognostic biomarker and correlates with immune cell infiltration in hepatocellular carcinoma. Aging 2021, 13, 21294–21308. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.L.; Chaudhry, S.; Lopes, G.S.; Levin, N.K.; Tainsky, M.A. FANCM, RAD1, CHEK1 and TP53I3 act as BRCA-like tumor suppressors and are mutated in hereditary ovarian cancer. Cancer Genet. 2019, 235–236, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Jang, S.J.; Shim, J.H.; Kim, D.; Hong, S.; Sung, C.O.; Baek, D.; Haq, F.; Ansari, A.A.; Lee, S.Y.; et al. Genomic portrait of resectable hepatocellular carcinomas: Implications of RB1 and FGF19 aberrations for patient stratification. Hepatology 2014, 60, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xiong, H.; Dong, S.; Wei, X. Identification and Validation of a Novel Immune Infiltration-Based Diagnostic Score for Early Detection of Hepatocellular Carcinoma by Machine-Learning Strategies. Gastroenterol. Res. Pract. 2022, 2022, 5403423. [Google Scholar] [CrossRef]
- Hoeller, D.; Hecker, C.-M.; Dikic, I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat. Rev. Cancer 2006, 6, 776–788. [Google Scholar] [CrossRef]
- Wong, Y.-H.; Chen, R.-H.; Chen, B.-S. Core and specific network markers of carcinogenesis from multiple cancer samples. J. Theor. Biol. 2014, 362, 17–34. [Google Scholar] [CrossRef]
- Yang, C.; Fu, R.; Zhuang, Z.; Wang, S. Studies on the biological functions of CPS1 in AFS(1) induced hepatocarcinogenesis. Gene 2016, 591, 255–261. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Li, X.; Liao, G.; Wang, Z.; Han, X.; Gu, J.; Mu, X.; Qiu, J.; Qian, Y. AFB1 Triggers Lipid Metabolism Disorders through the PI3K/Akt Pathway and Mediates Apoptosis Leading to Hepatotoxicity. Foods 2024, 13, 163. https://doi.org/10.3390/foods13010163
Wang T, Li X, Liao G, Wang Z, Han X, Gu J, Mu X, Qiu J, Qian Y. AFB1 Triggers Lipid Metabolism Disorders through the PI3K/Akt Pathway and Mediates Apoptosis Leading to Hepatotoxicity. Foods. 2024; 13(1):163. https://doi.org/10.3390/foods13010163
Chicago/Turabian StyleWang, Tiancai, Xiabing Li, Guangqin Liao, Zishuang Wang, Xiaoxu Han, Jingyi Gu, Xiyan Mu, Jing Qiu, and Yongzhong Qian. 2024. "AFB1 Triggers Lipid Metabolism Disorders through the PI3K/Akt Pathway and Mediates Apoptosis Leading to Hepatotoxicity" Foods 13, no. 1: 163. https://doi.org/10.3390/foods13010163
APA StyleWang, T., Li, X., Liao, G., Wang, Z., Han, X., Gu, J., Mu, X., Qiu, J., & Qian, Y. (2024). AFB1 Triggers Lipid Metabolism Disorders through the PI3K/Akt Pathway and Mediates Apoptosis Leading to Hepatotoxicity. Foods, 13(1), 163. https://doi.org/10.3390/foods13010163