Comparison of Multiple NIR Instruments for the Quantitative Evaluation of Grape Seed and Other Polyphenolic Extracts with High Chemical Similarities
Abstract
1. Introduction
2. Materials and Methods
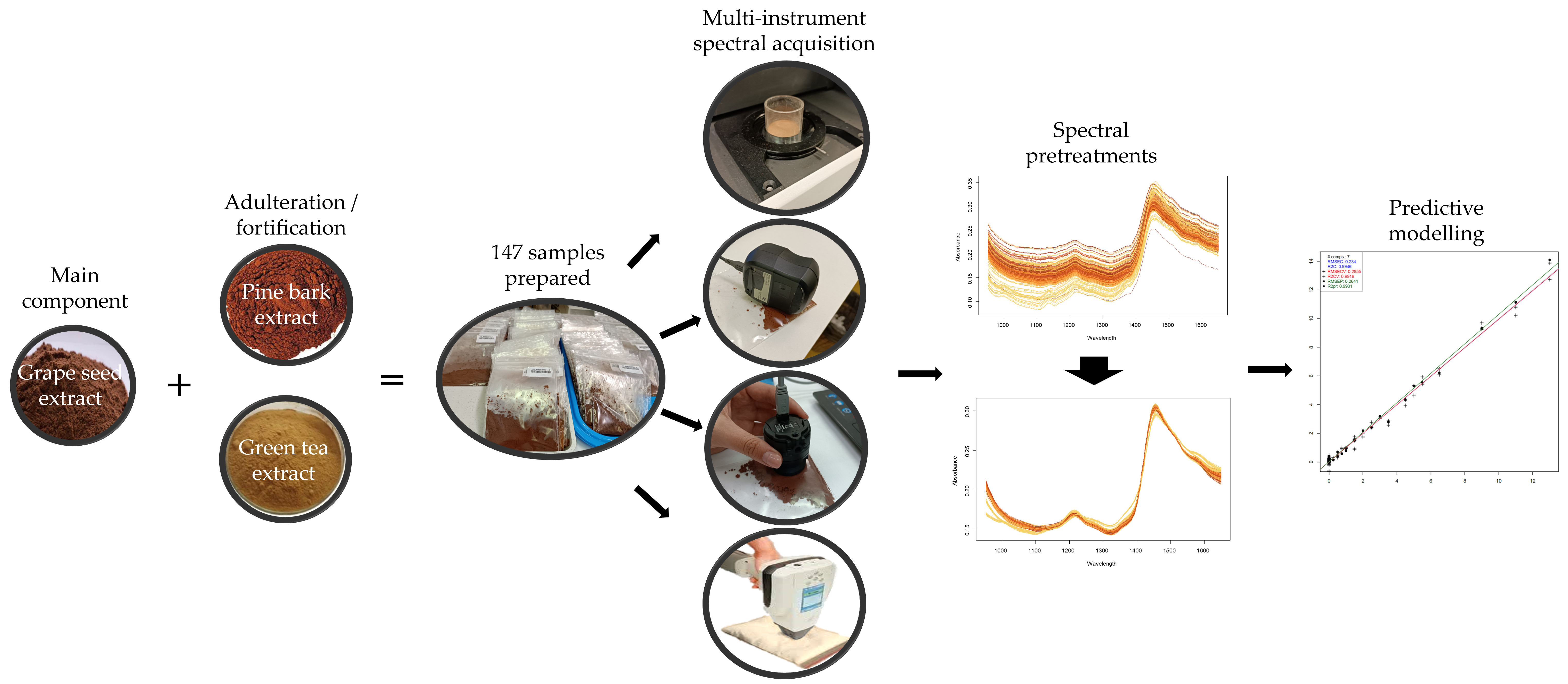
2.1. Samples and Their Preparation
2.2. Methods for the Characterization of Grape Seed Extract Adulteration and Fortification
2.2.1. Procyanidin Content Determination
2.2.2. Antioxidant Capacity Determination
2.2.3. HPLC Method for Proanthocyanidin Monomers (Gallic Acid, Catechin, Epicatechin) and Caffeic Acid Determination
2.2.4. NIR Spectral Acquisition
2.3. Statistical Methods
2.3.1. Univariate Statistical Comparison of the Chemical Reference Results
2.3.2. Exploratory Analysis of the NIR Spectroscopy Results
2.3.3. Predictive Modelling of the NIR Spectroscopy Results
3. Results
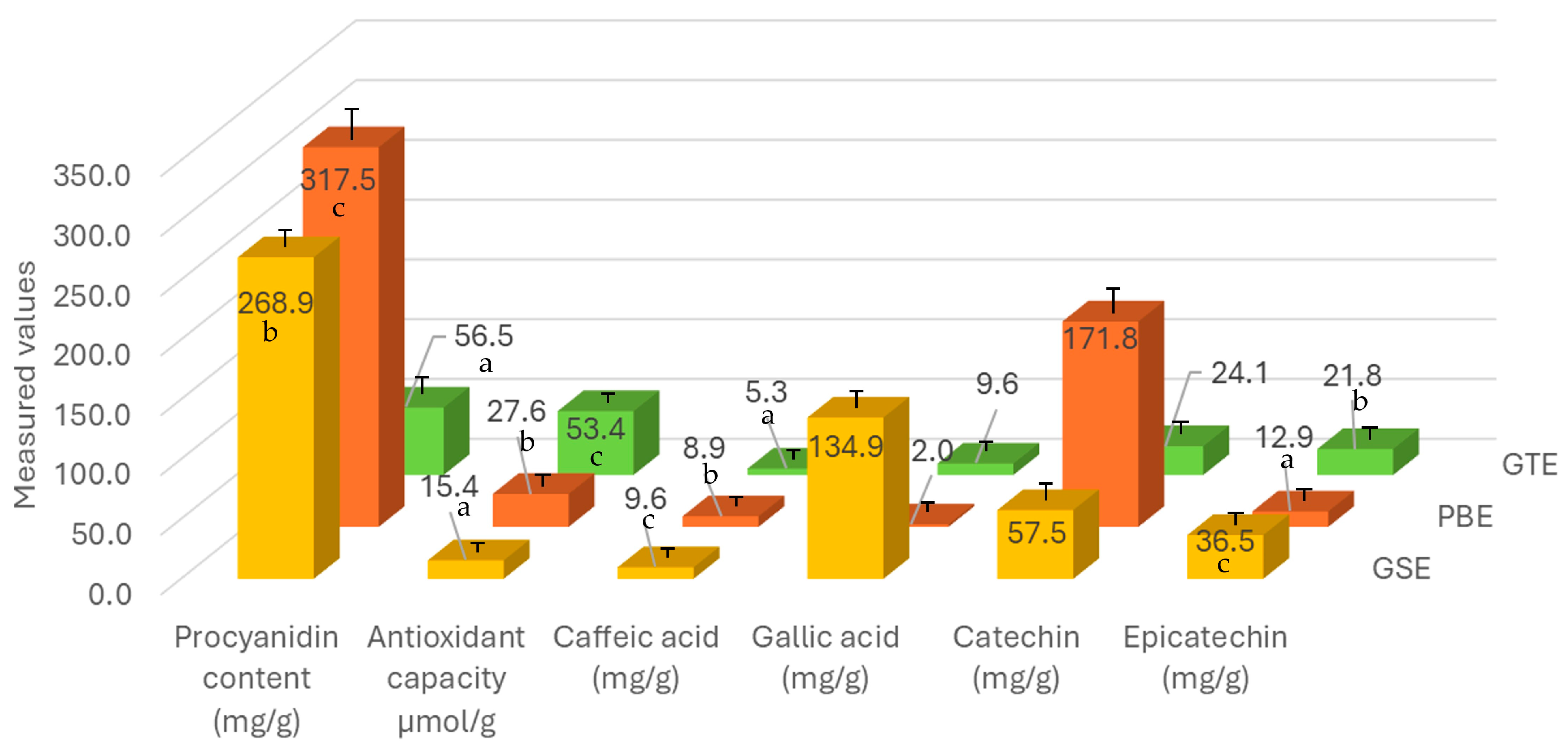
3.1. Chemical Measurement Results of the Extract Mixtures
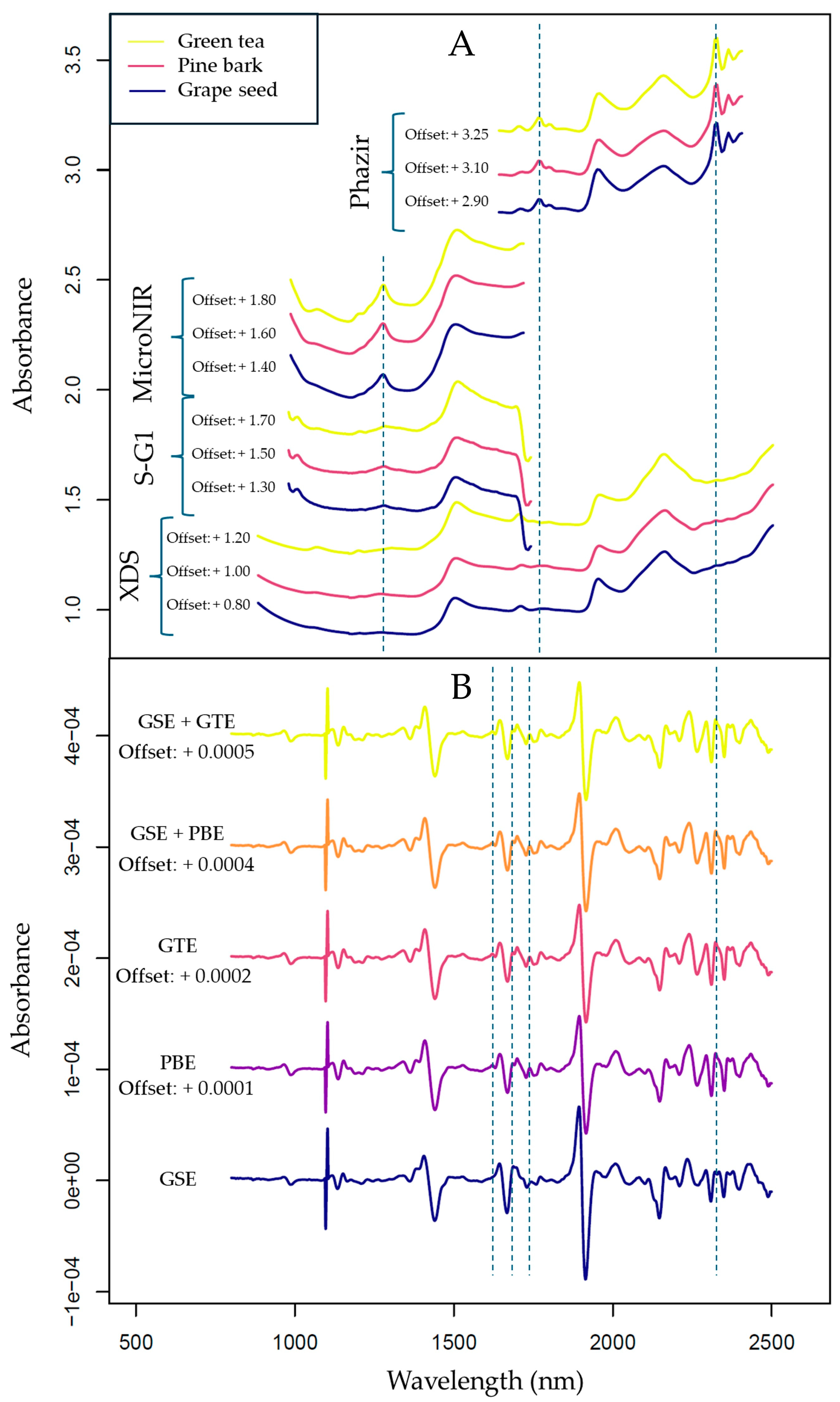
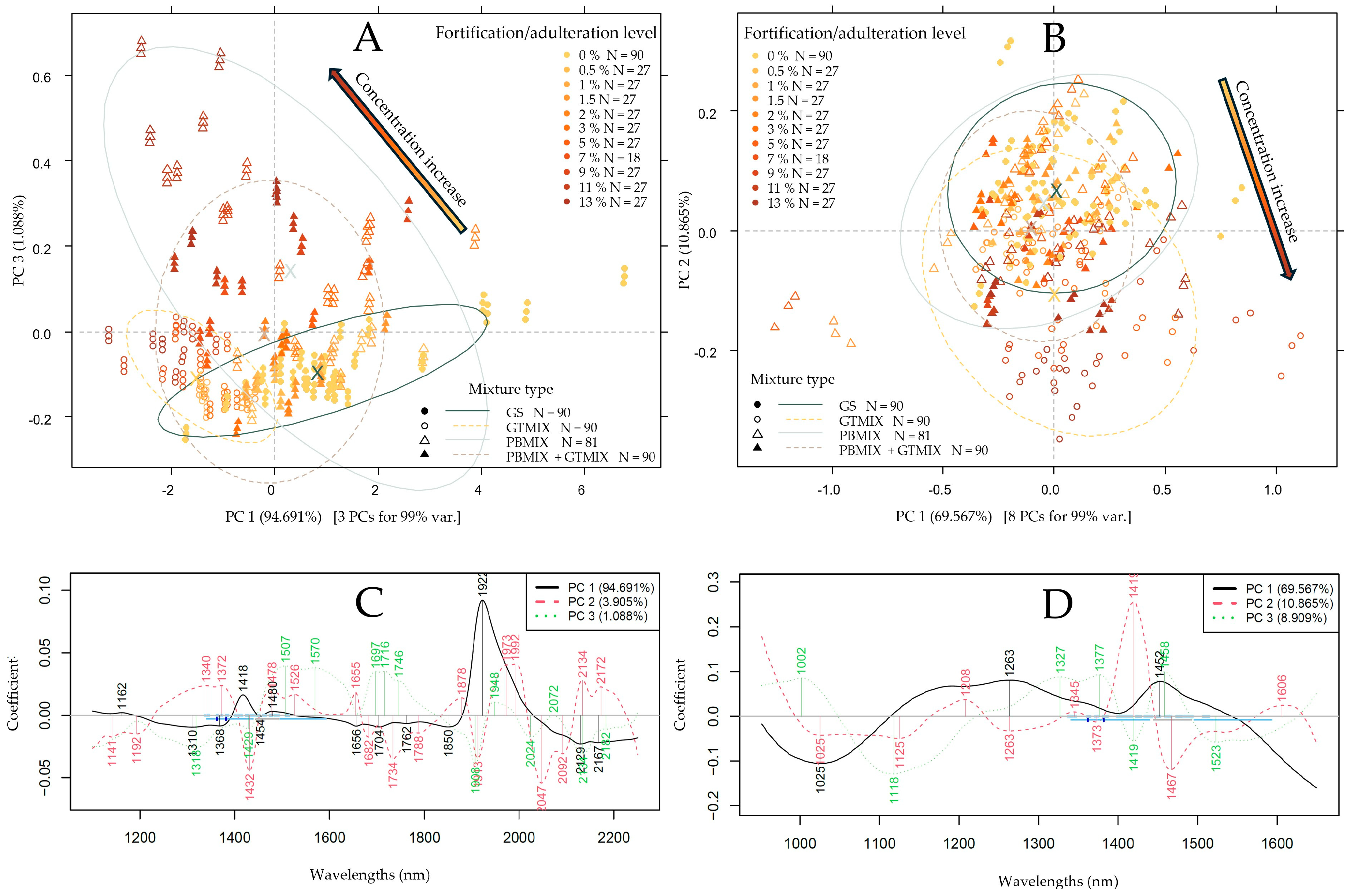
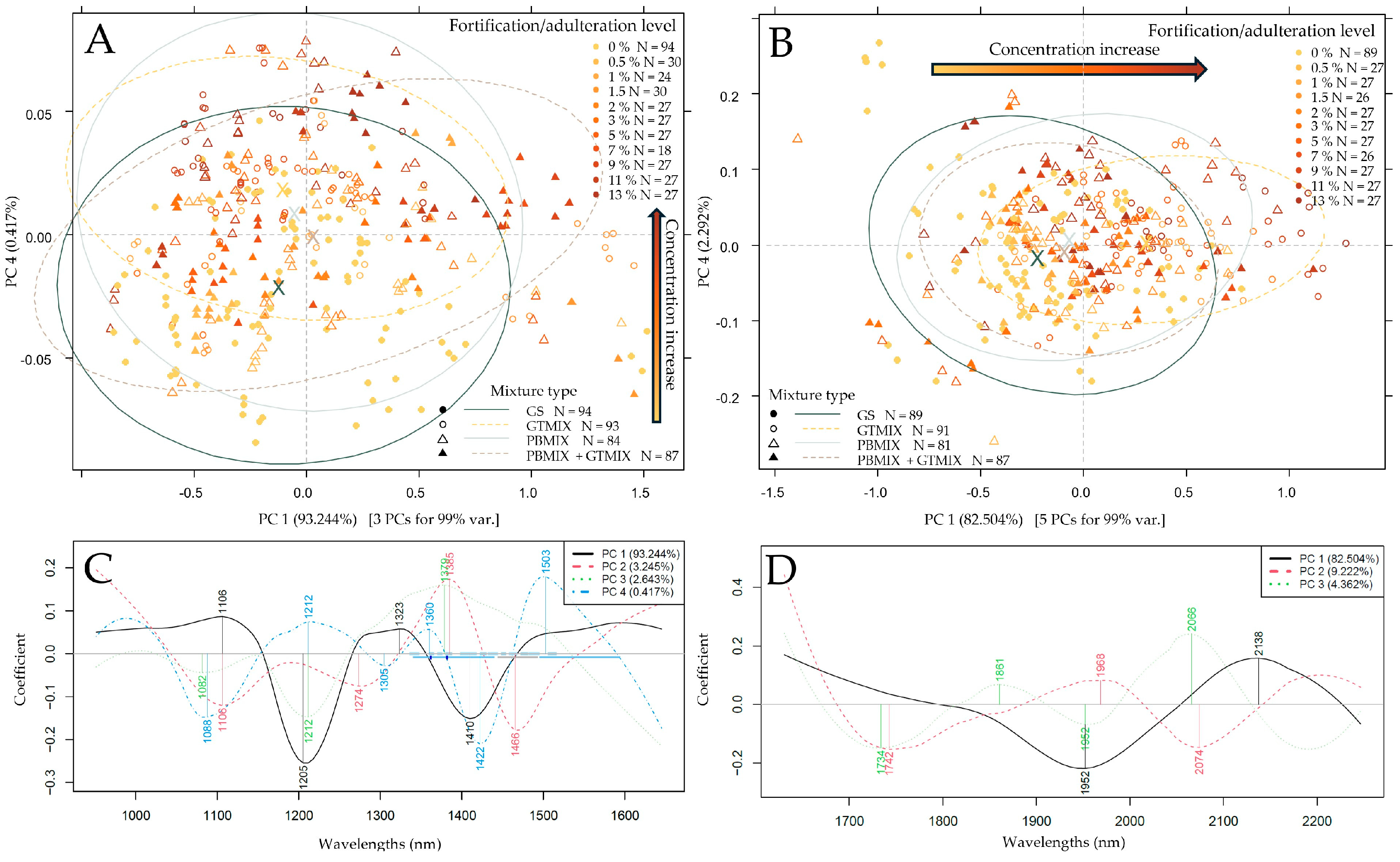
3.2. Exploratory Data Evaluation of the NIR Spectroscopy Results Based on Raw Spectra and PCA
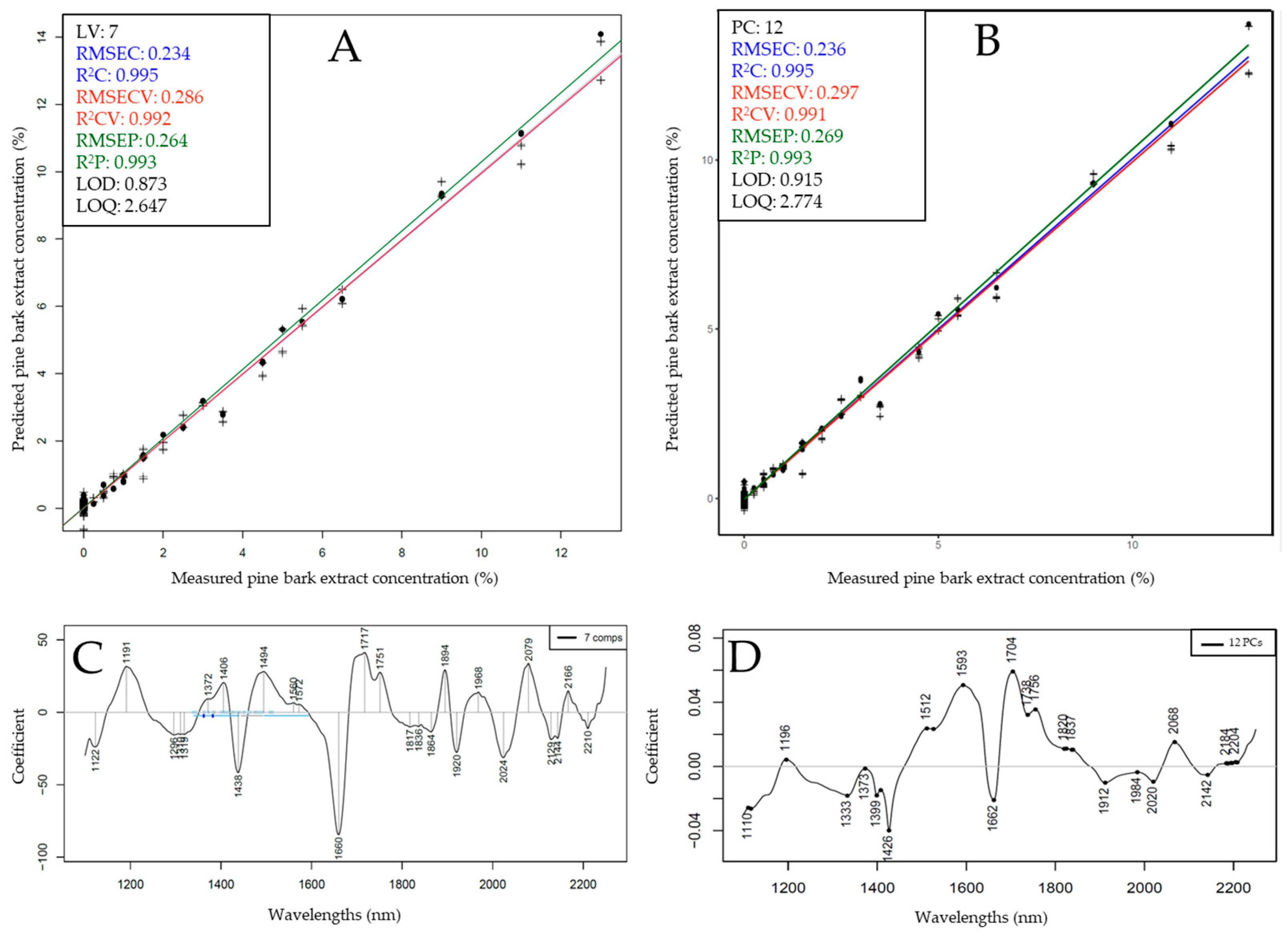
3.3. Predicting Extract Concentrations and Chemical Parameters Using PLSR and SVR
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FDA Questions and Answers on Dietary Supplements. Internet. Available online: https://www.fda.gov/food/information-consumers-using-dietary-supplements/questions-and-answers-dietary-supplements (accessed on 18 December 2024).
- Djaoudene, O.; Romano, A.; Bradai, Y.D.; Zebiri, F.; Ouchene, A.; Yousfi, Y.; Amrane-Abider, M.; Sahraoui-Remini, Y.; Madani, K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients 2023, 15, 3320. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Khoshkhat, P.; Chamani, M.; Shahsavari, S.; Dorkoosh, F.A.; Rajabi, A.; Maniruzzaman, M.; Nokhodchi, A. In-Depth Multidisciplinary Review of the Usage, Manufacturing, Regulations & Market of Dietary Supplements. J. Drug Deliv. Sci. Technol. 2022, 67, 102985. [Google Scholar]
- Dietary Supplements Market Size & Trends. Available online: https://www.Grandviewresearch.Com/Industry-Analysis/Dietary-Supplements-Market-Report (accessed on 14 November 2024).
- Hys, K. Identification of the Reasons Why Individual Consumers Purchase Dietary Supplements. In Perspectives on Consumer Behaviour: Theoretical Aspects and Practical Applications; Sroka, W., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 193–209. ISBN 978-3-030-47380-8. [Google Scholar]
- Aziz, M.H.; Kumar, R.; Ahmad, N. Cancer chemoprevention by resveratrol: In vitro and in vivo studies and the underlying mechanisms (review). Int. J. Oncol. 2003, 23, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Yemul, S.; Wang, J.; Pasinetti, G.M. Grape Seed Polyphenolic Extract as a Potential Novel Therapeutic Agent in Tauopathies. J. Alzheimer’s Dis. 2009, 16, 433–439. [Google Scholar] [CrossRef]
- Bertelli, A.A.A.; Das, D.K. Grapes, Wines, Resveratrol, and Heart Health. J. Cardiovasc. Pharmacol. 2009, 54, 468–476. [Google Scholar] [CrossRef]
- Kupina, S.; Gafner, S. On Adulteration of Grape Seed Extract. NCNPR Botanical Adulterants Bulletin. 2016. Available online: https://www.polyphenolics.com/wp-content/uploads/2016/06/052015-BAP-BABs-GrapeSeedEx-CC-v2.pdf (accessed on 14 November 2024).
- Villani, T.S.; Reichert, W.; Ferruzzi, M.G.; Pasinetti, G.M.; Simon, J.E.; Wu, Q. Chemical Investigation of Commercial Grape Seed Derived Products to Assess Quality and Detect Adulteration. Food Chem. 2015, 170, 271–280. [Google Scholar] [CrossRef]
- Betz, J.M. Botanical Quality Initiatives at the Office of Dietary Supplements; National Institutes of Health, American Chemical Society: Bethesda, MD, USA, 2006; Volume 6.
- Ho, C.-T.; Simon, J.E.; Shahidi, F.; Shao, Y. Dietary Supplements (ACS Symposium Series 987), 1st ed.; American Chemical Society: Washington, DC, USA, 2008. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Crit. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Lobo, J.K.; Janle, E.M.; Cooper, B.; Simon, J.E.; Wu, Q.L.; Welch, C.; Ho, L.; Weaver, C.; Pasinetti, G.M. Bioavailability of Gallic Acid and Catechins from Grape Seed Polyphenol Extract Is Improved by Repeated Dosing in Rats: Implications for Treatment in Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 18, 113–124. [Google Scholar] [CrossRef]
- Wang, J.; Ferruzzi, M.G.; Ho, L.; Blount, J.; Janle, E.M.; Gong, B.; Pan, Y.; Nagana Gowda, G.A.; Raftery, D.; Arrieta-Cruz, I.; et al. Brain-Targeted Proanthocyanidin Metabolites for Alzheimer’s Disease Treatment. J. Neurosci. 2012, 32, 5144–5150. [Google Scholar] [CrossRef]
- Kupina, S.A.; Kelm, M.A.; Monagas, M.J.; Gafner, S. Grape Seed Extract Laboratory Guidance Document; ABC-AHP-NCNPR Botanical Adulterants Prevention Program: Austin, TX, USA, 2019. [Google Scholar]
- Levy, J.; Boyer, R.R.; Neilson, A.P.; O’Keefe, S.F.; Chu, H.S.S.; Williams, R.C.; Dorenkott, M.R.; Goodrich, K.M. Evaluation of Peanut Skin and Grape Seed Extracts to Inhibit Growth of Foodborne Pathogens. Food Sci. Nutr. 2017, 5, 1130–1138. [Google Scholar] [CrossRef]
- Passos, C.P.; Cardoso, S.M.; Domingues, M.R.M.; Domingues, P.; Silva, C.M.; Coimbra, M.A. Evidence for Galloylated Type-A Procyanidins in Grape Seeds. Food Chem. 2007, 105, 1457–1467. [Google Scholar] [CrossRef]
- Perumalla, A.V.S.; Hettiarachchy, N.S. Green Tea and Grape Seed Extracts—Potential Applications in Food Safety and Quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.; de La, L.; Fernández-Arroyo, S.; Segura-Carretero, A. Pine Bark and Green Tea Concentrated Extracts: Antioxidant Activity and Comprehensive Characterization of Bioactive Compounds by HPLC-ESI-QTOF-MS. Int. J. Mol. Sci. 2014, 15, 20382–20402. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; El-Desouky, W.; Yousef, R.S. Synergistic Antioxidant Scavenging Activities of Grape Seed and Green Tea Extracts against Oxidative Stress. Not. Bot. Horti Agrobot. Cluj. Napoca 2016, 44, 367–374. [Google Scholar] [CrossRef]
- Moore, J.C.; Spink, J.; Lipp, M. Development and Application of a Database of Food Ingredient Fraud and Economically Motivated Adulteration from 1980 to 2010. J. Food Sci. 2012, 77, R118–R126. [Google Scholar] [CrossRef]
- Creydt, M.; Fischer, M. Food Authentication in Real Life: How to Link Nontargeted Approaches with Routine Analytics? Electrophoresis 2020, 41, 1665–1679. [Google Scholar] [CrossRef]
- Aouadi, B.; Zaukuu, J.L.Z.; Vitális, F.; Bodor, Z.; Fehér, O.; Gillay, Z.; Bazar, G.; Kovacs, Z. Historical Evolution and Food Control Achievements of near Infrared Spectroscopy, Electronic Nose, and Electronic Tongue—Critical Overview. Sensors 2020, 20, 5479. [Google Scholar] [CrossRef]
- Esslinger, S.; Riedl, J.; Fauhl-Hassek, C. Potential and Limitations of Non-Targeted Fingerprinting for Authentication of Food in Official Control. Food Res. Int. 2014, 60, 189–204. [Google Scholar] [CrossRef]
- Badak-Kerti, K.; Németh, S.; Zitek, A.; Firtha, F. Hyperspectral Monitoring of Fructose Content in Marzipan. Prog. Agric. Eng. Sci. 2018, 14, 79–88. [Google Scholar] [CrossRef][Green Version]
- Szalay, K.; Deákvári, J.; Firtha, F.; Tolner, I.; Csorba, A.; Fenyvesi, L. Identifying Nutrition Sensitive Spectral Changes in Various Winter Wheat Samples. Prog. Agric. Eng. Sci. 2011, 7, 47–63. [Google Scholar] [CrossRef][Green Version]
- Farkas, J.; Dalmadi, I. Near Infrared and Fluorescence Spectroscopic Methods and Electronic Nose Technology for Monitoring Foods. Prog. Agric. Eng. Sci. 2009, 5, 1–29. [Google Scholar] [CrossRef]
- Pappa, H.N. Near-Infrared Spectroscopy. Pharmacopeial Forum 2010, 36, 532. [Google Scholar]
- Douglas, M.R.; King, P.S.; Lee, B.L. Emerging Digital Micromirror Device Based Systems and Applications VIII. In Proceedings of the SPIE OPTO, San Francisco, CA, USA, 13–18 February 2016; Volume 9761. [Google Scholar]
- McGrath, T.F.; Haughey, S.A.; Islam, M.; Elliott, C.T. The Potential of Handheld near Infrared Spectroscopy to Detect Food Adulteration: Results of a Global, Multi-Instrument Inter-Laboratory Study. Food Chem. 2021, 353, 128718. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.Y.; Solihin, M.I.; Astuti, W.; Ang, C.K.; Zailah, W. Food Powders Classification Using Handheld Near-Infrared Spectroscopy and Support Vector Machine. Kournal Phys. Conf. Ser. 2019, 1367, 012029. [Google Scholar] [CrossRef]
- González-Mohino, A.; Pérez-Palacios, T.; Antequera, T.; Ruiz-Carrascal, J.; Olegario, L.S.; Grassi, S. Monitoring the Processing of Dry Fermented Sausages with a Portable NIRS Device. Foods 2020, 9, 1294. [Google Scholar] [CrossRef]
- Kademi, H.I.; Ulusoy, B.H.; Hecer, C. Applications of Miniaturized and Portable near Infrared Spectroscopy (NIRS) for Inspection and Control of Meat and Meat Products. Food Rev. Int. 2019, 35, 201–220. [Google Scholar] [CrossRef]
- Sohn, S.I.; Pandian, S.; Zaukuu, J.L.Z.; Oh, Y.J.; Lee, Y.H.; Shin, E.K.; Thamilarasan, S.K.; Kang, H.J.; Ryu, T.H.; Cho, W.S. Rapid Discrimination of Brassica Napus Varieties Using Visible and Near-Infrared (Vis-NIR) Spectroscopy. J. King Saud. Univ. Sci. 2023, 35, 102495. [Google Scholar] [CrossRef]
- Bian, X.; Liu, Y.; Zhang, R.; Sun, H.; Liu, P.; Tan, X. Rapid Quantification of Grapeseed Oil Multiple Adulterations Using Near-Infrared Spectroscopy Coupled with a Novel Double Ensemble Modeling Method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 311, 124016. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. A Comparative Study to Distinguish the Vineyard of Origin by NIRS Using Entire Grapes, Skins and Seeds. J. Sci. Food Agric. 2013, 93, 967–972. [Google Scholar] [CrossRef]
- Torchio, F.; Segade, S.R.; Giacosa, S.; Gerbi, V.; Rolle, L. Effect of Growing Zone and Vintage on the Prediction of Extractable Flavanols in Winegrape Seeds by A Ft-Nir Method. J. Agric. Food Chem. 2013, 38, 9076–9088. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Feasibility Study on the Use of near Infrared Spectroscopy to Determine Flavanols in Grape Seeds. Talanta 2010, 82, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pulido, F.J.; Hernández-Hierro, J.M.; Nogales-Bueno, J.; Gordillo, B.; González-Miret, M.L.; Heredia, F.J. A Novel Method for Evaluating Flavanols in Grape Seeds by near Infrared Hyperspectral Imaging. Talanta 2014, 122, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Deconinck, E.; Aouadi, C.; Bothy, J.L.; Courselle, P. Detection and Identification of Multiple Adulterants in Plant Food Supplements Using Attenuated Total Reflectance—Infrared Spectroscopy. J. Pharm. Biomed. Anal. 2018, 152, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, M.; Nebojan, I.; Mihalev, K.; Yoncheva, N.; Kljusurić, J.G.; Kurtanjek, Ž. Application of NIR Spectroscopy and Chemometrics in Quality Control of Wild Berry Fruit Extracts during Storage. Hrvat. Časopis Za Prehrambenu Tehnol. Biotehnol. I Nutr. 2013, 8, 67–73. [Google Scholar]
- Gardana, C.; Scialpi, A.; Fachechi, C.; Simonetti, P. Near-Infrared Spectroscopy and Chemometrics for the Routine Detection of Bilberry Extract Adulteration and Quantitative Determination of the Anthocyanins. J. Spectrosc. 2018, 2018, 4751247. [Google Scholar] [CrossRef]
- Walkowiak, A.; Ledziński, Ł.; Zapadka, M.; Kupcewicz, B. Detection of Adulterants in Dietary Supplements with Ginkgo Biloba Extract by Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy and Multivariate Methods PLS-DA and PCA. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 208, 222–228. [Google Scholar] [CrossRef]
- Nagy, M.M.; Wang, S.; Farag, M.A. Quality Analysis and Authentication of Nutraceuticals Using near IR (NIR) Spectroscopy: A Comprehensive Review of Novel Trends and Applications. Trends Food Sci. Technol. 2022, 123, 290–309. [Google Scholar] [CrossRef]
- Deconinck, E.; Djiogo, C.A.S.; Bothy, J.L.; Courselle, P. Detection of Regulated Herbs and Plants in Plant Food Supplements and Traditional Medicines Using Infrared Spectroscopy. J. Pharm. Biomed. Anal. 2017, 142, 210–217. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The Conversion of Procyanidins and Prodelphinidins to Cyanidin and Delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lukacs, M.; Zaukuu, J.L.Z.; Bazar, G.; Pollner, B.; Fodor, M.; Kovacs, Z. Comparison of Multiple NIR Spectrometers for Detecting Low-Concentration Nitrogen-Based Adulteration in Protein Powders. Molecules 2024, 29, 781. [Google Scholar] [CrossRef] [PubMed]
- Cowe, I.A.; McNicol, J.W. The Use of Principal Components in the Analysis of Near-Infrared Spectra. Appl. Spectrosc. 1985, 39, 257–266. [Google Scholar] [CrossRef]
- Næs, T. A User-Friendly Guide to Multivariate Calibration and Classification; NIR Publications: West Sussex, UK, 2002; ISBN 0952866625. [Google Scholar]
- Gowen, A.A.; Downey, G.; Esquerre, C.; O’Donnell, C.P. Preventing Over-Fitting in PLS Calibration Models of near-Infrared (NIR) Spectroscopy Data Using Regression Coefficients. J. Chemom. 2011, 25, 375–381. [Google Scholar] [CrossRef]
- Balabin, R.M.; Smirnov, S.V. Melamine Detection by Mid- and near-Infrared (MIR/NIR) Spectroscopy: A Quick and Sensitive Method for Dairy Products Analysis Including Liquid Milk, Infant Formula, and Milk Powder. Talanta 2011, 85, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Balabin, R.M.; Lomakina, E.I. Support Vector Machine Regression (SVR/LS-SVM)—An Alternative to Neural Networks (ANN) for Analytical Chemistry? Comparison of Nonlinear Methods on near Infrared (NIR) Spectroscopy Data. Analyst 2011, 136, 1703–1712. [Google Scholar] [CrossRef]
- Bazar, G.; Eles, V.; Kovacs, Z.; Romvari, R.; Szabo, A. Multicomponent Blood Lipid Analysis by Means of near Infrared Spectroscopy, in Geese. Talanta 2016, 155, 202–211. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Kovacs, Z.; Pollner, B. R-Package Aquap2—Multivariate Data Analysis Tools for R Including Aquaphotomics Methods. Available online: https://www.aquaphotomics.com/aquap2/ (accessed on 25 September 2024).
- Kuhnert, S.; Lehmann, L.; Winterhalter, P. Rapid Characterisation of Grape Seed Extracts by a Novel HPLC Method on a Diol Stationary Phase. J. Funct. Foods 2015, 15, 225–232. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Wu, Y.; Tan, H.; Meng, F.; Wang, Y.S.; Li, M.; Zhao, L.; Liu, L.; Qian, Y.; et al. Analysis of Accumulation Patterns and Preliminary Study on the Condensation Mechanism of Proanthocyanidins in the Tea Plant [Camellia sinensis]. Sci. Rep. 2015, 5, 8742. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.; Liu, S.; Yang, L. The Galloyl Catechins Contributing to Main Antioxidant Capacity of Tea Made from Camellia sinensis in China. Sci. World J. 2014, 2014, 863984. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Human Nutrition and Metabolism Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Tița, O.; Lengyel, E.; Stegăruș, D.I.; Săvescu, P.; Ciubara, A.B.; Constantinescu, M.A.; Tița, M.A.; Rață, D.; Ciubara, A. Identification and Quantification of Valuable Compounds in Red Grape Seeds. Appl. Sci. 2021, 11, 5124. [Google Scholar] [CrossRef]
- Bec, K.B.; Grabska, J.; Pfeifer, F.; Siesler, H.W.; Huck, C.W. Rapid On-Site Analysis of Soil Microplastics Using Miniaturized NIR Spectrometers: Key Aspect of Instrumental Variation. J. Hazard. Mater. 2024, 480, 135967. [Google Scholar] [CrossRef] [PubMed]
- Kirchler, C.G.; Pezzei, C.K.; Beć, K.B.; Mayr, S.; Ishigaki, M.; Ozaki, Y.; Huck, C.W. Critical Evaluation of Spectral Information of Benchtop vs. Portable near-Infrared Spectrometers: Quantum Chemistry and Two-Dimensional Correlation Spectroscopy for a Better Understanding of PLS Regression Models of the Rosmarinic Acid Content in Rosmarini Folium. Analyst 2017, 142, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Mayr, S.; Beć, K.B.; Grabska, J.; Wiedemair, V.; Pürgy, V.; Popp, M.A.; Bonn, G.K.; Huck, C.W. Challenging Handheld NIR Spectrometers with Moisture Analysis in Plant Matrices: Performance of PLSR vs. GPR vs. ANN Modelling. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 249, 119342. [Google Scholar] [CrossRef]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the Most Common Pre-Processing Techniques for near-Infrared Spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Workman, J.; Weyer, L. Practical Guide and Spectral Atlas for Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429110511. [Google Scholar]
- Zaukuu, J.L.Z.; Aouadi, B.; Lukács, M.; Bodor, Z.; Vitális, F.; Gillay, B.; Gillay, Z.; Friedrich, L.; Kovacs, Z. Detecting Low Concentrations of Nitrogen-Based Adulterants in Whey Protein Powder Using Benchtop and Handheld NIR Spectrometers and the Feasibility of Scanning through Plastic Bag. Molecules 2020, 25, 2522. [Google Scholar] [CrossRef]
- Whitacre, E.; Oliver, J.; Van Den Broek, R.; Van Engelen, P.; Kremers, B.; Van Der Horst, B.; Stewart, M.; Jansen-Beuvink, A. Predictive Analysis of Cocoa Procyanidins Using Near-Infrared Spectroscopy Techniques. J. Food Sci. 2003, 68, 2618–2622. [Google Scholar] [CrossRef]
- Wiedemair, V.; Huck, C.W. Evaluation of the Performance of Three Hand-Held near-Infrared Spectrometer through Investigation of Total Antioxidant Capacity in Gluten-Free Grains. Talanta 2018, 189, 233–240. [Google Scholar] [CrossRef]
- Huang, Y.; Du, G.; Ma, Y.; Zhou, J. Near-Infrared Determination of Polyphenols Using Linear and Nonlinear Regression Algorithms. Optik 2015, 126, 2030–2034. [Google Scholar] [CrossRef]
- Ye, S.; Weng, H.; Xiang, L.; Jia, L.; Xu, J. Synchronously Predicting Tea Polyphenol and Epigallocatechin Gallate in Tea Leaves Using Fourier Transform–Near-Infrared Spectroscopy and Machine Learning. Molecules 2023, 28, 5379. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, M.; Bazar, G.; Pollner, B.; Henn, R.; Kirchler, C.G.; Huck, C.W.; Kovacs, Z. Near Infrared Spectroscopy as an Alternative Quick Method for Simultaneous Detection of Multiple Adulterants in Whey Protein-Based Sports Supplement. Food Control 2018, 94, 331–340. [Google Scholar] [CrossRef]
- Busserolles, J.; Gueux, E.; Balasińska, B.; Piriou, Y.; Rock, E.; Rayssiguier, Y.; Mazur, A. In Vivo Antioxidant Activity of Procyanidin-Rich Extracts from Grape Seed and Pine (Pinus maritima) Bark in Rats. Int. J. Vitam. Nutr. Res. 2006, 76, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Thompson, M.A.; Collins, P.B. Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Nova Publishers: Hauppauge, NY, USA, 2013; ISBN 9781626189218. [Google Scholar]
- Veluri, R.; Singh, R.P.; Liu, Z.; Thompson, J.A.; Agarwal, R.; Agarwal, C. Fractionation of Grape Seed Extract and Identification of Gallic Acid as One of the Major Active Constituents Causing Growth Inhibition and Apoptotic Death of DU145 Human Prostate Carcinoma Cells. Carcinogenesis 2006, 27, 1445–1453. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Huang, J.; Ren, G.; Ning, J.; Deng, W.; Li, L.; Zhang, Z. Quality Assessment of Instant Green Tea Using Portable NIR Spectrometer. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 240, 118576. [Google Scholar] [CrossRef]
- Zareef, M.; Chen, Q.; Ouyang, Q.; Arslan, M.; Hassan, M.M.; Ahmad, W.; Viswadevarayalu, A.; Wang, P.; Ancheng, W. Rapid Screening of Phenolic Compounds in Congou Black Tea (Camellia sinensis) during in Vitro Fermentation Process Using Portable Spectral Analytical System Coupled Chemometrics. J. Food Process Preserv. 2019, 43, e13996. [Google Scholar] [CrossRef]
- Weyer, L.G.; Lo, S.-C. Spectra-Structure Correlations in the Near-Infrared. In Handbook of Vibrational Spectroscopy; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Arslan, M.; Xiaobo, Z.; Shi, J.; Elrasheid Tahir, H.; Zareef, M.; Rakha, A.; Bilal, M. In Situ Prediction of Phenolic Compounds in Puff Dried Ziziphus Jujuba Mill. Using Hand-Held Spectral Analytical System. Food Chem. 2020, 331, 127361. [Google Scholar] [CrossRef]
- Rodríguez-Pulido, F.J.; Barbin, D.F.; Sun, D.W.; Gordillo, B.; González-Miret, M.L.; Heredia, F.J. Grape Seed Characterization by NIR Hyperspectral Imaging. Postharvest Biol. Technol. 2013, 76, 74–82. [Google Scholar] [CrossRef]
- Schwieder, M.; Leitão, P.J.; Suess, S.; Senf, C.; Hostert, P. Estimating Fractional Shrub Cover Using Simulated Enmap Data: A Comparison of Three Machine Learning Regression Techniques. Remote Sens. 2014, 6, 3427–3445. [Google Scholar] [CrossRef]
- Majadi, M.; Barkó, A.; Varga-Tóth, A.; Maukenovna, Z.S.; Batirkhanovna, D.Z.; Dilora, S.; Lukacs, M.; Kaszab, T.; Mednyánszky, Z.; Kovacs, Z. Quality Assessment of Reconstructed Cow, Camel and Mare Milk Powders by Near-Infrared Spectroscopy and Chemometrics. Molecules 2024, 29, 3989. [Google Scholar] [CrossRef]

| Grape Seed Extract Content | Pine Bark Extract Content | Green Tea Extract Content | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrument | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR |
| Range (wl) | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 |
| Pre-treat. | SG-31 + SNV | SG-21 + deTr | SG-11 + deTr + SNV | SG-11 + deTr + SNV | SG-21 + MSC | SG-31 + deTr + SNV | SG-21 + deTr + SNV | SG-11 + SNV | SG-21 + SNV | SG-11 + deTr | SG-21 + deTr + SNV | SG-11 + SNV |
| LV | 7 | 8 | 12 | 9 | 7 | 12 | 14 | 10 | 4 | 8 | 12 | 6 |
| RMSEC | 0.393 | 2.037 | 1.769 | 1.929 | 0.234 | 1.822 | 1.704 | 1.580 | 0.394 | 0.550 | 0.795 | 0.982 |
| R2C | 0.992 | 0.786 | 0.839 | 0.809 | 0.995 | 0.688 | 0.717 | 0.744 | 0.985 | 0.973 | 0.943 | 0.915 |
| RMSECV | 0.446 | 2.271 | 2.128 | 2.140 | 0.277 | 2.083 | 2.061 | 1.790 | 0.423 | 0.630 | 0.914 | 1.092 |
| R2CV | 0.990 | 0.734 | 0.767 | 0.764 | 0.992 | 0.592 | 0.586 | 0.671 | 0.983 | 0.965 | 0.925 | 0.895 |
| Grape Seed Extract Content | Pine Bark Extract Content | Green Tea Extract Content | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrument | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR |
| Range (wl) | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 |
| Pre-treat. | SG-31 + SNV | SG-21 + deTr | SG-11 + deTr + SNV | SG-11 + deTr + SNV | SG-21 + MSC | SG-31 + deTr + SNV | SG-21 + deTr + SNV | SG-11 + SNV | SG-21 + SNV | SG-11 + deTr | SG-21 + deTr + SNV | SG-11 + SNV |
| PC | 7 | 23 | 8 | 17 | 12 | 14 | 8 | 17 | 13 | 16 | 26 | 17 |
| Kernel | linear | linear | linear | linear | linear | linear | linear | linear | linear | linear | linear | linear |
| ε | 0.1 | 0.5 | 0.1 | 0.01 | 0.01 | 0.5 | 0.5 | 0.5 | 0.01 | 0.1 | 0.1 | 0.5 |
| Cost | 10 | 1 | 0.25 | 110 | 10 | 0.24 | 0.4 | 10 | 10 | 10 | 10 | 1 |
| RMSEC | 0.512 | 2.131 | 2.659 | 2.772 | 0.233 | 2.186 | 2.262 | 2.231 | 0.374 | 0.561 | 0.809 | 1.565 |
| R2C | 0.986 | 0.762 | 0.626 | 0.596 | 0.995 | 0.559 | 0.486 | 0.510 | 0.987 | 0.971 | 0.938 | 0.766 |
| RMSECV | 0.566 | 2.448 | 2.768 | 2.957 | 0.268 | 2.372 | 2.678 | 2.492 | 0.396 | 0.644 | 0.903 | 1.673 |
| R2CV | 0.983 | 0.685 | 0.600 | 0.540 | 0.993 | 0.480 | 0.290 | 0.388 | 0.985 | 0.961 | 0.924 | 0.733 |
| Procyanidin Content | Antioxidant Capacity | Caffeic Acid Content | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrument | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | Micro- PHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR |
| Range (wl) | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 |
| Pre-treat. | MSC | SG-25 + deTr + SNV | SG-21 + deTr + SNV | SG-11 + SNV | SG-21 + deTr + MSC | SG-21 + deTr + SNV | SG-11 + deTr + SNV | SG-11 + MSC | SG-21 + deTr + SNV | SNV | SG-21 + SNV | SG-11 + SNV |
| LV | 4 | 12 | 11 | 6 | 3 | 4 | 6 | 10 | 4.000 | 8.000 | 14 | 10 |
| RMSEC | 0.948 | 1.329 | 2.167 | 2.165 | 0.202 | 0.379 | 0.478 | 0.400 | 0.018 | 0.026 | 0.031 | 0.039 |
| R2C | 0.983 | 0.967 | 0.919 | 0.913 | 0.976 | 0.918 | 0.863 | 0.905 | 0.984 | 0.963 | 0.951 | 0.915 |
| RMSECV | 1.012 | 1.629 | 2.530 | 2.391 | 0.214 | 0.403 | 0.528 | 0.456 | 0.019 | 0.031 | 0.038 | 0.045 |
| R2CV | 0.980 | 0.950 | 0.890 | 0.894 | 0.974 | 0.907 | 0.8331 | 0.877 | 0.982 | 0.951 | 0.929 | 0.890 |
| Procyanidin Content | Antioxidant Capacity | Caffeic Acid Content | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrument | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR |
| Range (wl) | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 |
| Pre-treat. | MSC | SG-25 + deTr + SNV | SG-21 + deTr + SNV | SG-11 + SNV | SG-21 + deTr + MSC | SG-21 + deTr + SNV | SG-11 + deTr + SNV | SG-11 + MSC | SG-21 + deTr + SNV | SNV | SG-21 + SNV | SG-11 + SNV |
| PC | 13 | 13 | 26 | 18 | 7 | 18 | 24 | 21 | 9 | 19 | 12 | 22 |
| Kernel | linear | linear | linear | linear | linear | linear | linear | linear | linear | linear | linear | linear |
| ε | 0.01 | 0.01 | 0.01 | 0.5 | 0.01 | 0.01 | 0.1 | 0.1 | 0.1 | 0.1 | 0.5 | 0.01 |
| Cost | 10 | 0.1 | 1 | 1 | 1 | 1 | 10 | 10 | 1 | 10 | 10 | 1 |
| RMSEC | 0.764 | 2.018 | 1.960 | 3.500 | 0.159 | 0.335 | 0.385 | 0.648 | 0.017 | 0.029 | 0.053 | 0.067 |
| R2C | 0.989 | 0.922 | 0.925 | 0.761 | 0.984 | 0.930 | 0.907 | 0.737 | 0.985 | 0.957 | 0.854 | 0.764 |
| RMSECV | 0.805 | 2.331 | 2.206 | 3.753 | 0.167 | 0.373 | 0.436 | 0.704 | 0.019 | 0.034 | 0.058 | 0.070 |
| R2CV | 0.988 | 0.896 | 0.907 | 0.726 | 0.983 | 0.914 | 0.883 | 0.690 | 0.982 | 0.938 | 0.823 | 0.737 |
| Gallic Acid Content | Catechin Content | Epicatechin Content | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrument | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR |
| Range (wl) | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 |
| Pre-treat. | SG-21 + deTr + SNV | SG-21 + deTr | SG-21 + deTr + MSC | SG-11 + SNV | SG-21 + deTr + SNV | SG-21 + FD | SG-21 + deTr + MSC | SG-11 + SNV | SG-21 + deTr + SNV | SG-21 + deTr | SG-21 + deTr + SNV | SG-11 + MSC |
| LV | 6 | 9 | 11 | 10 | 6 | 15 | 15 | 11 | 6.000 | 15.000 | 15 | 15 |
| RMSEC | 0.609 | 2.680 | 2.947 | 2.447 | 0.318 | 1.798 | 1.983 | 1.807 | 0.086 | 0.401 | 0.426 | 0.369 |
| R2C | 0.988 | 0.774 | 0.727 | 0.816 | 0.994 | 0.775 | 0.724 | 0.784 | 0.990 | 0.768 | 0.750 | 0.821 |
| RMSECV | 0.682 | 2.978 | 3.506 | 2.738 | 0.373 | 2.224 | 2.492 | 2.030 | 0.097 | 0.484 | 0.531 | 0.446 |
| R2CV | 0.985 | 0.721 | 0.614 | 0.769 | 0.991 | 0.655 | 0.5634 | 0.727 | 0.987 | 0.663 | 0.610 | 0.738 |
| Gallic Acid Content | Catechin Content | Epicatechin Content | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instrument | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR | NIRS XDS | NIR-S-G1 | MicroNIR | MicroPHAZIR |
| Range (wl) | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 | 1100–2250 | 950–1650 | 950–1650 | 1630–2250 |
| Pre-treat. | SG-21 + deTr + SNV | SG-21 + deTr | SG-21 + deTr + MSC | SG-11 + SNV | SG-21 + deTr + SNV | SG-21 + FD | SG-21 + deTr + MSC | SG-11 + SNV | SG-21 + deTr + SNV | SG-21 + deTr | SG-21 + deTr + SNV | SG-11 + MSC |
| PC | 7 | 8 | 6 | 18 | 9 | 9 | 5 | 22 | 9 | 8 | 5 | 21 |
| Kernel | linear | radial | linear | linear | linear | radial | radial | linear | linear | radial | radial | linear |
| ε | 0.01 | 0.5 | 0.5 | 0.1 | 0.01 | 0.1 | 0.1 | 0.5 | 0.01 | 0.1 | 0.5 | 0.1 |
| Cost | 10 | 0.3 | 0.25 | 10 | 10 | 0.3 | 0.5 | 10 | 1 | 0.25 | 0.3 | 10 |
| RMSEC | 0.727 | 3.506 | 3.666 | 3.496 | 0.313 | 2.609 | 2.808 | 2.521 | 0.086 | 0.539 | 0.563 | 0.555 |
| R2C | 0.983 | 0.613 | 0.574 | 0.614 | 0.994 | 0.546 | 0.466 | 0.578 | 0.990 | 0.605 | 0.566 | 0.582 |
| RMSECV | 0.809 | 3.696 | 3.852 | 3.764 | 0.360 | 3.248 | 3.447 | 2.816 | 0.093 | 0.662 | 0.682 | 0.608 |
| R2CV | 0.979 | 0.570 | 0.529 | 0.553 | 0.991 | 0.297 | 0.195 | 0.473 | 0.988 | 0.404 | 0.363 | 0.499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukacs, M.; Vitalis, F.; Bardos, A.; Tormási, J.; Bec, K.B.; Grabska, J.; Gillay, Z.; Tömösközi-Farkas, R.A.; Abrankó, L.; Albanese, D.; et al. Comparison of Multiple NIR Instruments for the Quantitative Evaluation of Grape Seed and Other Polyphenolic Extracts with High Chemical Similarities. Foods 2024, 13, 4164. https://doi.org/10.3390/foods13244164
Lukacs M, Vitalis F, Bardos A, Tormási J, Bec KB, Grabska J, Gillay Z, Tömösközi-Farkas RA, Abrankó L, Albanese D, et al. Comparison of Multiple NIR Instruments for the Quantitative Evaluation of Grape Seed and Other Polyphenolic Extracts with High Chemical Similarities. Foods. 2024; 13(24):4164. https://doi.org/10.3390/foods13244164
Chicago/Turabian StyleLukacs, Matyas, Flora Vitalis, Adrienn Bardos, Judit Tormási, Krzysztof B. Bec, Justyna Grabska, Zoltan Gillay, Rita A. Tömösközi-Farkas, László Abrankó, Donatella Albanese, and et al. 2024. "Comparison of Multiple NIR Instruments for the Quantitative Evaluation of Grape Seed and Other Polyphenolic Extracts with High Chemical Similarities" Foods 13, no. 24: 4164. https://doi.org/10.3390/foods13244164
APA StyleLukacs, M., Vitalis, F., Bardos, A., Tormási, J., Bec, K. B., Grabska, J., Gillay, Z., Tömösközi-Farkas, R. A., Abrankó, L., Albanese, D., Malvano, F., Huck, C. W., & Kovacs, Z. (2024). Comparison of Multiple NIR Instruments for the Quantitative Evaluation of Grape Seed and Other Polyphenolic Extracts with High Chemical Similarities. Foods, 13(24), 4164. https://doi.org/10.3390/foods13244164












