Effects of Lactic Acid Bacteria Fermentation and In Vitro Simulated Digestion on the Bioactivities of Purple Sweet Potato Juice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fermentation of PSPJ
2.3. In Vitro Simulated Digestion
2.4. Viable Counts of LAB
2.5. Phytochemical Concentration Analysis
2.5.1. Total Phenolic Content (TPC)
2.5.2. Total Flavonoid Content (TFC)
2.5.3. Total Anthocyanin Content (TAC)
2.6. Phenolic Profiles
2.7. α-Glucosidase Inhibition Rate Analysis
2.8. Analysis of Antioxidant Activity
2.9. Determination of Phenols Bioaccessibility and Phenols Bioavailability
2.10. Statistical Analysis
3. Results
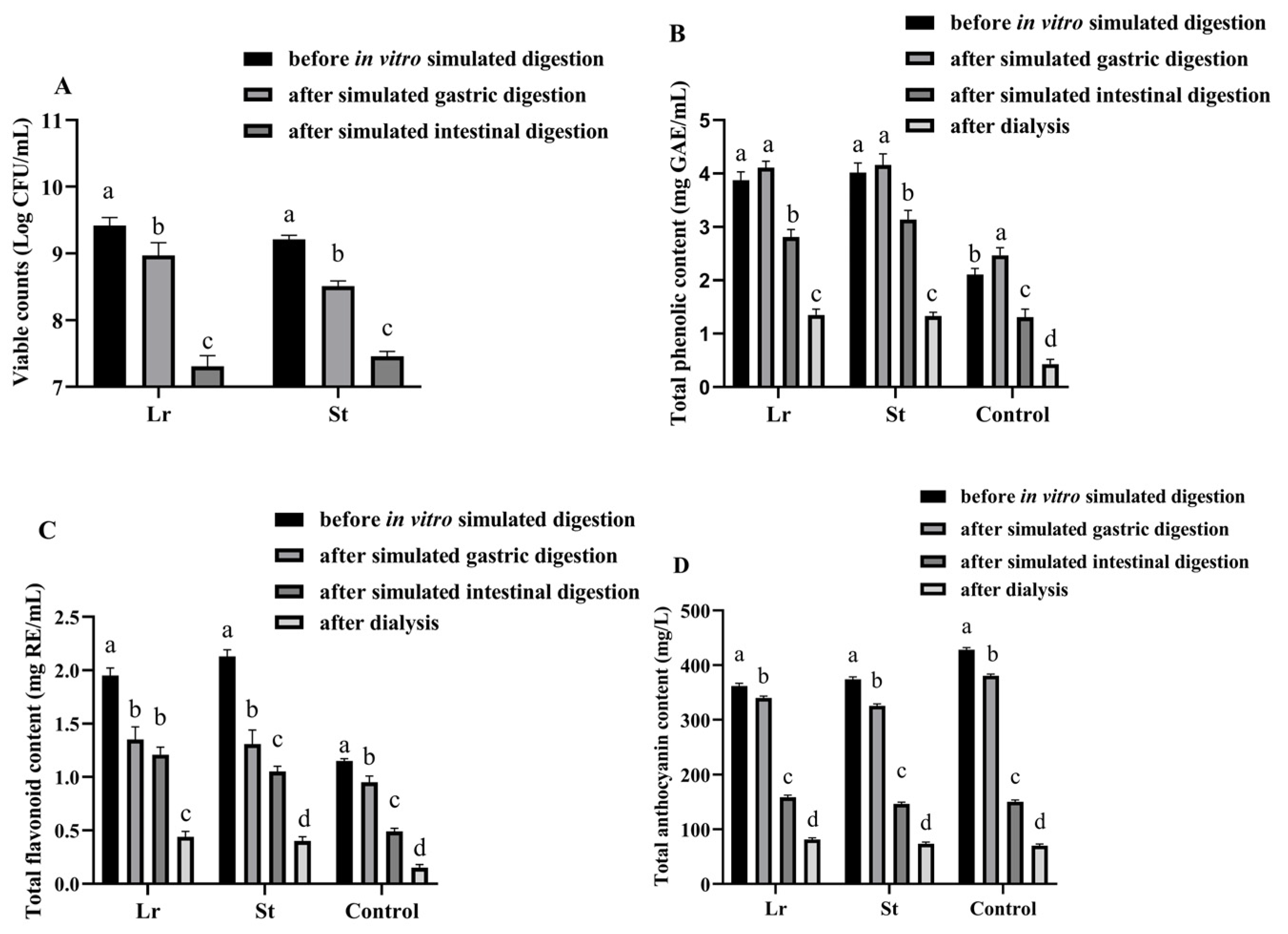
3.1. Variations in Viable Counts Throughout the In Vitro Simulated Digestion Process
3.2. Phytochemical Concentrations During In Vitro Simulated Digestion Process
3.3. Effects of In Vitro Simulated Digestion on Phenolic Composition
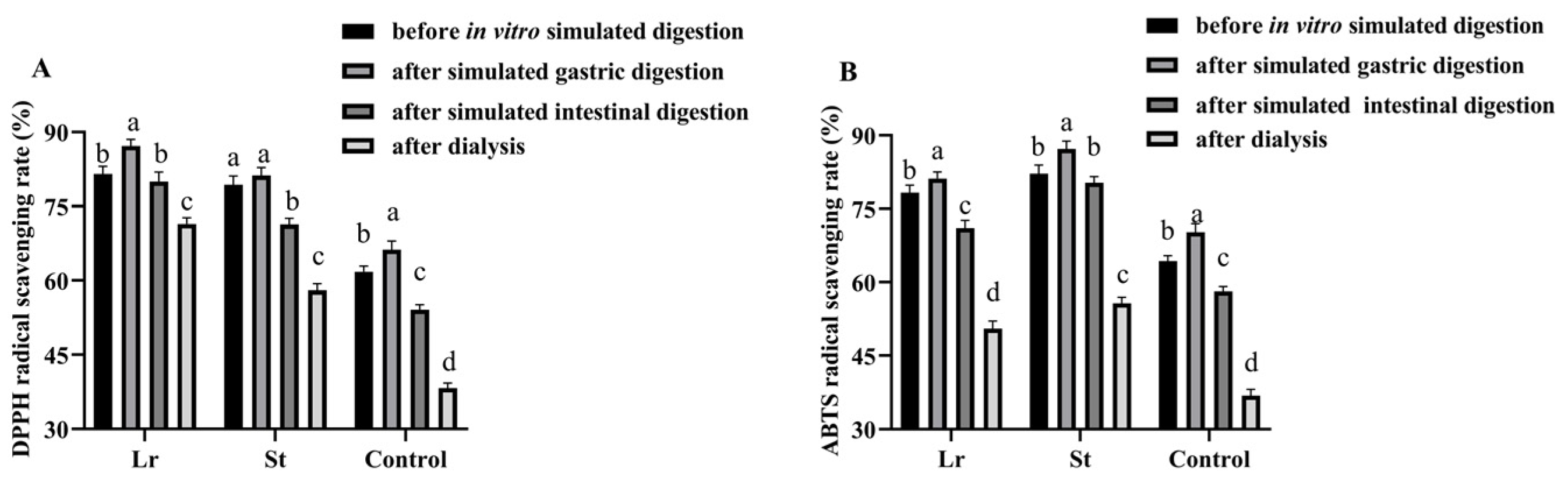
3.4. Effects of In Vitro Simulated Digestion on Antioxidant Activities
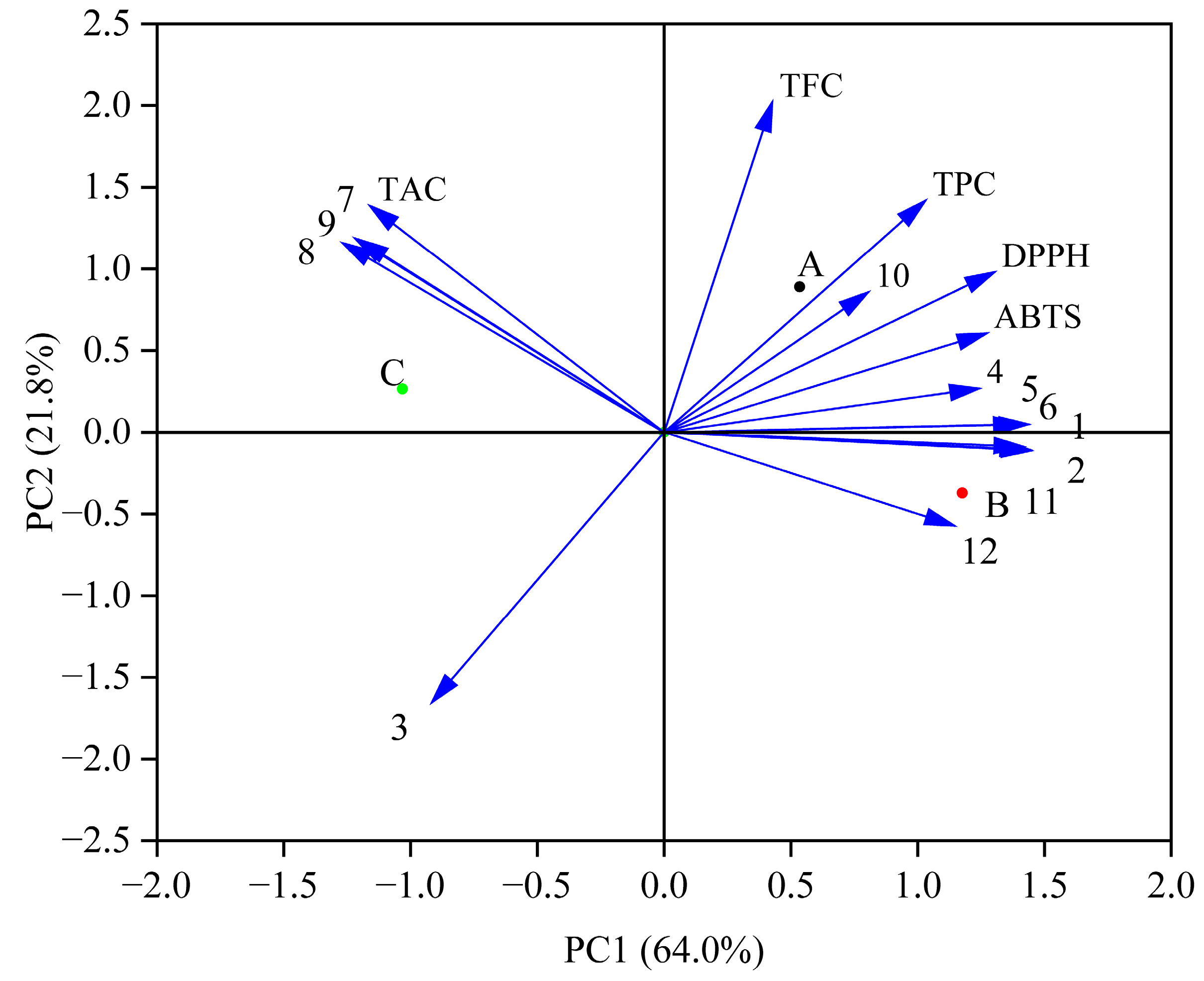
3.5. Principal Component Analysis (PCA) of Antioxidant Activities and Phenolic Profiles During In Vitro Simulated Digestion Process
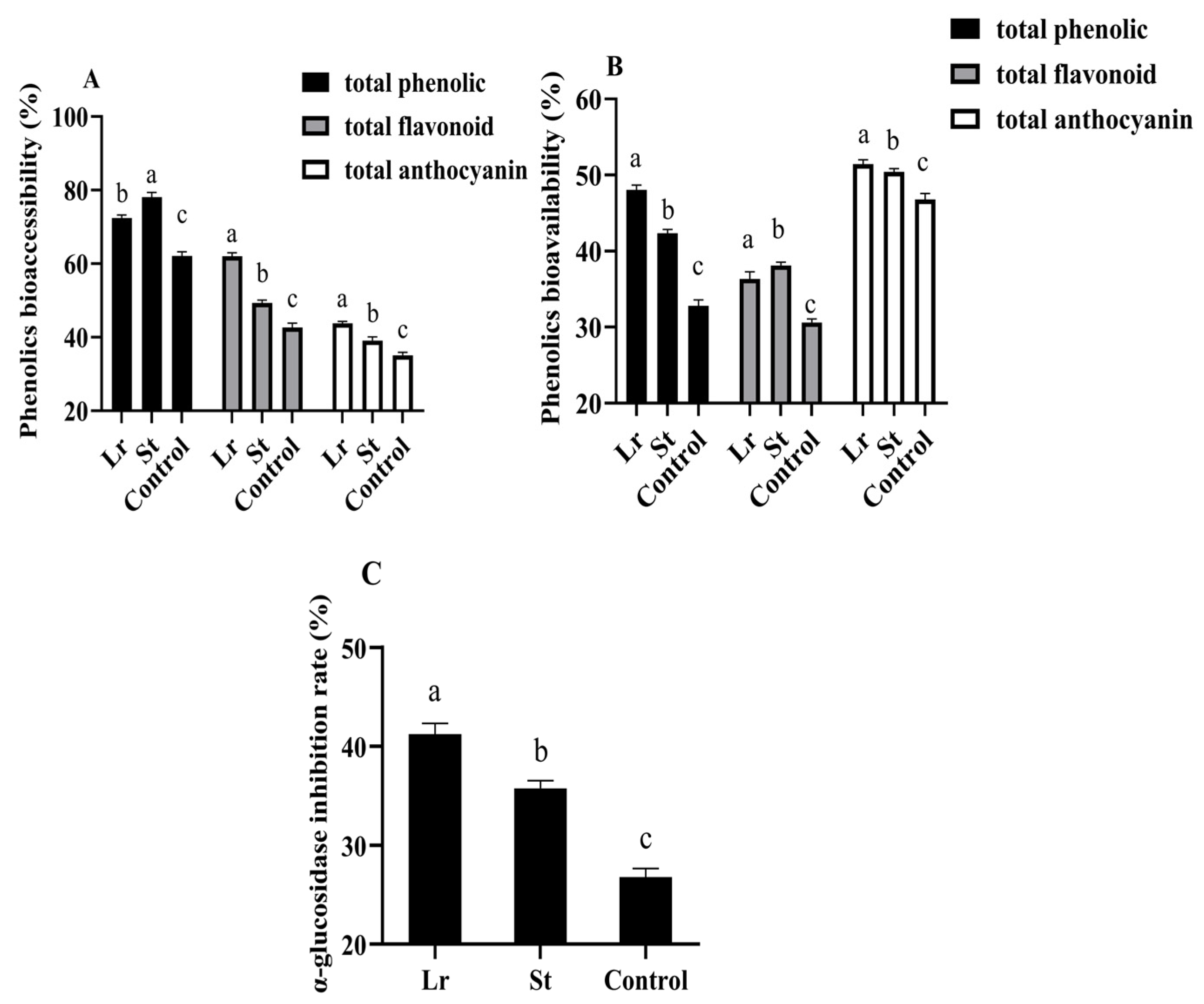
3.6. Changes in α-Glucosidase Inhibition Rate During Fermentation
3.7. Changes in Phenols Bioaccessibility and Phenols Bioavailability During In Vitro Simulated Digestion Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laveriano-Santos, E.P.; López-Yerena, A.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Romanyà, J.; Pérez, M. Sweet Potato Is Not Simply an Abundant Food Crop: A Comprehensive Review of Its Phytochemical Constituents, Biological Activities, and the Effects of Processing. Antioxidants 2022, 11, 1648. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Y.; Shen, M.Y.; Wei, D.M.; Xu, L.; Sui, T.; Cao, C.; Zhou, Y.B. Study on compatible characteristics of wheat and purple sweet potato starches. Food Hydrocoll. 2020, 107, 105961. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Kim, Y. Effect of Purple Sweet Potato Using Different Cooking Methods on Cytoprotection against Ethanol-Induced Oxidative Damage through Nrf2 Activation in HepG2 Cells. Antioxidants 2023, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.H.; Wang, J.W.; Wang, Y.H.; Xu, Z.H.; Li, B.; Meng, X.J.; Sun, X.Y.; Zhu, J.Y. Influence of fermentation by lactic acid bacteria and in vitro digestion on the biotransformations of blueberry juice phenols. Food Control 2022, 133, 108603. [Google Scholar] [CrossRef]
- Kilua, A.; Han, K.H.; Fukushima, M. Effect of polyphenols isolated from purple sweet potato (Ipomoea batatas cv. Ayamurasaki) on the microbiota and the biomarker of colonic fermentation in rats fed with cellulose or inulin. Food Funct. 2020, 11, 10182–10192. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.P.; Wei, Z.H.; Yin, B.X.; Man, C.X.; Jiang, Y.J. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT-Food Sci. Technol. 2021, 139, 110590. [Google Scholar] [CrossRef]
- Bocchi, S.; Rocchetti, G.; Elli, M.; Lucini, L.; Lim, C.Y.; Morelli, L. The combined effect of fermentation of lactic acid bacteria and in vitro digestion on metabolomic and oligosaccharide profile of oat beverage. Food Res. Int. 2021, 142, 110216. [Google Scholar] [CrossRef]
- Guo, Y.X.; Chen, X.F.; Gong, P.; Chen, F.X.; Cui, D.D.; Wang, M.R. Advances in the in vitro digestion and fermentation of polysaccharides. Int. J. Food Sci. Technol. 2021, 56, 4970–4982. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Tong, Y.J.; Tong, Q.Y.; Liu, Y.T.; Xu, W.T. Effects of different lactic acid bacteria on phenolic profiles, antioxidant capacities, and volatile compounds in purple sweet potato juice. J. Food Sci. Technol. 2024, 61, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.Y.; Yang, X.Y.; Li, L. Non-beany flavor soymilk fermented by lactic acid bacteria: Characterization, stability, antioxidant capacity and in vitro digestion. Food Chem. X. 2023, 17, 100578. [Google Scholar] [CrossRef]
- Li, T.L.; Jiang, T.; Liu, N.; Wu, C.Y.; Xu, H.D.; Lei, H.J. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef]
- Penidez, S.H.S.; Manini, M.A.V.; LeBlanc, J.G.; Gerez, C.L.; Rollan, G.C. Quinoa sourdough-based biscuits with high antioxidant activity fermented with autochthonous lactic acid bacteria. J. Appl. Microbiol. 2022, 132, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.X.; Li, B.; Gong, E.S.; Shu, C.; Si, X.; Gao, N.X.; Zhang, W.J.; Cui, H.J.; Meng, X.J. Effects of α-casein and β-casein on the stability, antioxidant activity and bioaccessibility of blueberry anthocyanins with an in vitro simulated digestion. Food Chem. 2021, 334, 127526. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Lv, J.M.; Gu, Y.; He, Y.K.; Chen, J.C.; Ye, X.Q.; Zhou, Z.Q. Mixed fermentation of Chinese bayberry pomace using yeast, lactic acid bacteria and acetic acid bacteria: Effects on color, phenols and antioxidant ingredients. LWT-Food Sci. Technol. 2022, 163, 113503. [Google Scholar] [CrossRef]
- Wang, X.W.; Han, M.Z.; Zhang, M.N.; Wang, Y.; Ren, Y.P.; Yue, T.L.; Gao, Z.P. In vitro evaluation of the hypoglycemic properties of lactic acid bacteria and its fermentation adaptability in apple juice. LWT-Food Sci. Technol. 2021, 136, 110363. [Google Scholar] [CrossRef]
- Swieca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B. Wheat bread enriched with green coffee—In Vitro bioaccessibility and bioavailability of phenols and antioxidant activity. Food Chem. 2017, 221, 1451–1457. [Google Scholar] [CrossRef]
- Wang, Z.N.; Feng, Y.Z.; Yang, N.N.; Jiang, T.; Xu, H.D.; Lei, H.J. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenols, antioxidant activities and flavor volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Hayes, J.J.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Protection of candidate probiotic lactobacilli by Cheddar cheese matrix during simulated gastrointestinal digestion. J. Funct. Foods 2022, 92, 105042. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.J.; Tao, Y.; Li, D.D.; Han, Y.B.; Show, P.L.; Wen, G.Z.; Zhou, J.Z. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenols, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Yeo, J.D.; Tsao, R.; Sun, Y.; Shahidi, F. Liberation of insoluble-bound phenols from lentil hull matrices as affected by Rhizopus oryzae fermentation: Alteration in phenolic profiles and their inhibitory capacities against low-density lipoprotein (LDL) and DNA oxidation. Food Chem. 2021, 363, 130275. [Google Scholar] [CrossRef]
- Dogan, K.; Akman, P.K.; Tornuk, F. Role of non-thermal treatments and fermentation with probiotic Lactobacillus plantarum on in vitro bioaccessibility of bioactives from vegetable juice. J. Sci. Food Agric. 2021, 101, 4779–4788. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.R.C.; de Souza Mesquita, L.M.; Martins, P.L.G.; Habu, S.; de Rosso, V.V. Lactobacillus fermentation of Jussara pulp leads to the enzymatic conversion of anthocyanins increasing antioxidant activity. J. Food Compos. Anal. 2018, 69, 162–170. [Google Scholar] [CrossRef]
- Devi, A.; Aiyappaa, A.K.; Waterhouse, A.L. Adsorption and biotransformation of anthocyanin glucosides and quercetin glycosides by Oenococcus oeni and Lactobacillus plantarum in model wine solution. J. Sci. Food Agric. 2020, 100, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- Mashitoa, F.M.; Akinola, S.A.; Manhevi, V.E.; Garcia, C.; Remize, F.; Slabbert, R.M.; Sivakumar, D. Influence of Fermentation of Pasteurised Papaya Puree with Different Lactic Acid Bacterial Strains on Quality and Bioaccessibility of Phenolic Compounds during In Vitro Digestion. Foods 2021, 10, 962. [Google Scholar] [CrossRef]
- Jang, D.; Jung, Y.S.; Seong, H.; Kim, M.S.; Rha, C.S.; Nam, T.G.; Han, N.S.; Kim, D.O. Stability of Enzyme-Modified Flavonoid C- and O-Glycosides from Common Buckwheat Sprout Extracts during In Vitro Digestion and Colonic Fermentation. J. Agric. Food Chem. 2021, 69, 5764–5773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, Y.; Zhang, X.; Shao, S.; Han, Y.; Chu, D.T.; Xie, G.; Ye, X. Metabolic profile of ginkgo kernel juice fermented with lactic aicd bacteria: A potential way to degrade ginkgolic acids and enrich terpene lactones and phenols. Process Biochem. 2019, 76, 25–33. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.Z.; Ying, D.Y.; Adhikari, B.; Fang, Z.X. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Canalis, M.S.B.; Baroni, M.V.; León, A.E.; Ribotta, P.D. Effect of peach puree incorportion on cookie quality and on simulated digestion of polyphenols and antioxidant properties. Food Chem. 2020, 333, 127464. [Google Scholar] [CrossRef]
- Ryu, D.; Koh, E. Stability assessment of anthocyanins from black soybean, grape, and purple sweet potato under in vitro gastrointestinal digestion. Food Sci. Biotechnol. 2022, 31, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Xie, C.Y.; Li, H.N.; Lei, S.C.; Li, J.; Huang, X.W.; Yang, F. Effect of in vitro digestion on chestnut outer-skin and inner-skin bioaccessibility: The relationship between biotransformation and antioxidant activity of polyphenols by metabolomics. Food Chem. 2021, 363, 130277. [Google Scholar] [CrossRef]
- Ziólkiewicz, A.; Kasprzak-Drozd, K.; Wójtowicz, A.; Oniszczuk, T.; Gancarz, M.; Kowalska, I.; Moldoch, J.; Kondracka, A.; Oniszczuk, A. The Effect of In Vitro Digestion on Polyphenolic Compounds and Antioxidant Properties of Sorghum (Sorghum bicolor (L.) Moench) and Sorghum-Enriched Pasta. Molecules 2023, 28, 1706. [Google Scholar] [CrossRef]
- Ye, C.Y.; Zhang, R.F.; Dong, L.M.; Chi, J.W.; Huang, F.; Dong, L.H.; Zhang, M.W.; Jia, X.C. α-Glucosidase inhibitors from brown rice bound phenols extracts (BRBPE): Identification and mechanism. Food Chem. 2022, 372, 131306. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.B.; Qi, J.; Xu, K.; Li, B.; Xu, H.D.; Tian, X.J.; Lei, H.J. Effect of lactic acid fermentation and in vitro digestion on the bioactive compounds in Chinese wolfberry (Lycium barbarum) pulp. Food Biosci. 2023, 53, 102558. [Google Scholar] [CrossRef]
- Pinton, S.; Dias, F.F.G.; Lerno, L.A.; Barile, D.; Bell, J. Revitalizing Unfermented Cabernet Sauvignon Pomace Using an Eco-Friendly, Two-Stage Countercurrent Process: Role of pH on the Extractability of Bioactive Phenols. Processes 2022, 10, 2093. [Google Scholar] [CrossRef]
| Control | Lr | St | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Digestion | After SGD | After SGD + SID | Before Digestion | After SGD | After SGD + SID | Before Digestion | After SGD | After SGD + SID | |
| Gallic acid | 4.11 ± 0.12 b | 2.30 ± 0.29 c | 8.11 ± 0.31 a | 14.05 ± 0.11 b | 11.21 ± 0.11 c | 18.61 ± 0.12 a | 8.75 ± 0.15 c | 10.98 ± 0.14 b | 15.61 ± 0.12 a |
| Vanillic acid | ND | ND | 3.15 ± 0.07 a | 7.42 ± 0.06 b | 3.21 ± 0.09 c | 8.61 ± 0.07 a | 13.02 ± 0.08 b | 6.13 ± 0.12 c | 15.13 ± 0.09 a |
| Syringic acid | 43.21 ± 0.09 c | 47.21 ± 0.05 b | 52.46 ± 0.21 a | 26.13 ± 0.09 b | 22.25 ± 0.17 c | 33.12 ± 0.06 a | 31.28 ± 0.17 c | 37.36 ± 0.13 b | 41.22 ± 0.16 a |
| Protocatechuic acid | 0.48 ± 0.03 a | 0.23 ± 0.02 b | ND | 3.13 ± 0.05 c | 6.23 ± 0.04 b | 8.01 ± 0.12 a | 5.08 ± 0.13 b | 7.35 ± 0.11 a | 3.05 ± 0.06 c |
| Ferulic acid | 6.58 ± 0.06 c | 11.32 ± 0.06 a | 7.84 ± 0.14 b | 17.23 ± 0.03 c | 23.21 ± 0.13 a | 21.23 ± 0.26 b | 11.25 ± 0.03 c | 16.11 ± 0.23 b | 19.17 ± 0.13 a |
| Caffeic acid | 4.67 ± 0.05 c | 6.21 ± 0.07 b | 11.45 ± 0.19 a | 24.24 ± 0.18 c | 27.11 ± 0.09 b | 37.14 ± 0.17 a | 15.37 ± 0.17 b | 13.21 ± 0.16 c | 27.15 ± 0.08 a |
| Chlorogenic acid | 72.23 ± 0.19 a | 69.25 ± 0.05 b | 57.14 ± 0.16 c | 66.19 ± 0.05 a | 56.25 ± 0.23 c | 58.12 ± 0.14 b | 62.38 ± 0.16 a | 54.12 ± 0.15 b | 51.63 ± 0.07 c |
| Peonidin-3-O-glucoside | 6.79 ± 0.02 a | 5.21 ± 0.07 b | 1.67 ± 0.15 c | 4.59 ± 0.08 a | 4.12 ± 0.05 b | 0.67 ± 0.08 c | 3.03 ± 0.04 a | 2.04 ± 0.03 b | ND |
| Cyanidin-3-O-glucoside | 2.15 ± 0.07 a | 1.78 ± 0.11 b | ND | 1.46 ± 0.04 a | 0.23 ± 0.02 b | ND | 1.12 ± 0.05 b | 0.11 ± 0.02 c | ND |
| Rutin | 13.37 ± 0.24 b | 18.13 ± 0.10 a | 8.97 ± 0.21 c | 16.57 ± 0.18 b | 21.32 ± 0.27 a | 12.57 ± 0.32 c | 15.13 ± 0.24 b | 8.82 ± 0.14 c | 20.23 ± 0.18 a |
| Quercetin | 15.11 ± 0.13 b | 11.21 ± 0.12 c | 18.32 ± 0.28 a | 28.45 ± 0.23 b | 12.63 ± 0.19 c | 35.26 ± 0.07 a | 22.79 ± 0.18 b | 15.36 ± 0.20 c | 29.15 ± 0.12 a |
| Kaempferol | 3.05 ± 0.03 c | 7.21 ± 0.15 a | 4.21 ± 0.07 b | 5.16 ± 0.13 c | 9.21 ± 0.06 a | 6.53 ± 0.16 b | 7.03 ± 0.13 c | 8.01 ± 0.06 b | 15.12 ± 0.08 a |
| TPC | TFC | TAC | DPPH | ABTS | |
|---|---|---|---|---|---|
| TPC | 1 | ||||
| TFC | 0.725 * | 1 | |||
| TAC | 0.335 | 0.536 | 1 | ||
| DPPH | 0.826 ** | 0.607 * | 0.096 | 1 | |
| ABTS | 0.936 ** | 0.502 * | 0.064 | 0.726 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Wang, Z.; Tong, Q.; Liu, Y. Effects of Lactic Acid Bacteria Fermentation and In Vitro Simulated Digestion on the Bioactivities of Purple Sweet Potato Juice. Foods 2024, 13, 4094. https://doi.org/10.3390/foods13244094
Tong Y, Wang Z, Tong Q, Liu Y. Effects of Lactic Acid Bacteria Fermentation and In Vitro Simulated Digestion on the Bioactivities of Purple Sweet Potato Juice. Foods. 2024; 13(24):4094. https://doi.org/10.3390/foods13244094
Chicago/Turabian StyleTong, Yingjia, Zeqing Wang, Qunyi Tong, and Yutong Liu. 2024. "Effects of Lactic Acid Bacteria Fermentation and In Vitro Simulated Digestion on the Bioactivities of Purple Sweet Potato Juice" Foods 13, no. 24: 4094. https://doi.org/10.3390/foods13244094
APA StyleTong, Y., Wang, Z., Tong, Q., & Liu, Y. (2024). Effects of Lactic Acid Bacteria Fermentation and In Vitro Simulated Digestion on the Bioactivities of Purple Sweet Potato Juice. Foods, 13(24), 4094. https://doi.org/10.3390/foods13244094






