Influence of Smoking and Paprika Spice on the Content of Polycyclic Aromatic Hydrocarbons (PAHs) in the Traditional Spanish Smoked Sausage ‘Botillo del Bierzo’
Abstract
1. Introduction
2. Materials and Methods
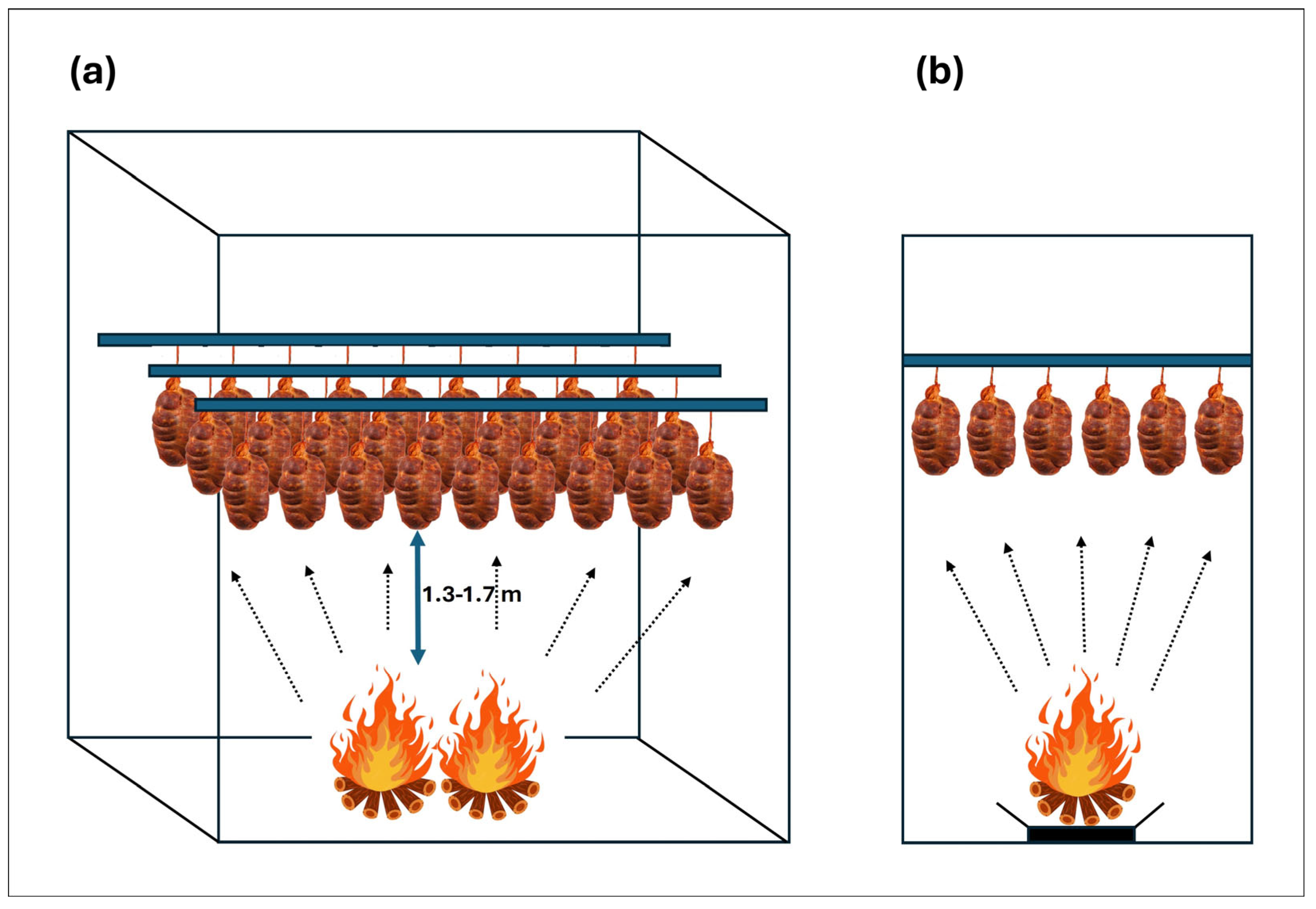
2.1. Botillo del Bierzo Processing and Sampling
2.2. Analytical Methods
2.3. PAH Determination
2.3.1. Chemicals
2.3.2. Preparation of Samples for GC–MS Analysis
2.3.3. Quantification of PAH by GC–MS
2.3.4. GC–MS Method Validation
2.4. Statistical Methods
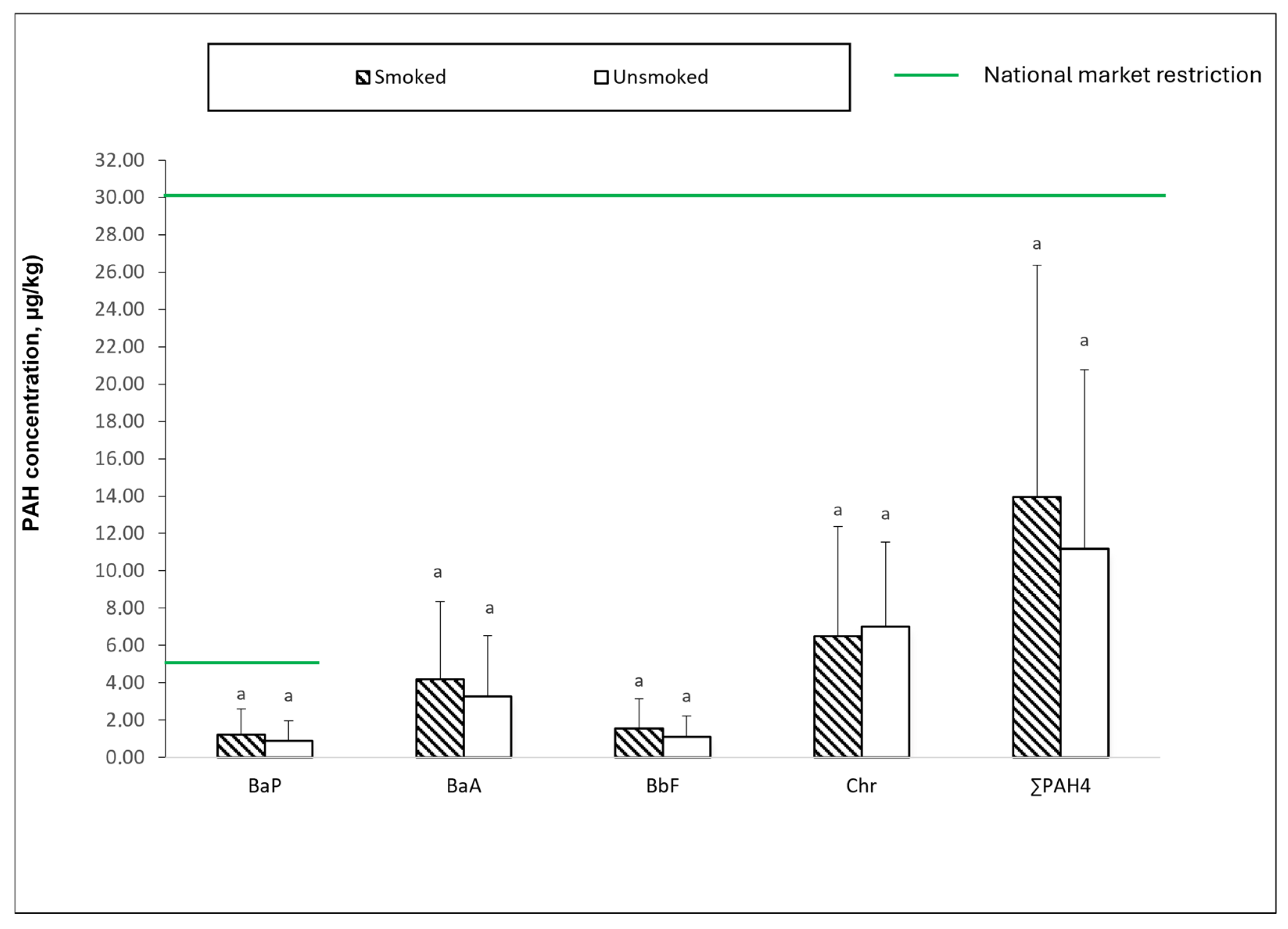
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeyeye, S.A.O.; Ashaolu, T.J. Polycyclic aromatic hydrocarbons formation and mitigation in meat and meat products. Polycycl. Aromat. Compd. 2022, 42, 3401–3411. [Google Scholar] [CrossRef]
- Ledesma, E.; Rendueles, M.; Díaz, M. Contamination of meat products during smoking by polycyclic aromatic hydrocarbons: Processes and prevention. Food Control 2016, 60, 64–87. [Google Scholar] [CrossRef]
- Sikorski, Z.E.; Kołakow, E. Smoking. In Handbook of Meat Processing; Toldrá, F., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 231–245. [Google Scholar]
- Lorenzo, J.M.; Purriños, L.; García Fontán, M.C.; Franco, D. Polycyclic aromatic hydrocarbons (PAHs) in two Spanish traditional smoked sausage varieties: “Androlla” and “Botillo”. Meat Sci. 2010, 86, 660–664. [Google Scholar] [CrossRef] [PubMed]
- BOE. Orden de 15 de noviembre de 2000 por la que se ratifica el Reglamento de la Indicación Geográfica Protegida «Botillo del Bierzo». In Boletín Oficial del Estado nº 285; Boletín Oficial del Estado (BOE): Madrid, Spain, 2000; pp. 41231–41236. [Google Scholar]
- DOCE. Reglamento (CE) Nº 1971/2001 de la Comisión de 9 de octubre de 2001 que completa el anexo del Reglamento (CE) nº 2400/96 relativo a la inscripción de determinadas denominaciones en el Registro de Denominaciones de Origen Protegidas y de Indicaciones Geográficas Protegidas establecido en el Reglamento (CEE nº 2081/92 del Consejo, relativo a la protección de las indicaciones geográficas y de las denominaciones de origen de los productos agrícolas y alimenticios. D. Of. Comunidades Eur. 2001, L 269, 5–6.
- IGP BB. Pliego de Condiciones de la Indicación Geográfica Protegida ‘Botillo del Bierzo’; Indicación Geográfica Protegida ‘Botillo del, bierzo’; Ministerio de Agricultura, Pesca y Alimentación; Dirección General de Alimentación; Subdirección General de Denominaciones de Calidad: Madrid, Spain, 2001; pp. 1–13.
- Conde, F.J.; Ayala, J.H.; Afonso, A.M.; González, V. Polycyclic aromatic hydrocarbons in smoke used to smoke cheese produced by the combustion of rock rose (Cistus monspeliensis) and tree heather (Erica arborea) wood. J. Agric. Food Chem. 2005, 53, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Purrinos, L.; Bermudez, R.; Cobas, N.; Figueiredo, M.; García-Falcón, M.C. Polycyclic aromatic hydrocarbons (PAHs) in two Spanish traditional smoked sausage varieties: “Chorizo gallego” and “Chorizo de cebolla”. Meat Sci. 2011, 89, 105–109. [Google Scholar] [CrossRef]
- Berki, M.; Daood, H.G.; Adányi, N.; Tömösközi-Farkas, R. Polycyclic aromatic hydrocarbons in smoked and non-smoked paprika samples. J. Food Process. Preserv. 2020, 44, e14861. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Y.; Huang, T.; Yu, Y.; Bassey, A.P.; Huang, M. The contamination, formation, determination and control of polycyclic aromatic hydrocarbons in meat products. Food Control 2022, 141, 109194. [Google Scholar] [CrossRef]
- Palade, L.M.; Negoită, M.; Adascălului, A.C.; Mihai, A.L. Polycyclic Aromatic Hydrocarbon Occurrence and Formation in Processed Meat, Edible Oils, and Cereal-Derived Products: A Review. Appl. Sci. 2023, 13, 7877. [Google Scholar] [CrossRef]
- Škaljac, S.; Jokanović, M.; Peulić, T.; Vranešević, J.; Kartalović, B.; Tomović, V.; Ikonić, P.; Šojić, B. Content of Polycyclic Aromatic Hydrocarbons in Traditionally Smoked Meat Products from North Serbia (Vojvodina). Fermentation 2024, 10, 104. [Google Scholar] [CrossRef]
- SCF. Opinion of the Scientific Committee on Food on the Risks to Human Health of Polycyclic Aromatic Hydrocarbons in Food. Scientific Committee on Food (SCF), 2002. Available online: https://ec.europa.eu/food/fs/sc/scf/out153_en.pdf (accessed on 26 September 2024).
- European Union. Commission Regulation (EC) No 208/2005 of 4 February 2005 amending Regulation (EC) No 466/2001 as regards polycyclic aromatic hydrocarbons. Off. J. Eur. Union 2011, L34, 3–5. [Google Scholar]
- European Union. Commission regulation (EC) N1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- EFSA. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on polycyclic aromatic hydrocarbons in Food. EFSA J. 2008, 6, 724. [Google Scholar] [CrossRef]
- Velázquez, R.; Córdoba, M.G.; Hernández, A.; Casquete, R.; Aranda, E.; Bartolome, T.; Martín, A. Effects of use of modified traditional driers in making smoked paprika “Pimentón de La Vera”, on pepper quality and mitigation of PAH contamination. J. Food Compos. Anal. 2022, 110, 104566. [Google Scholar] [CrossRef]
- European Union. Commission regulation (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, L215, 4–8. [Google Scholar]
- European Union. Commission regulation (EU) No 1327/2014 of 12 December 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of polycyclic aromatic hydrocarbons (PAHs) in traditionally smoked meat and meat products and traditionally smoked fish and fishery products. Off. J. Eur. Union 2014, L358, 13–14. [Google Scholar]
- European Union. Commission regulation (EU) No 1255/2020 of 7 September 2020 amending Regulation (EC) No 1881/2006 as regards maximum levels of polycyclic aromatic hydrocarbons (PAHs) in traditionally smoked meat and smoked meat products and traditionally smoked fish and smoked fishery products and establishing a maximum level of PAHs in powders of food of plant origin used for the preparation of beverages. Off. J. Eur. Union 2020, L293, 1–4. [Google Scholar]
- European Union. Commission regulation (EU) No 915/2023 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 2006/1881. Off. J. Eur. Union 2023, L119, 109–148. [Google Scholar]
- Velázquez, R.; Hernández, A.; Martín, A.; Aranda, E.; Gallardo, G.; Bartolomé, T.; Córdoba, M.G. Quality assessment of commercial paprikas. Int. J. Food Sci. Technol. 2014, 49, 830–839. [Google Scholar] [CrossRef]
- Bartolomé, T.; Coleto, J.M.; Velázquez, R. Pimentón de la Vera: Un caso paradigmático de la Denominación de Origen Protegida. In A Qualidade Numa Perspectiva Multi e Interdisciplinary; Lucas, M.R., Saraiva, M., Rosa, A., Eds.; Ediçoes Sílabo, Lda.: Lisboa, Portugal, 2011; pp. 117–125. [Google Scholar]
- Bartolomé, T.; Coleto, J.M.; Velázquez, R. Historias de las plantas II: La historia del pimiento. In La Agricultura y la Ganadería Extremeñas; Fundación CB: Badajoz, Spain, 2016; pp. 241–254. [Google Scholar]
- Purcaro, G.; Moret, S.; Conte, L.S. Overview on polycyclic aromatic hydrocarbons: Occurrence, legislation and innovative determination in foods. Talanta 2013, 105, 292–305. [Google Scholar] [CrossRef]
- Monago-Maraña, O.; Galeano-Díaz, T.; Muñoz de la Peña, A. Chemometric Discrimination Between Smoked and Non-Smoked Paprika Samples. Quantification of PAHs in Smoked Paprika by Fluorescence-U-PLS/RBL. Food Anal. Methods 2017, 10, 1128–1137. [Google Scholar] [CrossRef]
- European Union. Commission regulation (EU) No 2015/1933 of 27 October 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in cocoa fibre, banana chips, food supplements, dried herbs and dried spices. Off. J. Eur. Union 2015, L282, 11–13. [Google Scholar]
- ISO 1442:2023; Meat and Meat Products. Determination of Moisture Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 1444.1996; Meat and Meat Products. Determination of Free Fat Content. International Organization for Standardization: Geneva, Switzerland, 1996.
- ISO 937.2023; Meat and Meat Products. Determination of Nitrogen Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2023.
- Zhao, L. Determination of 14 Polycyclic Aromatic Hidrocarbon Compounds in Edible Oil. Food Testing and Agriculture. Application Note. 2019. Available online: https://www.agilent.com/cs/library/applications/application-pah-oil-captiva-emr-lipid-5994-1483en-agilent.pdf (accessed on 26 September 2024).
- Zhao, L.; Wong, D. Determination of 19 Polycyclic Aromatic Hydrocarbon Compounds in Salmon and Beef. Using Captiva EMR-Lipid Cleanup by GC/MS/MS. Food Testing and Agriculture. Application Note. 2019. Available online: https://www.agilent.com/cs/library/applications/application-pahs-salmon-beef-5994-0553en-agilent.pdf (accessed on 26 September 2024).
- European Union. Commission regulation (EU) No. 836/2011 of 19 August 2011 amending Regulation (EC) No. 333/2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo(a)pyrene in foodstuffs. Off. J. Eur. Union 2011, L215, 9–16. [Google Scholar]
- Thompson, M. Recent Trends in Inter-Laboratory Precision at Ppb and Sub-Ppb Concentrations in Relation to Fitness for Purpose Criteria in Proficiency Testing. Analyst 2000, 125, 385–386. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Michinel, M.; López, M.; Carballo, J. Biochemical Characteristics of Two Spanish Traditional Dry-cured Sausage Varieties: Androlla and Botillo. J. Food Compos. Anal. 2000, 13, 809–817. [Google Scholar] [CrossRef]
- Santamaría, I.; Lizarraga, T.; Astiasarán, I.; Bello, J. Contribución a la tipificación del chorizo de Pamplona. Estudio físicoquímico y sensorial. Rev. Esp. Cienc. Tecnol. Aliment. 1992, 32, 431–445. [Google Scholar]
- León, F.; Millán, R.; Serrano, A. Características del salchichón comercial tipo “casero”. Alimentaria 1978, 98, 29–34. [Google Scholar]
- Gomes, A.; Santos, C.; Almeida, J.; Elias, M.; Roseiro, L.C. Effect of fat content, casing type and smoking procedures on PAHs contents of Portuguese traditional dry fermented sausages. Food Chem. Toxicol. 2013, 58, 369–374. [Google Scholar] [CrossRef]
- Wang, Z.; Ng, K.; Warner, R.D.; Stockmann, R.; Fang, Z. Reduction strategies for polycyclic aromatic hydrocarbons in processed foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1598–1626. [Google Scholar] [CrossRef]
- Pöhlmann, M.; Hitzel, A.; Schwägele, F.; Speer, K.; Jira, W. Polycyclic aromatic hydrocarbons (PAH) and phenolic substances in smoked Frankfurter-type sausages depending on type of casing and fat content. Food Control 2013, 31, 136–144. [Google Scholar] [CrossRef]
- Waszkiewicz-Robak, B.; Szterk, A.; Rogalski, M.; Kruk, M.; Rokowska, E.; Zarodkiewicz, M.; Mikiciuk, J. Wpływ procesu wędzenia wyrobów wieprzowych otrzymanych z mięsa o różnej jakości początkowej na zawartość wielopierścieniowych węglowodorów aromatycznych. Żywność Nauka Technol. Jakość 2014, 2, 73–92. (In Polish) [Google Scholar] [CrossRef]
- Ciecierska, M.; Obiedzinski, M. Influence of smoking process on polycyclic Aromatic hydrocarbons’ content in meat products. Acta Sci. Pol. Technol. Aliment. 2007, 6, 17–28. [Google Scholar]
- Mastanjevíc, K.; Kartalovíc, B.; Puljíc, L.; Kovačevíc, D.; Habschied, K. Influence of Dierent Smoking Procedures on Polycyclic Aromatic Hydrocarbons Formation in Traditional Dry Sausage Hercegovačka kobasica. Processes 2020, 8, 918. [Google Scholar] [CrossRef]
- Migdal, W.; Walczycka, M.; Zając, M.; Tkaczewska, J.; Kulawik, P.; Węsierska, E.; Migdal, L. The chemical composition and quality of traditionally smoked polish regional products, produced from of raw material obtained from native animal breed. J. Hyg. Eng. Des. 2020, 33, 12–21. [Google Scholar]
- Migdal, W.; Marcinčák, S.; Walczycka, M.; Domagała, J.; Pluta-Kubica, A.; Filipczak-Fiutak, M.; Migdal, A.; Migdal, L. Traditional smoking of Wallachian cheeses and sausages in Polish and Slovak parts of the Carpathian Mountains. J. Ethn. Foods 2024, 11, 1. [Google Scholar] [CrossRef]
- Santos, C.; Gomes, A.; Roseiro, L.C. Polycyclic aromatic hydrocarbons incidence in Portuguese traditional smoked meat products. Food Chem. Toxicol. 2011, 49, 2343–2347. [Google Scholar] [CrossRef]
- Fasano, E.; Yebra-Pimentel, I.; Martínez-Carballo, E.; Simal-Gándara, J. Profiling, distribution and levels of carcinogenic polycyclic aromatic hydrocarbons in traditional smoked plant and animal foods. Food Control 2016, 59, 581–590. [Google Scholar] [CrossRef]
- Monago-Maraña, O.; Perez, R.L.; Escandar, G.M.; Muñoz De La Peña, A.; Galeano-Díaz, T. Combination of liquid chromatography with multivariate curve resolution-alternating least-squares (MCR-ALS) in the quantitation of polycyclic aromatic hydrocarbons present in paprika samples. J. Agric. Food Chem. 2016, 64, 8254–8262. [Google Scholar] [CrossRef]
- Ishizaki, A.; Saito, K.; Hanioka, N.; Narimatsu, S.; Kataoka, H. Determination of polycyclic aromatic hydrocarbons in food samples by automated on-line in-tube solid-phase microextraction coupled with high-performance liquid chromatographyfluorescence detection. J. Chromatogr. A 2010, 1217, 5555–5563. [Google Scholar] [CrossRef]
- Rozentale, I.; Lun, A.Y.; Zacs, D.; Bartkevics, V. The occurrence of polycyclic aromatic hydrocarbons in dried herbs and spices. Food Control 2018, 83, 45–53. [Google Scholar] [CrossRef]
- Hwang, M.J.; Kang, S.J.; Kim, H.S.; Lee, K.W. Reduction of the polycyclic aromatic hydrocarbon levels in dried red peppers (Capsicum annuum L.) using heat pump assisted drying. Food Chem. 2019, 297, 124977. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Topuz, A.; Feng, H.; Kushad, M. The effect of drying method and storage on color characteristics of paprika. LWT Food Sci. Technol. 2009, 42, 1667–1673. [Google Scholar] [CrossRef]
- Tormo, M.A.; Campillo, J.E.; Viña, J.; Gómez-Encinas, J.; Borrás, C.; Torres, M.D.; Campillo, C. The mechanism of the antioxidant effect of smoked paprika from La Vera, Spain. Mecanismo del efecto antioxidante del pimentón ahumado de La Vera, España. CyTA-J. Food 2013, 11, 114–118. [Google Scholar] [CrossRef][Green Version]
- Lingbeck, J.M.; Cordero, P.; O’Bryan, C.A.; Johnson, M.G.; Ricke, S.C.; Crandall, P.G. Functionality of liquid smoke as an all-natural antimicrobial in food preservation. Meat Sci. 2014, 97, 197–206. [Google Scholar] [CrossRef]
- Rosa, A.; Deiana, M.; Casu, V.; Paccagnini, S.; Appendin, G.; Ballero, M.; Dessí, M.A. Antioxidant activity of capsinoids. J. Agric. Food Chem. 2002, 50, 7396–7401. [Google Scholar] [CrossRef]
- Coleto, J.M.; Martín, A.; Horrillo, A.; Mesías, F.J.; Velázquez, R. An Approach to the Consumption of Smoked Paprika in Spain and Its Impact on the Intake of Polycyclic Aromatic Hydrocarbons. Foods 2021, 10, 973. [Google Scholar] [CrossRef]

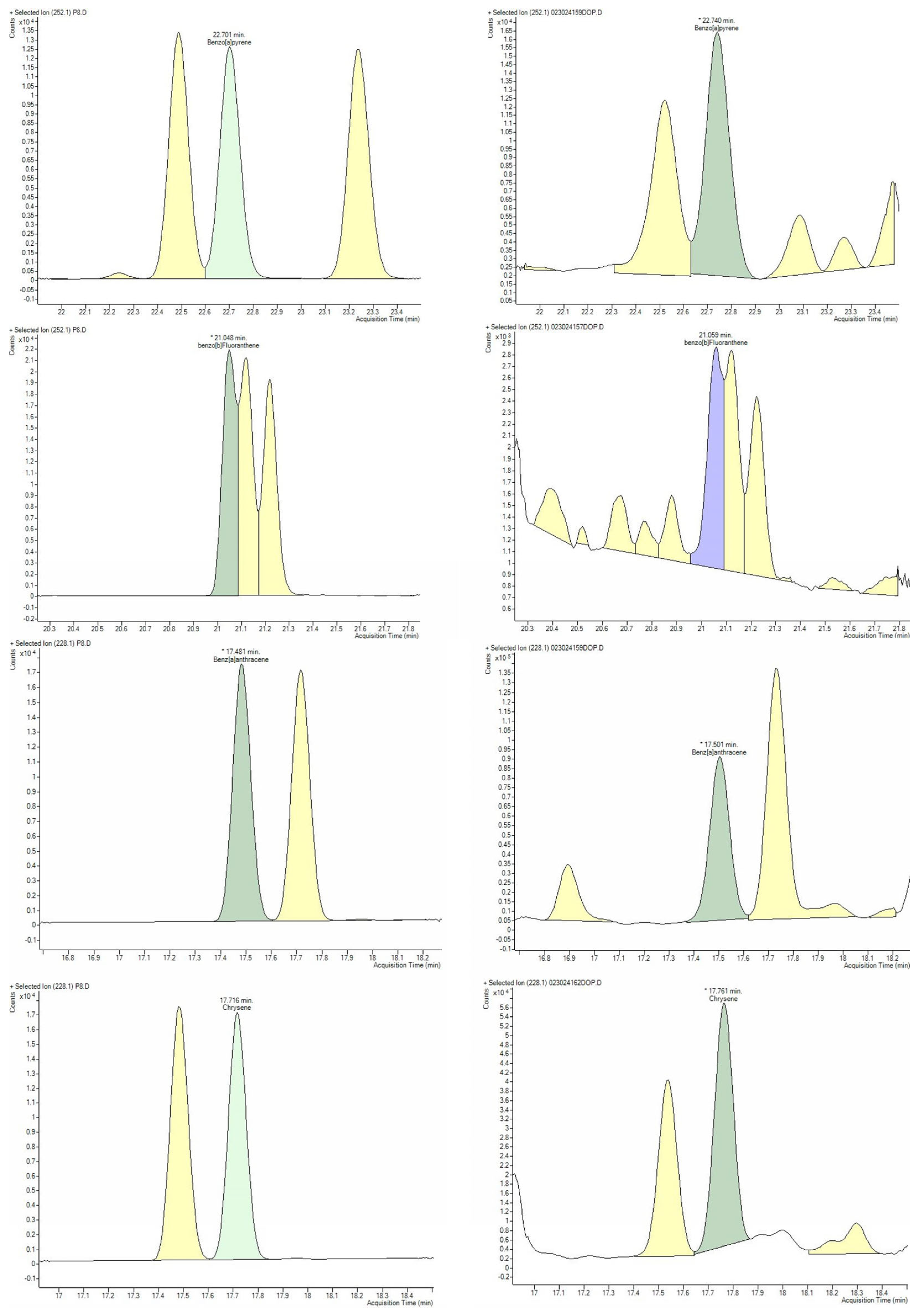
| Benz[a]anthracene | Chrysene | Benzo[b]fluoranthene | Benzo[a]pyrene | |
|---|---|---|---|---|
| Average % RSDReproducibility | 10.2 | 9.8 | 11.5 | 9.6 |
| Recovery % Range | 75–104 | 83–97 | 85–103 | 65–104 |
| LOQ µg/kg | 0.9 | 0.9 | 0.9 | 0.9 |
| LOD µg/kg | 0.31 | 0.32 | 0.38 | 0.27 |
| % Uncertainty | 20.6 | 20 | 22.8 | 19.4 |
| Weight (kg) | Moisture (g/100 g) | Dry Matter (g/100 g) | Protein (%DM) 1 | Fat Content (%DM) |
|---|---|---|---|---|
| 1.22 ± 0.22 2 | 57.39 ± 5.63 | 42.61 ± 5.63 | 41.53 ± 3.37 | 44.84 ± 5.19 |
| Botillo | Paprika | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Company | BaP | BaA | BbF | Chr | ∑PAHs4 | Company | BaP | BaA | BbF | Chr | ∑PHA4 |
| 1 | 1.99 a * ± 1.85 | 7.23 a ± 6.94 | 2.20 a ± 2.14 | 11.05 a ± 7.31 | 22.46 a ± 18.05 | 1 | 62.86 a ± 39.44 | 191.27 ab ± 85.21 | 68.66 a ± 44.60 | 291.55 ab ± 111.44 | 614.33 ab ± 275.65 |
| 2 | NDab | 0.90 bc ± 1.21 | NDab | 3.00 bcd ± 1.70 | 4.43 bcd ± 3.77 | 2 | 13.00 a ± 12.02 | 12.15 b ± 1.20 | 5.65 a ± 4.03 | 20.65 b ± 5.16 | 51.45 b ± 9.69 |
| 3 | 1.03 ab ± 0.94 | 4.67 abc ± 1.51 | 1.66 ab ± 1.27 | 7.86 abcd ± 2.26 | 15.23 ab ± 4.45 | 3 | 74.57 a ± 48.35 | 193.27 ab ± 63.76 | 65.94 a ± 31.57 | 306.81 ab ± 111.88 | 640.59 ab ± 206.31 |
| 4 | 1.45 ab ± 1.41 | 5.50 ab ± 3.58 | 1.87 ab ± 1.34 | 9.16 ab ± 6.02 | 17.98 ab ± 11.50 | 4 | 72.97 a ± 54.59 | 253.25 a ± 210.61 | 75.94 a ± 68.97 | 400.15 a ± 329.24 | 802.32 a ± 648.72 |
| 5 | 1.12 ab ± 1.29 | 4.93 abc ± 3.16 | 1.63 ab ± 1.30 | 8.01 abcd ± 4.27 | 15.69 ab ± 8.62 | 5 | 99.45 a ± 124.82 | 250.10 a ± 205.92 | 84.09 a ± 82.73 | 354.97 a ± 256.26 | 788.61 a ± 665.44 |
| 6 | 1.61 ab ± 1.55 | 4.64 abc ± 3.16 | 1.75 ab ± 1.46 | 8.42 abc ± 5.86 | 16.43 ab ± 9.53 | 6 | 64.09 a ± 46.34 | 154.43 ab ± 93.09 | 57.67 a ± 42.69 | 250.36 ab ± 145.02 | 526.56 ab ± 324.49 |
| 7 | ND ab | ND c | ND b | 1.83 cd ± 1.34 | 2.49 cd ± 1.38 | 7 | 67.33 a ± 37.37 | 151.93 ab ± 69.56 | 56.42 a ± 37.69 | 241.32 ab ± 117.61 | 517.00 ab ± 236.46 |
| 8 | ND b | 0.91 bc ± 0.99 | ND ab | 1.79 cd ± 1.50 | 3.01 cd ± 2.40 | 8 | 51.33 a ± 41.06 | 79.44 ab ± 93.68 | 28.67 a ± 29.32 | 106.91 ab ± 128.68 | 266.36 ab ± 265.83 |
| 9 | ND ab | 2.31 bc ± 2.29 | 1.00 ab ± 1.22 | 3.55 abcd ± 3.19 | 7.58 bcd ± 7.47 | ||||||
| 10 | ND b | ND c | ND b | 1.35 d ± 0.21 | 1.80 d ± 0.85 | ||||||
| Years | Years | ||||||||||
| 2019 | ND a | 2.57 b ± 2.04 | ND b | 4.57 b ± 2.90 | 8.69 b ± 6.70 | 2021 | 68.06 a ± 47.47 | 190.22 ab ± 134.27 | 66.03 ab ± 46.94 | 306.49 ab ± 213.11 | 630.80 ab ± 429.52 |
| 2020 | 0.92 a ± 1.33 | 3.21 b ± 2.82 | 1.10 b ± 1.05 | 6.12 ab ± 4.26 | 11.34 ab ± 8.95 | 2022 | 88.21 a ± 98.94 | 250.06 a ± 206.91 | 80.62 a ± 75.55 | 378.14 a ± 290.43 | 797.03 a ± 658.93 |
| 2021 | 1.12 a ± 1.38 | 3.91 ab ± 3.68 | 1.20 b ± 1.55 | 8.17 a ± 6.10 | 14.41 ab ± 12.36 | 2023 | 63.65 a ± 32.96 | 202.17 ab ± 146.77 | 74.20 a ± 49.46 | 302.52 ab ± 230.41 | 642.54 ab ± 437.81 |
| 2022 | 1.23 a ± 1.60 | 5.68 a ± 5.93 | 2.12 a ± 1.97 | 6.83 ab ± 7.38 | 15.81 a ± 16.32 | 2024 | 59.09 a ± 47.11 | 110.68 b ± 93.81 | 30.04 b ± 30.88 | 178.57 b ± 158.26 | 378.38 b ± 307.86 |
| Paprika | |||||
|---|---|---|---|---|---|
| Type of Paprika Processing | BaP | BaA | BbF | Chr | ∑PAH4 |
| Smoked | 83.17 a * ± 68.51 | 253.22 a ± 163.30 | 83.84 a ± 59.06 | 390.99 a ± 239.74 | 811.22 a ± 508.95 |
| Low-smoked | 68.01 ab ± 42.73 | 136.16 ab ± 89.01 | 47.67 ab ± 35.95 | 206.67 ab ± 143.79 | 458.51 ab ± 278.98 |
| Mix ** | 26.90 ab ± 14.43 | 64.12 b ± 37.24 | 17.62 ab ± 16.25 | 99.36 bc ± 57.27 | 208.00 bc ± 113.25 |
| Unsmoked | 17.63 b ± 15.02 | 3.92 b ± 3.62 | 4.72 b ± 3.95 | 6.71 c ± 5.56 | 32.99 c ± 18.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuertes-Martínez, J.R.; Guerra, M.; Rodríguez-González, Á.; del Valle-Herrero, H.; Valenciano, J.B.; Marcelo, V. Influence of Smoking and Paprika Spice on the Content of Polycyclic Aromatic Hydrocarbons (PAHs) in the Traditional Spanish Smoked Sausage ‘Botillo del Bierzo’. Foods 2024, 13, 4089. https://doi.org/10.3390/foods13244089
Fuertes-Martínez JR, Guerra M, Rodríguez-González Á, del Valle-Herrero H, Valenciano JB, Marcelo V. Influence of Smoking and Paprika Spice on the Content of Polycyclic Aromatic Hydrocarbons (PAHs) in the Traditional Spanish Smoked Sausage ‘Botillo del Bierzo’. Foods. 2024; 13(24):4089. https://doi.org/10.3390/foods13244089
Chicago/Turabian StyleFuertes-Martínez, Jaime R., Marcos Guerra, Álvaro Rodríguez-González, Héctor del Valle-Herrero, José B. Valenciano, and Víctor Marcelo. 2024. "Influence of Smoking and Paprika Spice on the Content of Polycyclic Aromatic Hydrocarbons (PAHs) in the Traditional Spanish Smoked Sausage ‘Botillo del Bierzo’" Foods 13, no. 24: 4089. https://doi.org/10.3390/foods13244089
APA StyleFuertes-Martínez, J. R., Guerra, M., Rodríguez-González, Á., del Valle-Herrero, H., Valenciano, J. B., & Marcelo, V. (2024). Influence of Smoking and Paprika Spice on the Content of Polycyclic Aromatic Hydrocarbons (PAHs) in the Traditional Spanish Smoked Sausage ‘Botillo del Bierzo’. Foods, 13(24), 4089. https://doi.org/10.3390/foods13244089






