Combining Metal(loid) and Secondary Metabolite Levels in Olea europaea L. Samples for Geographical Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
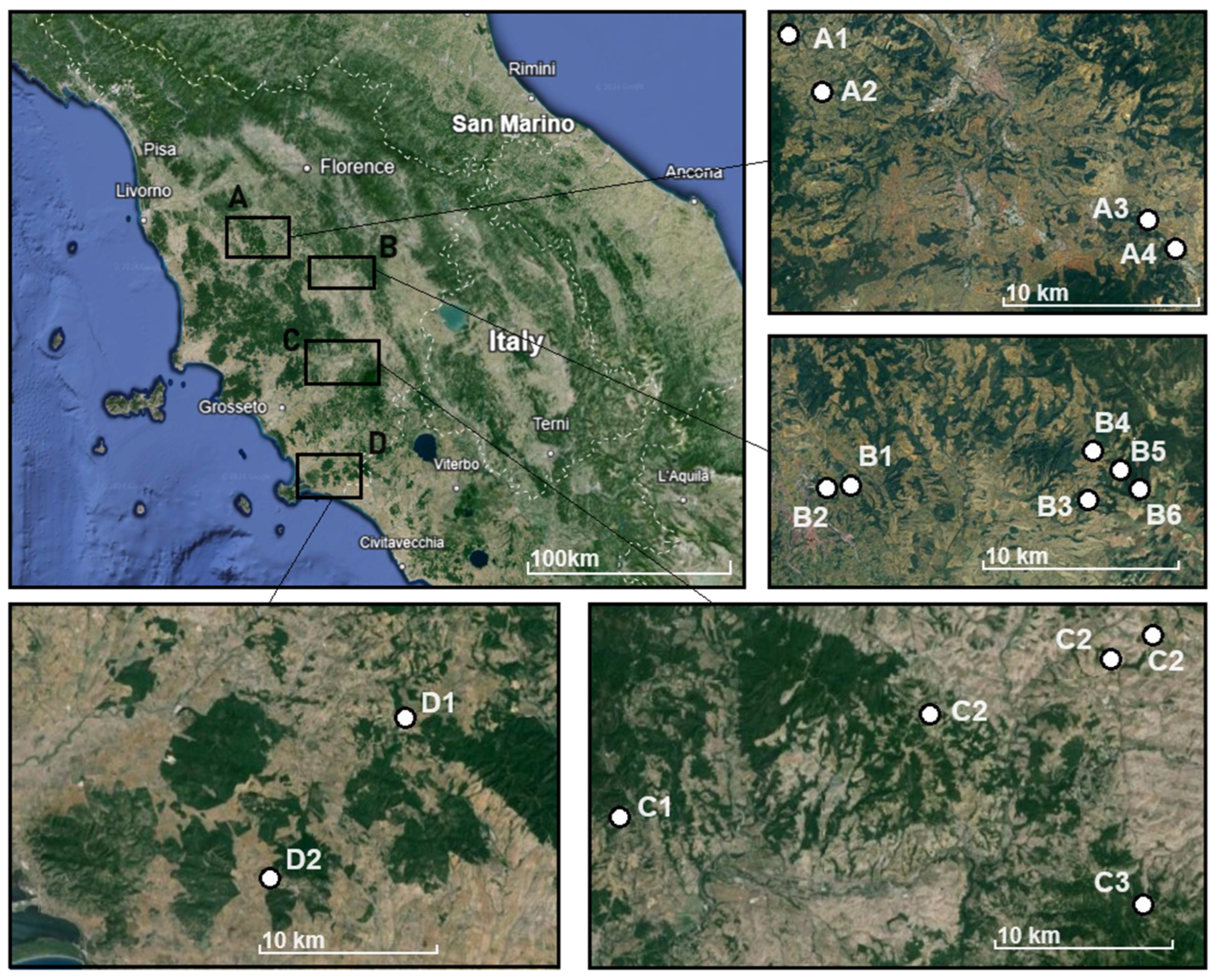
2.2. Sampling Procedure
2.3. Sample Treatment—Metabolites Extraction
2.4. Sample Treatment—Acid Digestion
2.5. Spectrophotometric Measurements
2.6. HPLC-DAD Metabolites Analysis
2.7. ICP-MS Metal(loid)s Analysis
| Plasma | |
|---|---|
| Plasma Mode | HMI |
| RF Power | 1600 W |
| RF Matching | 1.80 V |
| Nebulizer Gas | 0.68 L/min |
| Makeup/Dilution Gas | 0.27 L/min |
| Plasma Gas | 15.0 L/min |
| Auxiliary Gas | 0.9 L/min |
| Pre Run | |
|---|---|
| Uptake speed (Nebulizer Pump) | 0.3 RPS |
| Uptake time | 30 s |
| Stabilize | 40 s |
| Post Run | |
| Rinse speed (Nebulizer Pump) | 0.3 RPS |
| Rinse time (2% HNO3, 0.5% HCl) | 10 s |
| Rinse time (1% HNO3) | 30 s |
| Uptake speed (Nebulizer Pump) | 0.3 RPS |
| Uptake time | 30 s |
| Stabilize | 40 s |
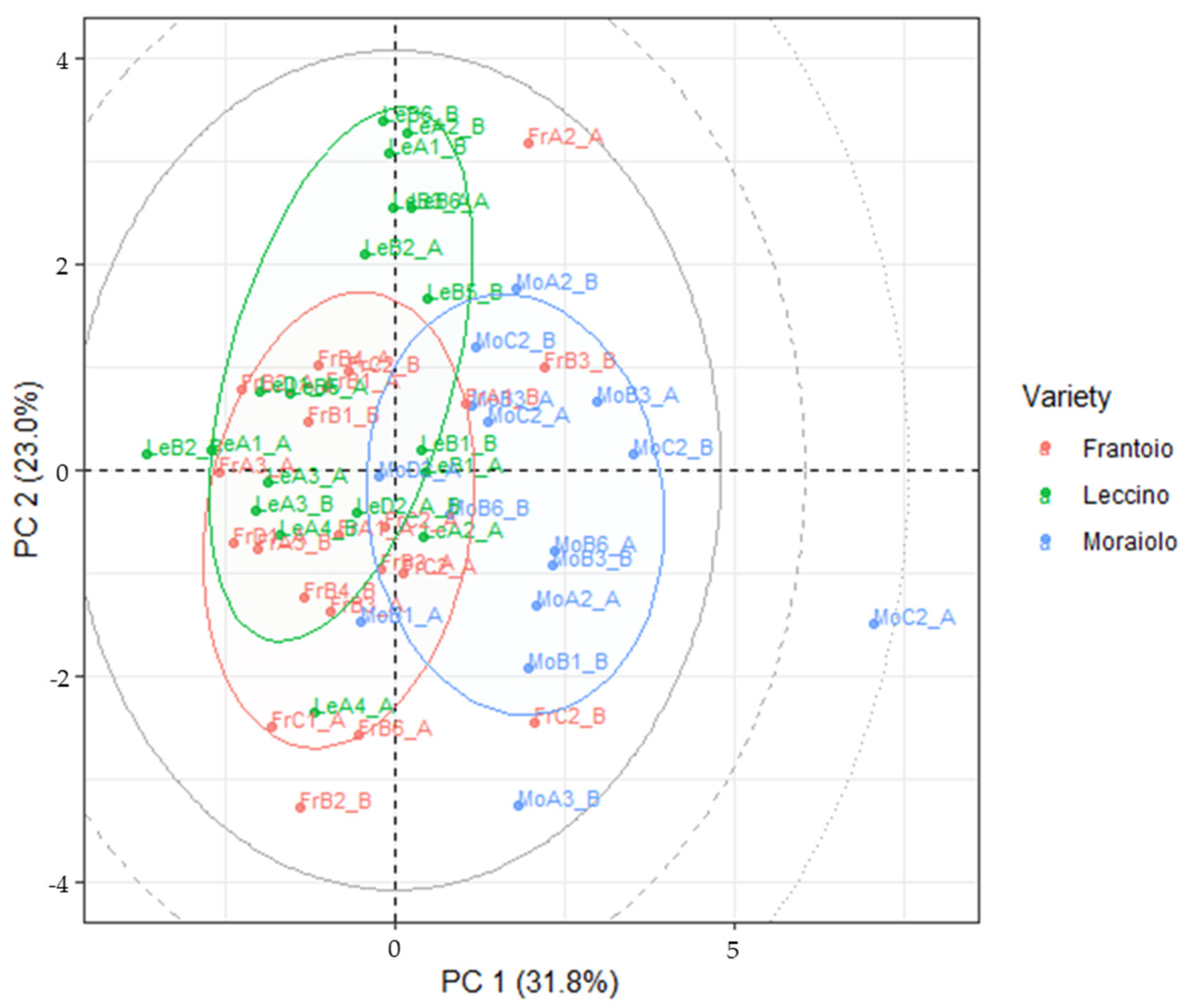
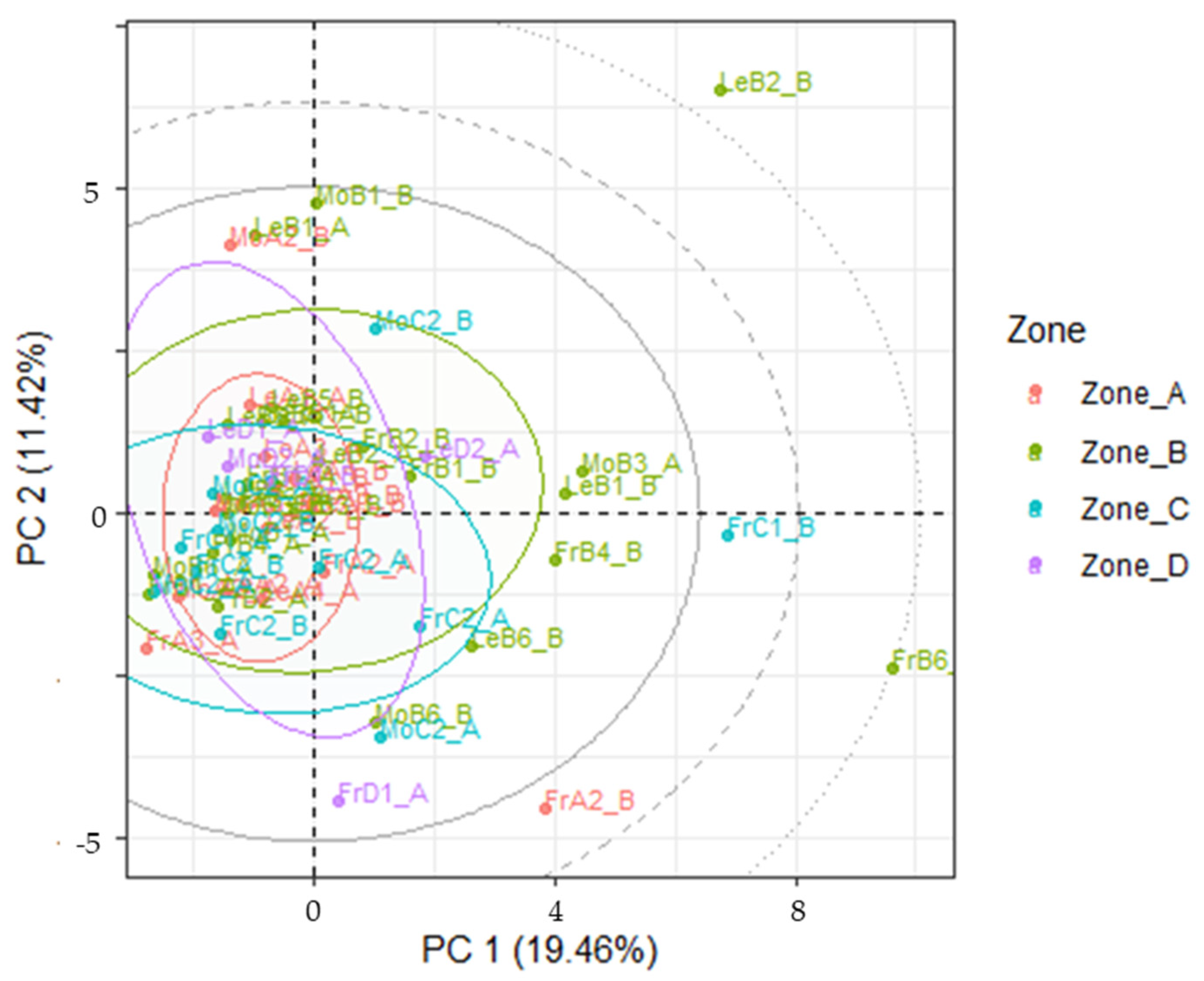
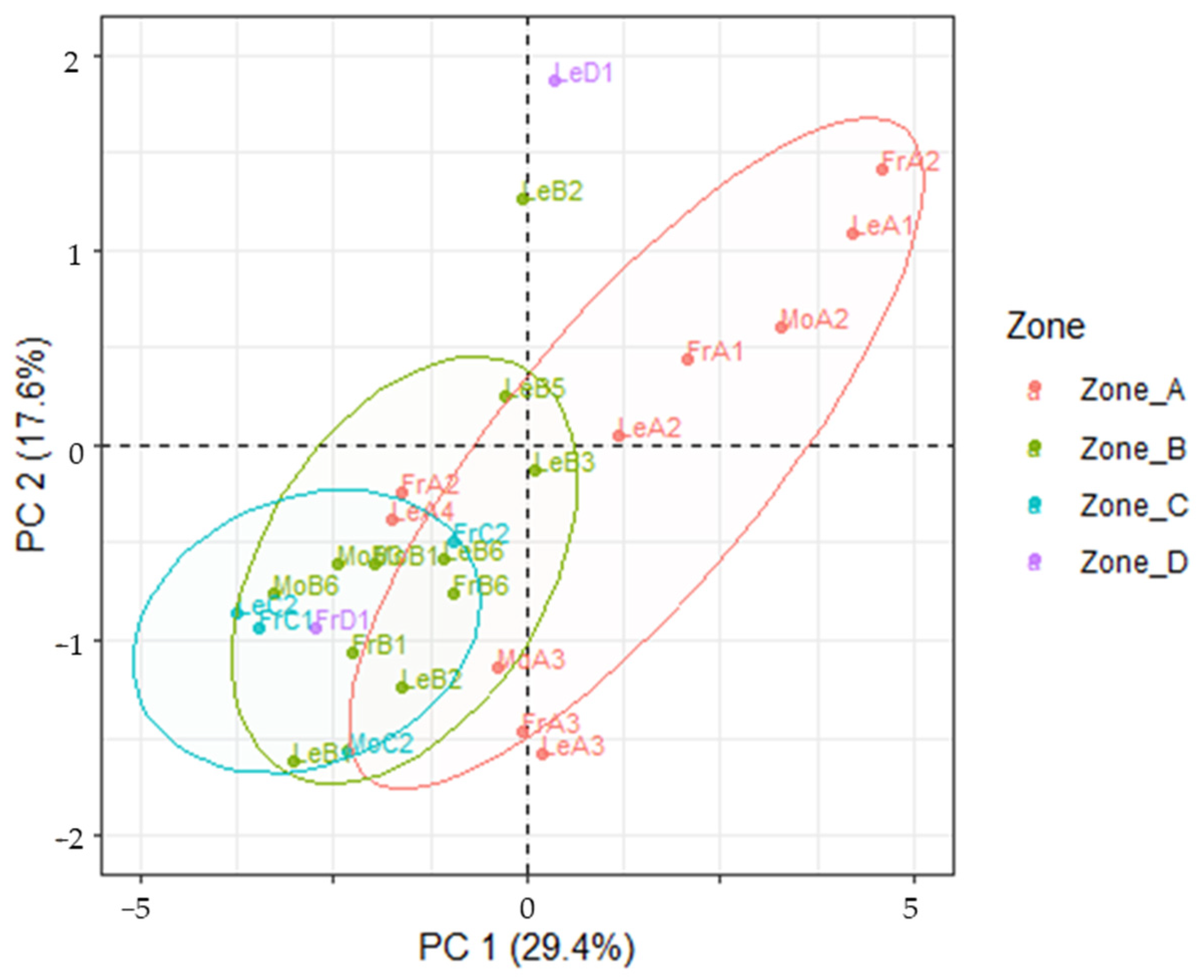
2.8. Multivariate Analysis
3. Results
3.1. Soil Analysis
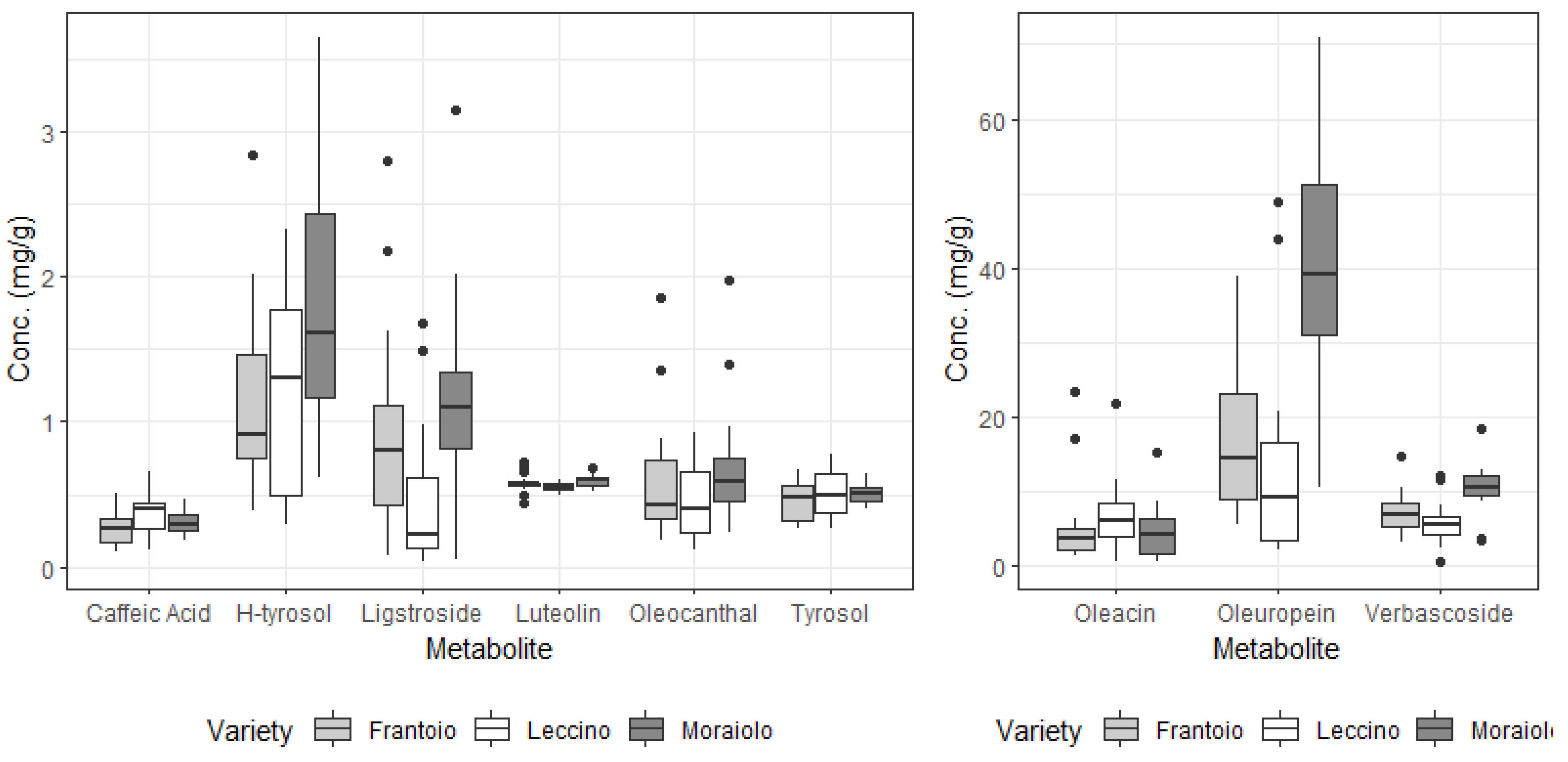
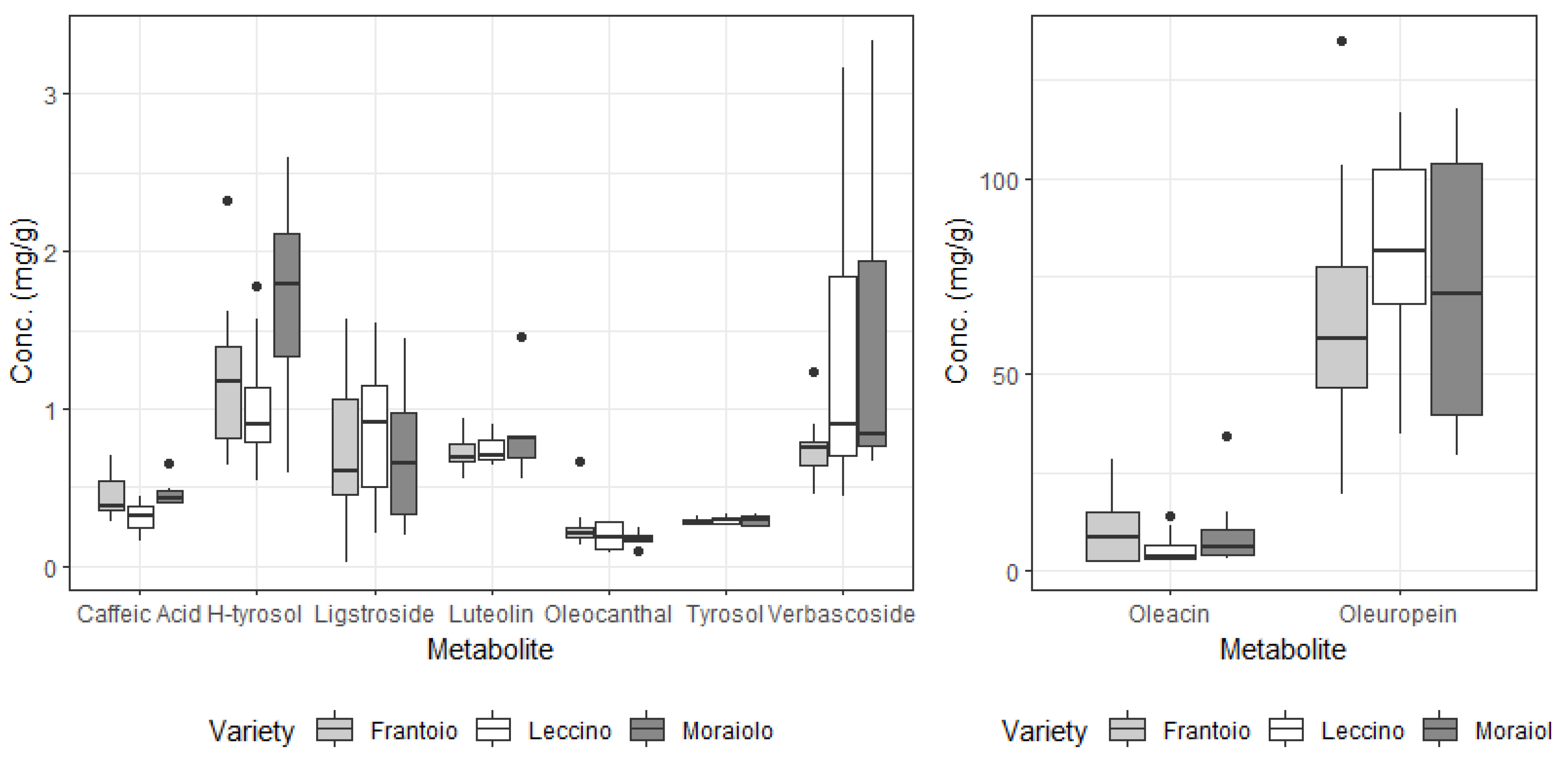
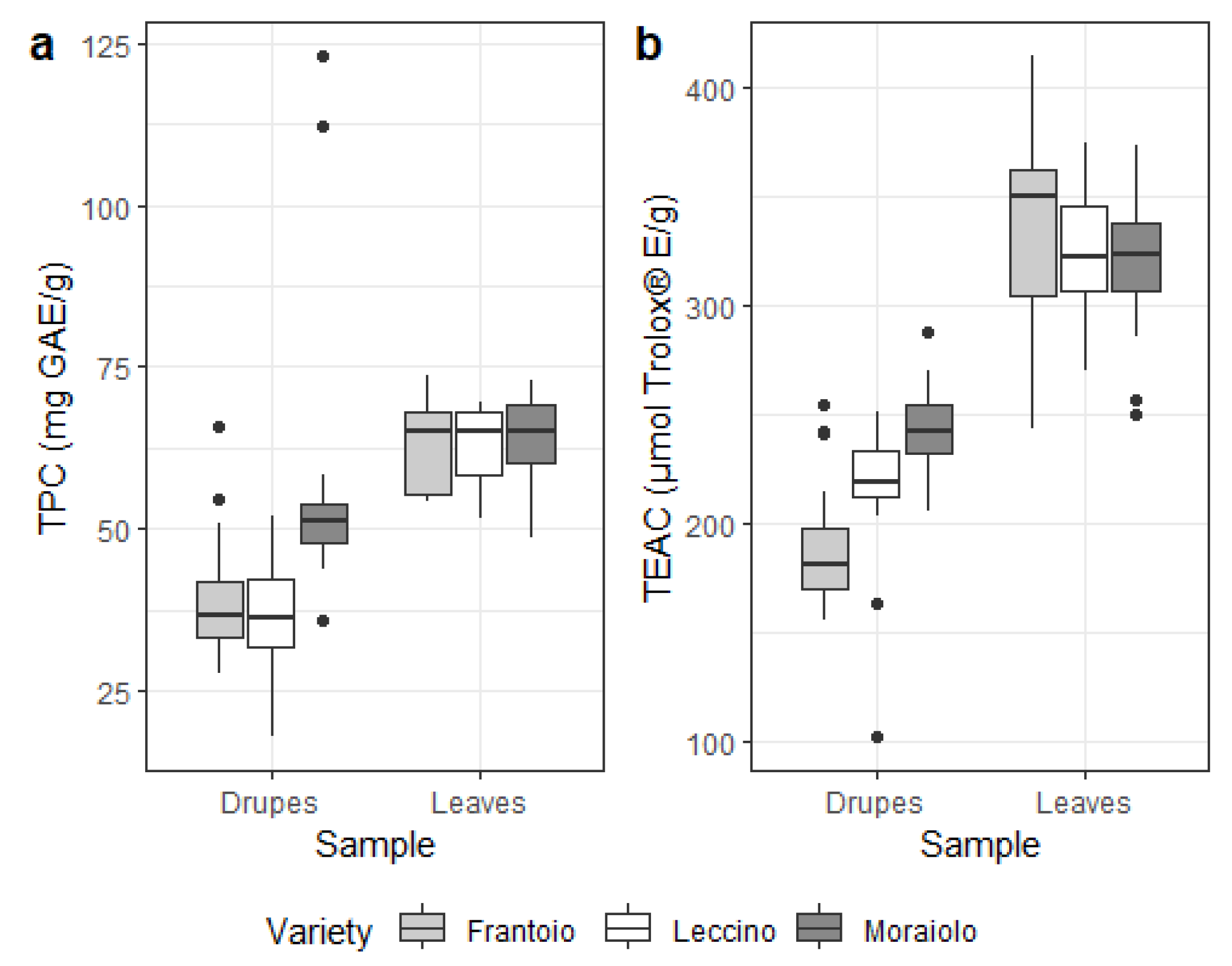
3.2. Secondary Metabolites in Drupes and Leaves
3.3. Mineral Content in Drupes and Leaves
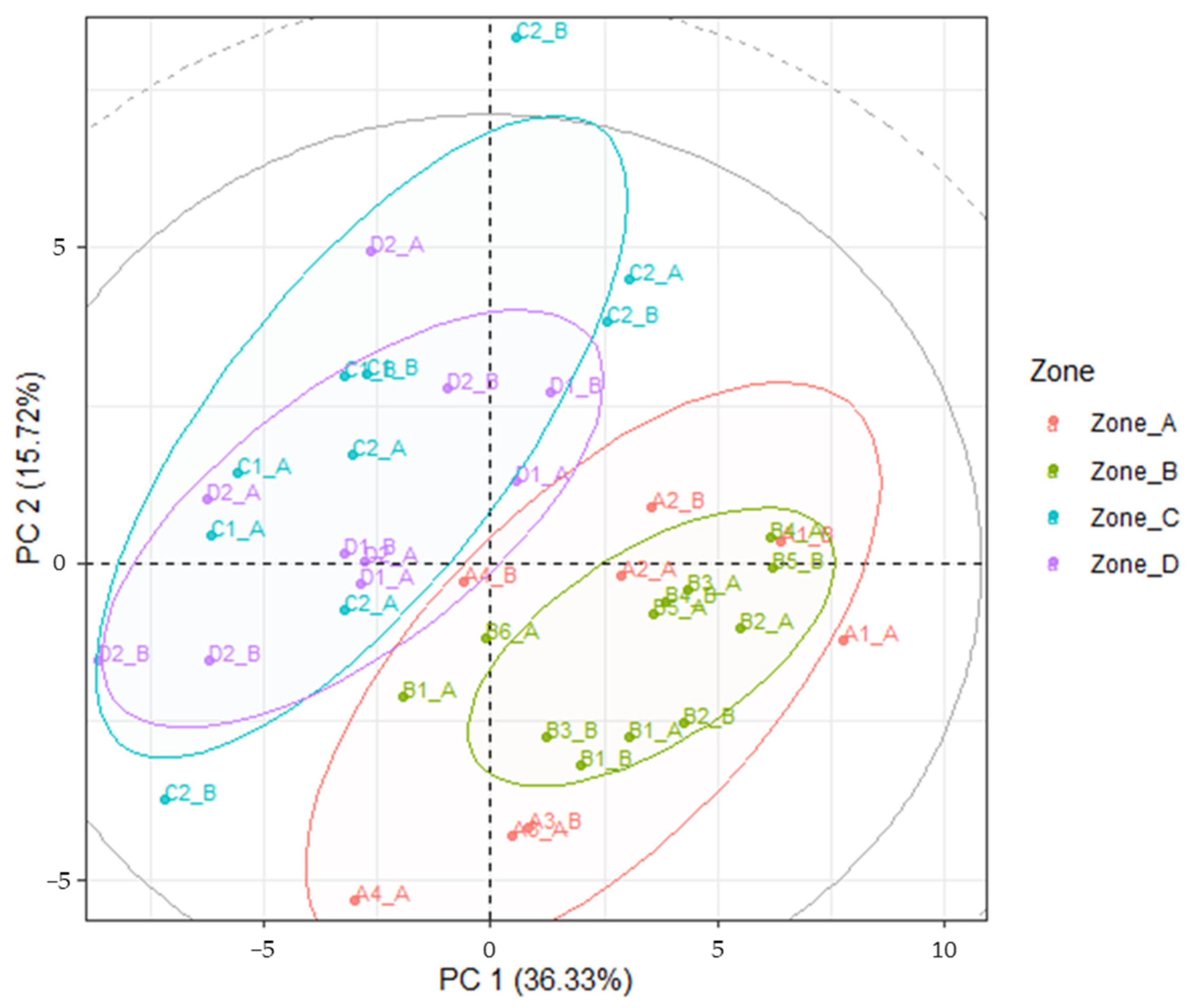
3.4. LDA Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liphschitz, N.; Gophna, R.; Hartman, M.; Biger, G. The Beginning of Olive (Olea europaea) Cultivation in the Old World: A Reassessment. J. Archaeol. Sci. 1991, 18, 441–453. [Google Scholar] [CrossRef]
- Trichopoulou, A. Mediterranean Diet as Intangible Heritage of Humanity: 10 Years On. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1943–1948. [Google Scholar] [CrossRef]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The Effect of Polyphenols in Olive Oil on Heart Disease Risk Factors. Ann. Intern. Med. 2006, 145, 333. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Haris Omar, S. Oleuropein in Olive and Its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, J.; Xin, Q.; Yuan, R.; Miao, Y.; Yang, M.; Mo, H.; Chen, K.; Cong, W. Protective Effects of Oleic Acid and Polyphenols in Extra Virgin Olive Oil on Cardiovascular Diseases. Food Sci. Hum. Wellness 2024, 13, 529–540. [Google Scholar] [CrossRef]
- Amiot, M.-J.; Fleuriet, A.; Macheix, J.-J. Accumulation of Oleuropein Derivatives during Olive Maturation. Phytochemistry 1989, 28, 67–69. [Google Scholar] [CrossRef]
- Di Vaio, C.; Graziani, G.; Gaspari, A.; De Luca, L.; Aiello, A.; Cirillo, A.; Bruno, A.; Romano, R.; Ritieni, A. Drupe Characters, Fatty Acids, Polyphenolic and Aromatic Profile of Olive Oil Obtained from “Oliva Bianca”, Minor Autochthonous Cultivar of Campania. Plants 2021, 10, 1119. [Google Scholar] [CrossRef]
- Visioli, F.; Caruso, D.; Galli, C.; Viappiani, S.; Galli, G.; Sala, A. Olive Oils Rich in Natural Catecholic Phenols Decrease Isoprostane Excretion in Humans. Biochem. Biophys. Res. Commun. 2000, 278, 797–799. [Google Scholar] [CrossRef]
- Casadei, E.; Valli, E.; Panni, F.; Donarski, J.; Farrús Gubern, J.; Lucci, P.; Conte, L.; Lacoste, F.; Maquet, A.; Brereton, P.; et al. Emerging Trends in Olive Oil Fraud and Possible Countermeasures. Food Control 2021, 124, 107902. [Google Scholar] [CrossRef]
- Ghisoni, S.; Lucini, L.; Angilletta, F.; Rocchetti, G.; Farinelli, D.; Tombesi, S.; Trevisan, M. Discrimination of Extra-Virgin-Olive Oils from Different Cultivars and Geographical Origins by Untargeted Metabolomics. Food Res. Int. 2019, 121, 746–753. [Google Scholar] [CrossRef]
- Mohamed, M.B.; Rocchetti, G.; Montesano, D.; Ali, S.B.; Guasmi, F.; Grati-Kamoun, N.; Lucini, L. Discrimination of Tunisian and Italian Extra-Virgin Olive Oils According to Their Phenolic and Sterolic Fingerprints. Food Res. Int. 2018, 106, 920–927. [Google Scholar] [CrossRef]
- Geana, E.I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Stefanescu, I.; Ionete, R.E.; Bala, C. Verifying the Red Wines Adulteration through Isotopic and Chromatographic Investigations Coupled with Multivariate Statistic Interpretation of the Data. Food Control 2016, 62, 1–9. [Google Scholar] [CrossRef]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.-L. From Olive Drupes to Olive Oil. An HPLC-Orbitrap-Based Qualitative and Quantitative Exploration of Olive Key Metabolites. Planta Med. 2013, 79, 1576–1587. [Google Scholar] [CrossRef]
- Olmo-García, L.; Kessler, N.; Neuweger, H.; Wendt, K.; Olmo-Peinado, J.; Fernández-Gutiérrez, A.; Baessmann, C.; Carrasco-Pancorbo, A. Unravelling the Distribution of Secondary Metabolites in Olea europaea L.: Exhaustive Characterization of Eight Olive-Tree Derived Matrices by Complementary Platforms (LC-ESI/APCI-MS and GC-APCI-MS). Molecules 2018, 23, 2419. [Google Scholar] [CrossRef]
- Di Donna, L.; Mazzotti, F.; Naccarato, A.; Salerno, R.; Tagarelli, A.; Taverna, D.; Sindona, G. Secondary Metabolites of Olea Europaea Leaves as Markers for the Discrimination of Cultivars and Cultivation Zones by Multivariate Analysis. Food Chem. 2010, 121, 492–496. [Google Scholar] [CrossRef]
- Geana, E.-I.; Ciucure, C.T.; Apetrei, I.M.; Clodoveo, M.L.; Apetrei, C. Discrimination of Olive Oil and Extra-Virgin Olive Oil from Other Vegetable Oils by Targeted and Untargeted HRMS Profiling of Phenolic and Triterpenic Compounds Combined with Chemometrics. Int. J. Mol. Sci. 2023, 24, 5292. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Wildung, R.E. Soil and Plant Factors Influencing the Accumulation of Heavy Metals by Plants. Environ. Health Perspect. 1978, 27, 149–159. [Google Scholar] [CrossRef]
- Jan, S.; Parray, J.A. Heavy Metal Uptake in Plants. In Approaches to Heavy Metal Tolerance in Plants; Springer: Singapore, 2016; pp. 1–18. [Google Scholar]
- Wieczorek, J.; Baran, A.; Bubak, A. Mobility, Bioaccumulation in Plants, and Risk Assessment of Metals in Soils. Sci. Total Environ. 2023, 882, 163574. [Google Scholar] [CrossRef]
- Borst, A.M.; Smith, M.P.; Finch, A.A.; Estrade, G.; Villanova-de-Benavent, C.; Nason, P.; Marquis, E.; Horsburgh, N.J.; Goodenough, K.M.; Xu, C.; et al. Adsorption of Rare Earth Elements in Regolith-Hosted Clay Deposits. Nat. Commun. 2020, 11, 4386. [Google Scholar] [CrossRef]
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The Story of Rare Earth Elements (REEs): Occurrences, Global Distribution, Genesis, Geology, Mineralogy and Global Production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Nanos, N.; Rodríguez Martín, J.A. Multiscale Analysis of Heavy Metal Contents in Soils: Spatial Variability in the Duero River Basin (Spain). Geoderma 2012, 189–190, 554–562. [Google Scholar] [CrossRef]
- Zeiner, M.; Steffan, I.; Cindric, I.J. Determination of Trace Elements in Olive Oil by ICP-AES and ETA-AAS: A Pilot Study on the Geographical Characterization. Microchem. J. 2005, 81, 171–176. [Google Scholar] [CrossRef]
- Telloli, C.; Tagliavini, S.; Passarini, F.; Salvi, S.; Rizzo, A. ICP-MS Triple Quadrupole as Analytical Technique to Define Trace and Ultra-Trace Fingerprint of Extra Virgin Olive Oil. Food Chem. 2023, 402, 134247. [Google Scholar] [CrossRef]
- Aceto, M.; Bonello, F.; Musso, D.; Tsolakis, C.; Cassino, C.; Osella, D. Wine Traceability with Rare Earth Elements. Beverages 2018, 4, 23. [Google Scholar] [CrossRef]
- Aceto, M.; Calà, E.; Musso, D.; Regalli, N.; Oddone, M. A Preliminary Study on the Authentication and Traceability of Extra Virgin Olive Oil Made from Taggiasca Olives by Means of Trace and Ultra-Trace Elements Distribution. Food Chem. 2019, 298, 125047. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Marconi, E.; Vitiello, G.; Massimi, L. An Optimized Method for Sample Preparation and Elemental Analysis of Extra-Virgin Olive Oil by Inductively Coupled Plasma Mass Spectrometry. Food Chem. 2021, 360, 130027. [Google Scholar] [CrossRef]
- Cajka, T.; Riddellova, K.; Klimankova, E.; Cerna, M.; Pudil, F.; Hajslova, J. Traceability of Olive Oil Based on Volatiles Pattern and Multivariate Analysis. Food Chem. 2010, 121, 282–289. [Google Scholar] [CrossRef]
- Nasr, E.G.; Epova, E.N.; de Diego, A.; Souissi, R.; Hammami, M.; Abderrazak, H.; Donard, O.F.X. Trace Elements Analysis of Tunisian and European Extra Virgin Olive Oils by ICP-MS and Chemometrics for Geographical Discrimination. Foods 2021, 11, 82. [Google Scholar] [CrossRef]
- Pucci, E.; Palumbo, D.; Puiu, A.; Lai, A.; Fiorani, L.; Zoani, C. Characterization and Discrimination of Italian Olive (Olea europaea sativa) Cultivars by Production Area Using Different Analytical Methods Combined with Chemometric Analysis. Foods 2022, 11, 1085. [Google Scholar] [CrossRef]
- Luka, M.F.; Akun, E. Investigation of Trace Metals in Different Varieties of Olive Oils from Northern Cyprus and Their Variation in Accumulation Using ICP-MS and Multivariate Techniques. Environ. Earth Sci. 2019, 78, 578. [Google Scholar] [CrossRef]
- Benincasa, C.; Lewis, J.; Perri, E.; Sindona, G.; Tagarelli, A. Determination of Trace Element in Italian Virgin Olive Oils and Their Characterization According to Geographical Origin by Statistical Analysis. Anal. Chim. Acta 2007, 585, 366–370. [Google Scholar] [CrossRef]
- Nardin, R.; Tamasi, G.; Baglioni, M.; Bisozzi, F.; Consumi, M.; Costa, J.; Fattori, G.; Tozzi, C.; Riccaboni, A.; Rossi, C. Determination of Elemental Content in Vineyard Soil, Leaves, and Grapes of Sangiovese Grapes from the Chianti Region Using ICP-MS for Geographical Identification. ACS Food Sci. Technol. 2024, 4, 2585–2599. [Google Scholar] [CrossRef]
- Borghini, F.; Tamasi, G.; Loiselle, S.A.; Baglioni, M.; Ferrari, S.; Bisozzi, F.; Costantini, S.; Tozzi, C.; Riccaboni, A.; Rossi, C. Phenolic Profiles in Olive Leaves from Different Cultivars in Tuscany and Their Use as a Marker of Varietal and Geographical Origin on a Small Scale. Molecules 2024, 29, 3617. [Google Scholar] [CrossRef]
- Calò, F.; Girelli, C.R.; Wang, S.C.; Fanizzi, F.P. Geographical Origin Assessment of Extra Virgin Olive Oil via NMR and MS Combined with Chemometrics as Analytical Approaches. Foods 2022, 11, 113. [Google Scholar] [CrossRef]
- Carmignani, L.; Conti, P.; Cornamusini, G.; Pirro, A. Geological Map of Tuscany (Italy). J. Maps 2013, 9, 487–497. [Google Scholar] [CrossRef]
- Deng, S.; Yin, Q.; Zhang, S.; Shi, K.; Jia, Z.; Ma, L. Drip Irrigation Affects the Morphology and Distribution of Olive Roots. HortScience 2017, 52, 1298–1306. [Google Scholar] [CrossRef]
- Masmoudi-Charfi, C.; Masmoudi, M.; Ben Mechlia, N. Root Distribution in Young Olive Trees (Olea europaea cv. Chétoui) and Agronomic Application. Adv. Hortic. Sci. 2013, 25, 112–122. [Google Scholar]
- Pequerul, A.; Pérez, C.; Madero, P.; Val, J.; Monge, E. A Rapid Wet Digestion Method for Plant Analysis. In Optimization of Plant Nutrition; Springer: Dordrecht, The Netherlands, 1993; pp. 3–6. [Google Scholar]
- Tamasi, G.; Baratto, M.C.; Bonechi, C.; Byelyakova, A.; Pardini, A.; Donati, A.; Leone, G.; Consumi, M.; Lamponi, S.; Magnani, A.; et al. Chemical Characterization and Antioxidant Properties of Products and By-products from Olea europaea L. Food Sci. Nutr. 2019, 7, 2907–2920. [Google Scholar] [CrossRef]
- R Core Team. R A Language and Environment for Statistical Computing, version 4.4.2.; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the Caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Karabourniotis, G. Boron Deficiency and Concentrations and Composition of Phenolic Compounds in Olea Europaea Leaves: A Combined Growth Chamber and Field Study. Tree Physiol 2005, 25, 307–315. [Google Scholar] [CrossRef]
- Toker, C.; Yavuz, N. The Effect of Boron Application on Chemical Characterization and Volatile Compounds of Virgin Olive Oil of Ayvalik Olive Cultivar. J. Am. Oil Chem. Soc. 2015, 92, 1421–1428. [Google Scholar] [CrossRef]
- Arbonés, A.; Rufat, J.; Pérez, M.A.; Pascual, M.; Benito, A.; De Lorenzo, C.; Villar, J.M.; Sastre, B. The Influence of Olive Tree Fertilization on the Phenols in Virgin Olive Oils. A Review. Grasas Aceites 2022, 73, e470. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Poirier, Y.; Jaskolowski, A.; Clúa, J. Phosphate Acquisition and Metabolism in Plants. Curr. Biol. 2022, 32, R623–R629. [Google Scholar] [CrossRef]
- Tyler, G. Rare Earth Elements in Soil and Plant Systems—A Review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Asare, M.O.; Száková, J.; Tlustoš, P. The Fate of Secondary Metabolites in Plants Growing on Cd-, As-, and Pb-Contaminated Soils—A Comprehensive Review. Environ. Sci. Pollut. Res. 2022, 30, 11378–11398. [Google Scholar] [CrossRef]
- Asiminicesei, D.-M.; Fertu, D.I.; Gavrilescu, M. Impact of Heavy Metal Pollution in the Environment on the Metabolic Profile of Medicinal Plants and Their Therapeutic Potential. Plants 2024, 13, 913. [Google Scholar] [CrossRef] [PubMed]
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional Aspects of Plant Secondary Metabolites in Metal Stress Tolerance and Their Importance in Pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Cavaca, L.A.S.; Lopez-Coca, I.M.; Silvero, G.; Afonso, C.A.M. The Olive-Tree Leaves as a Source of High-Added Value Molecules: Oleuropein. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 64, pp. 131–180. [Google Scholar]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological Activities of the Natural Antioxidant Oleuropein: Exceeding the Expectation—A Mini-Review. J. Funct. foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Del Carlo, M.; Gallina-Toschi, T.; Lercker, G.; Compagnone, D.; Fernández-Gutiérrez, A. Evaluation of the Antioxidant Capacity of Individual Phenolic Compounds in Virgin Olive Oil. J. Agric. Food Chem. 2005, 53, 8918–8925. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and Other Biological Activities of Phenols from Olives and Olive Oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Moreno-Sanz, P.; Lombardo, L.; Lorenzi, S.; Michelotti, F.; Grando, M.S. Genetic Resources of Olea europaea L. in the Garda Trentino Olive Groves Revealed by Ancient Trees Genotyping and Parentage Analysis of Drupe Embryos. Genes 2020, 11, 1171. [Google Scholar] [CrossRef]
- Cantini, C.; Cimato, A.; Autino, A.; Redi, A.; Cresti, M. Assessment of the Tuscan Olive Germplasm by Microsatellite Markers Reveals Genetic Identities and Different Discrimination Capacity among and within Cultivars. J. Am. Soc. Hortic. Sci. 2008, 133, 598–604. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals and Metalloids as Micronutrients for Plants and Animals. In Heavy Metals in Soils; Springer: Berlin/Heidelberg, Germany, 2013; pp. 195–209. [Google Scholar]
- DalCorso, G.; Manara, A.; Piasentin, S.; Furini, A. Nutrient Metal Elements in Plants. Metallomics 2014, 6, 1770–1788. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of Heavy Metal Ions on Soils and Soils Constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Batarseh, M.I.; Rawajfeh, A.; Ioannis, K.K.; Prodromos, K.H. Treated Municipal Wastewater Irrigation Impact on Olive Trees (Olea europaea L.) at Al-Tafilah, Jordan. Water Air Soil Pollut. 2011, 217, 185–196. [Google Scholar] [CrossRef]
- Prabagar, S.; Dharmadasa, R.M.; Lintha, A.; Thuraisingam, S.; Prabagar, J. Accumulation of Heavy Metals in Grape Fruit, Leaves, Soil and Water: A Study of Influential Factors and Evaluating Ecological Risks in Jaffna, Sri Lanka. Environ. Sustain. Indic. 2021, 12, 100147. [Google Scholar] [CrossRef]
- Alessandri, S.; Ieri, F.; Romani, A. Minor Polar Compounds in Extra Virgin Olive Oil: Correlation between HPLC-DAD-MS and the Folin-Ciocalteu Spectrophotometric Method. J. Agric. Food Chem. 2014, 62, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, Y. Comparative Study of Class Data Analysis with PCA-LDA, SIMCA, PLS, ANNs, and k-NN. Chemom. Intell. Lab. Syst. 1999, 49, 105–115. [Google Scholar] [CrossRef]
| Step | Time (min) | Power (W) | Temperature (°C) |
|---|---|---|---|
| Soil samples | |||
| 1 | 10 | 1800 | 160 |
| 2 | 15 | 1800 | 210 |
| 3 | 10 | 1800 | 210 |
| 4 | 20 | 0—VENT * | 50 * |
| Leaf samples | |||
| 1 | 10 | 1800 | 150 |
| 2 | 10 | 1800 | 180 |
| 3 | 10 | 1800 | 180 |
| 4 | 20 | 0—VENT * | 50 * |
| Teflon tube clean-up process | |||
| 1 | 10 | 1800 | 150 |
| 2 | 10 | 1800 | 180 |
| 3 | 10 | 1800 | 180 |
| 4 | 20 | 0—VENT * | 50 * |
| Time (min) | % MeOH |
|---|---|
| 0.0 | 5.0 |
| 1.0 | 5.0 |
| 30.0 | 60.0 * |
| 31.0 | 95.0 * |
| 35.0 | 95.0 |
| 36.0 | 5.0 * |
| 48.0 | 5.0 |
| Variety | Zone | ||||||
|---|---|---|---|---|---|---|---|
| F | F Crit | p-Value | F | F Crit | p-Value | ||
| Hydroxytyrosol | 4.88 | 3.18 | 0.0114 | 2.10 | 2.78 | 0.112 | |
| Tyrosol | 0.67 | 3.18 | 0.512 | 1.27 | 2.78 | 0.331 | |
| Caffeic Acid | 5.51 | 3.18 | 0.0067 | 4.18 | 2.78 | 0.0101 | |
| Verbascoside | 10.08 | 3.18 | 0.0002 | 1.78 | 2.78 | 0.161 | |
| Oleacin | 0.92 | 3.18 | 0.404 | 2.81 | 2.78 | 0.0485 | |
| Oleuropein | 20.68 | 3.18 | 2.46 × 10−7 | 1.04 | 2.78 | 0.350 | |
| Luteolin | 3.66 | 3.18 | 0.0325 | 1.99 | 2.78 | 0.125 | |
| Oleocanthal | 2.02 | 3.18 | 0.142 | 2.52 | 2.78 | 0.0682 | |
| Ligstroside | 5.35 | 3.18 | 0.00767 | 4.04 | 2.78 | 0.0118 | |
| TPC | 10.22 | 3.18 | 0.00018 | 1.70 | 2.78 | 0.178 | |
| TEAC | 17.72 | 3.18 | 1.35 × 10−6 | 0.187 | 2.78 | 0.905 |
| Variety | Zone | ||||||
|---|---|---|---|---|---|---|---|
| F | F Crit | p-Value | F | F Crit | p-Value | ||
| H-Tyr | 3.68 | 3.42 | 0.041 | 2.47 | 3.05 | 0.0897 | |
| Tyrosol | 0.355 | 3.42 | 0.704 | 2.21 | 3.05 | 0.116 | |
| Caffeic acid | 5.10 | 3.42 | 0.015 | 0.344 | 3.05 | 0.793 | |
| Verbascoside | 1.72 | 3.42 | 0.200 | 2.04 | 3.05 | 0.137 | |
| Oleacin | 1.03 | 3.42 | 0.372 | 3.02 | 3.05 | 0.051 | |
| Oleuropein | 0.394 | 3.42 | 0.679 | 2.19 | 3.05 | 0.118 | |
| Luteolin | 0.973 | 3.42 | 0.393 | 1.58 | 3.05 | 0.223 | |
| Oleocanthal | 1.28 | 3.42 | 0.297 | 0.898 | 3.05 | 0458 | |
| Ligstroside | 0.278 | 3.42 | 0.760 | 1.51 | 3.05 | 0.239 | |
| TPC | 0.182 | 3.42 | 0.835 | 2.89 | 3.05 | 0.058 | |
| TEAC | 0.451 | 3.42 | 0.643 | 0.473 | 3.05 | 0.704 |
| Variety | Zone | ||||||
|---|---|---|---|---|---|---|---|
| Test Validation | min | avg | max | min | avg | max | |
| Metal(loid)s | 0.16 | 0.56 | 0.91 | 0.08 | 0.58 | 1.00 | |
| Metabolites | 0.00 | 0.45 | 0.91 | 0.07 | 0.34 | 0.69 | |
| Combined PCA | 0.08 | 0.60 | 1.00 | 0.16 | 0.62 | 1.00 | |
| Variety | Zone | ||||||
| Cross Validation | min | avg | max | min | avg | max | |
| Metal(loid)s | 0.33 | 0.76 | 1.00 | 0.50 | 0.81 | 1.00 | |
| Metabolites | 0.25 | 0.61 | 0.91 | 0.16 | 0.54 | 0.84 | |
| Combined PCA | 0.58 | 0.91 | 1.00 | 0.69 | 0.92 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardin, R.; Tamasi, G.; Baglioni, M.; Fattori, G.; Boldrini, A.; Esposito, R.; Rossi, C. Combining Metal(loid) and Secondary Metabolite Levels in Olea europaea L. Samples for Geographical Identification. Foods 2024, 13, 4017. https://doi.org/10.3390/foods13244017
Nardin R, Tamasi G, Baglioni M, Fattori G, Boldrini A, Esposito R, Rossi C. Combining Metal(loid) and Secondary Metabolite Levels in Olea europaea L. Samples for Geographical Identification. Foods. 2024; 13(24):4017. https://doi.org/10.3390/foods13244017
Chicago/Turabian StyleNardin, Raffaello, Gabriella Tamasi, Michele Baglioni, Giacomo Fattori, Amedeo Boldrini, Rodolfo Esposito, and Claudio Rossi. 2024. "Combining Metal(loid) and Secondary Metabolite Levels in Olea europaea L. Samples for Geographical Identification" Foods 13, no. 24: 4017. https://doi.org/10.3390/foods13244017
APA StyleNardin, R., Tamasi, G., Baglioni, M., Fattori, G., Boldrini, A., Esposito, R., & Rossi, C. (2024). Combining Metal(loid) and Secondary Metabolite Levels in Olea europaea L. Samples for Geographical Identification. Foods, 13(24), 4017. https://doi.org/10.3390/foods13244017





