Chemometric Classification and Bioactivity Correlation of Black Instant Coffee and Coffee Bean Extract by Chlorogenic Acid Profiling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Sample Pretreatments
2.3. UPLC-MS Analysis
2.4. Data Processing
2.5. Cell Toxicity and Antioxidant Activity in HepG2 Cells
2.5.1. Cell Viability Assay
2.5.2. Cell Antioxidant Assay
3. Results and Discussion
3.1. Identification of Phenolic Acids
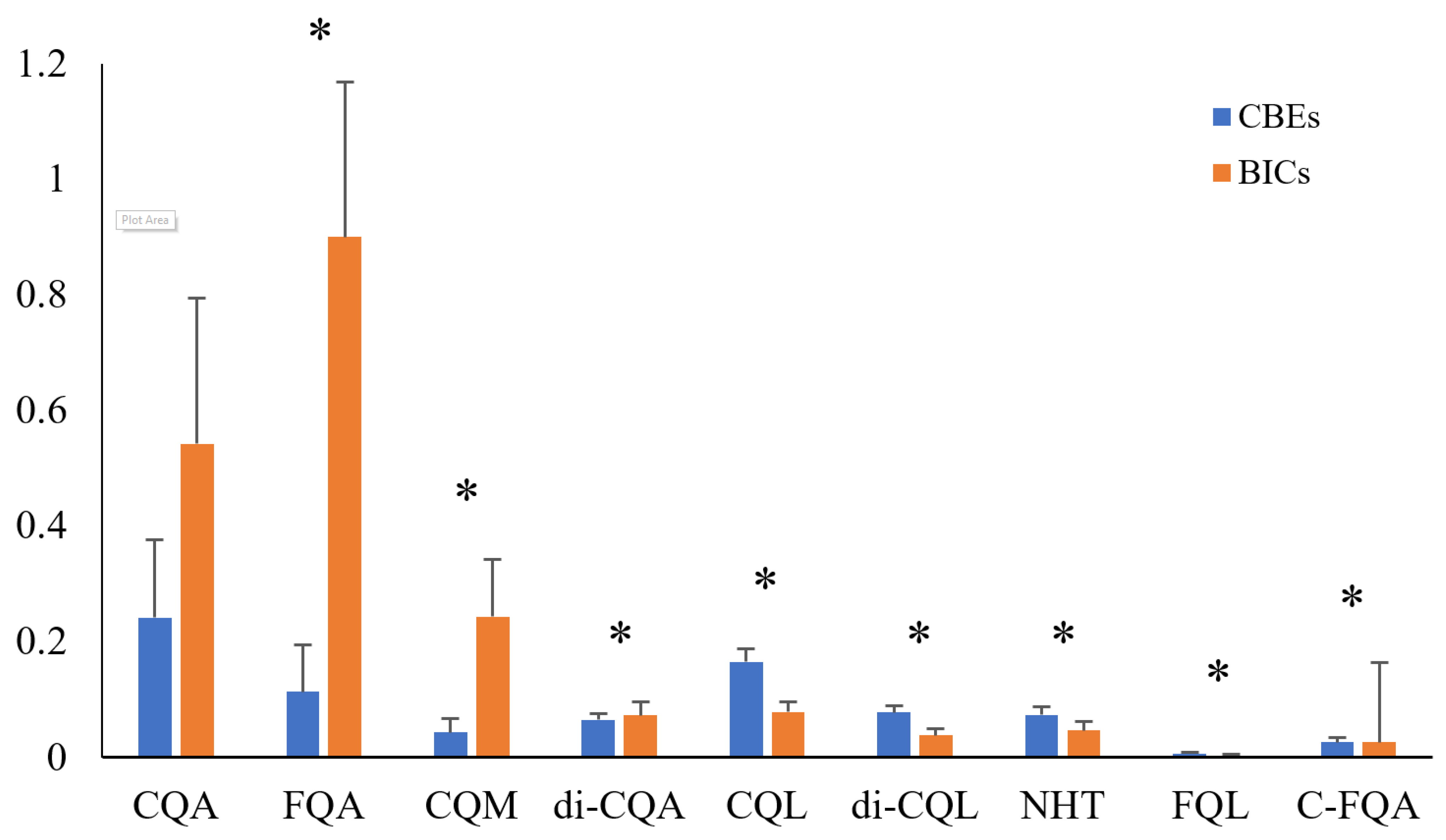
3.2. Difference in Phenolics Among Coffee Products
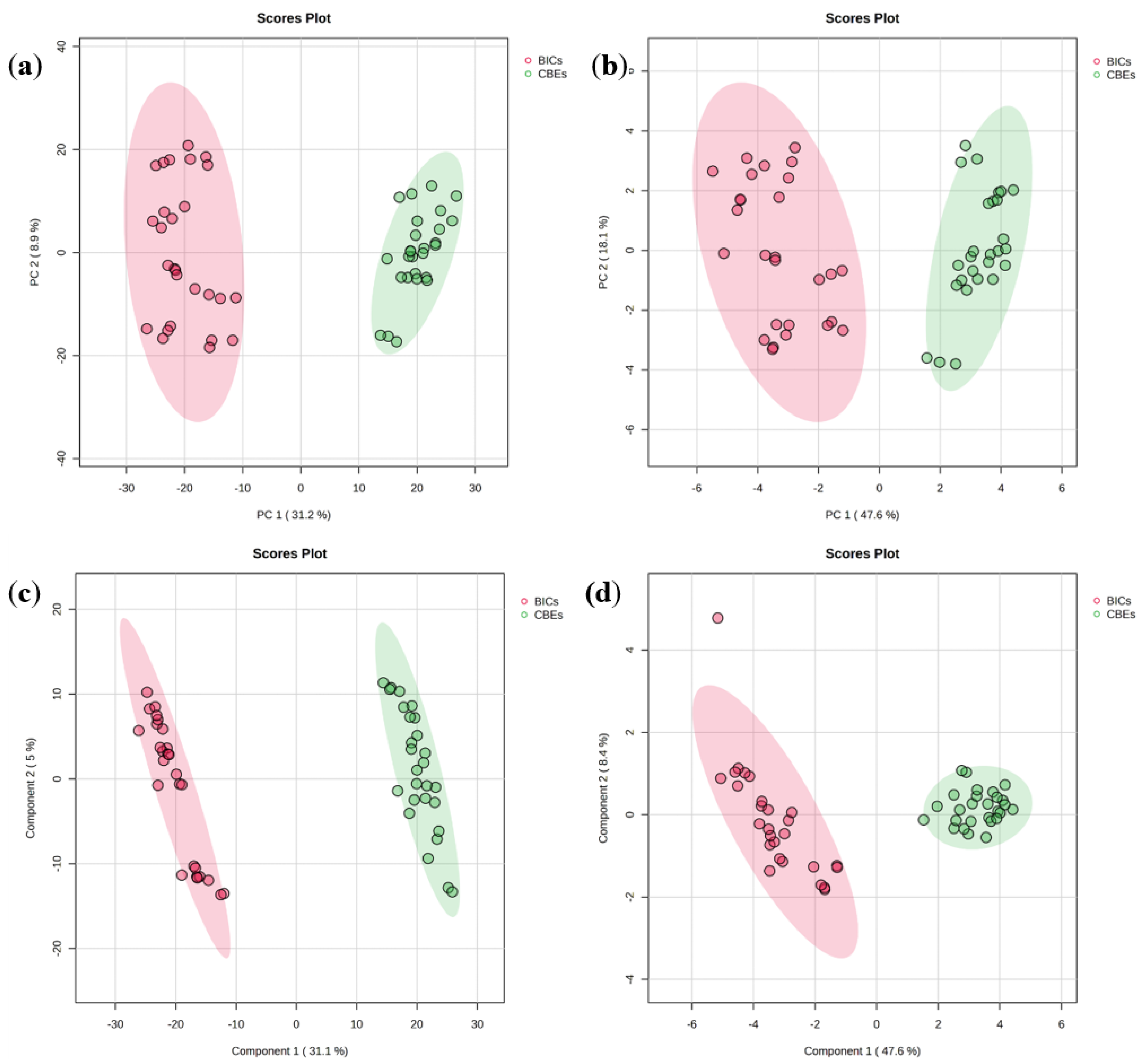
3.3. Classification of Black Instant Coffees and Coffee Bean Extracts Using Chemometrics
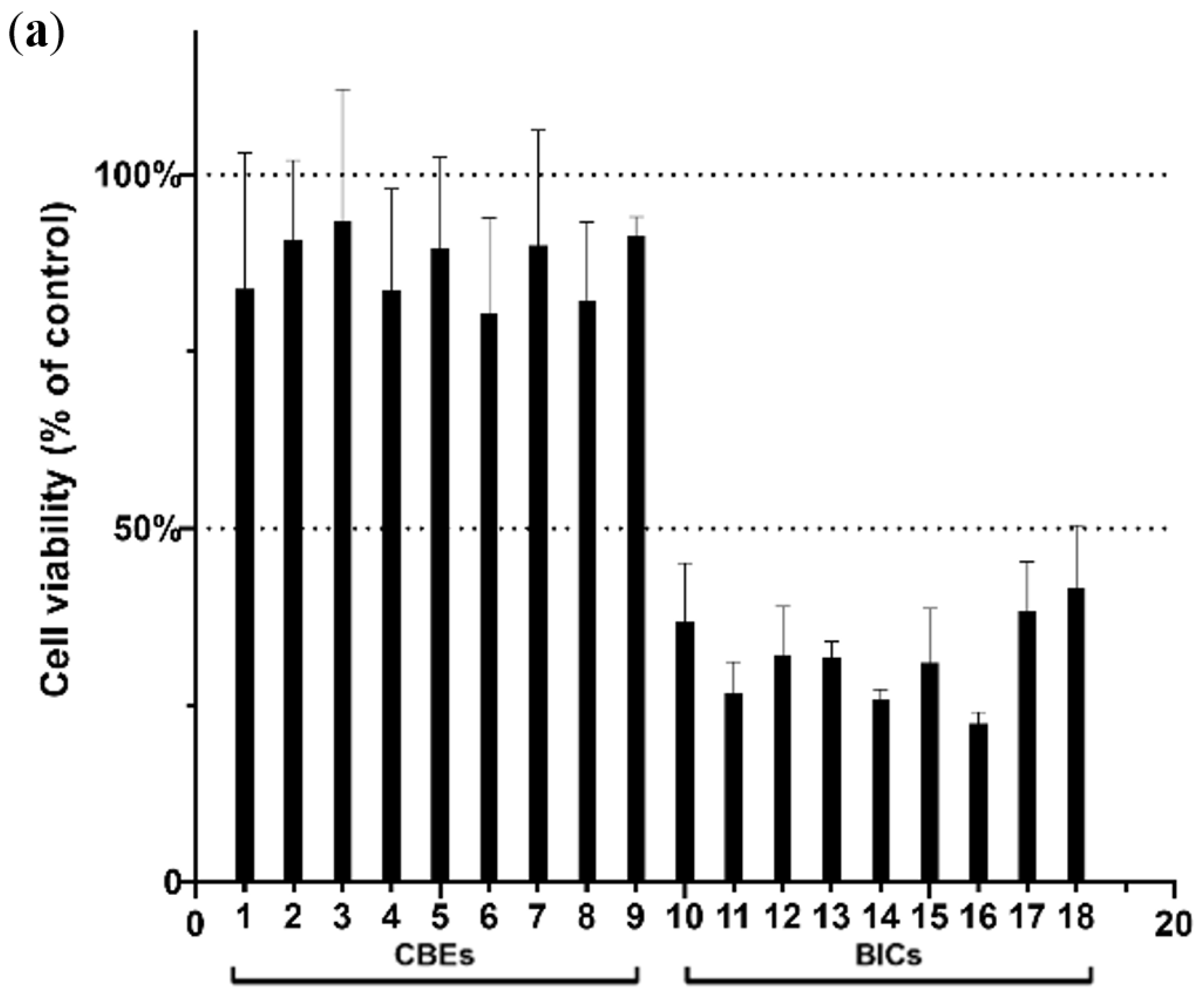
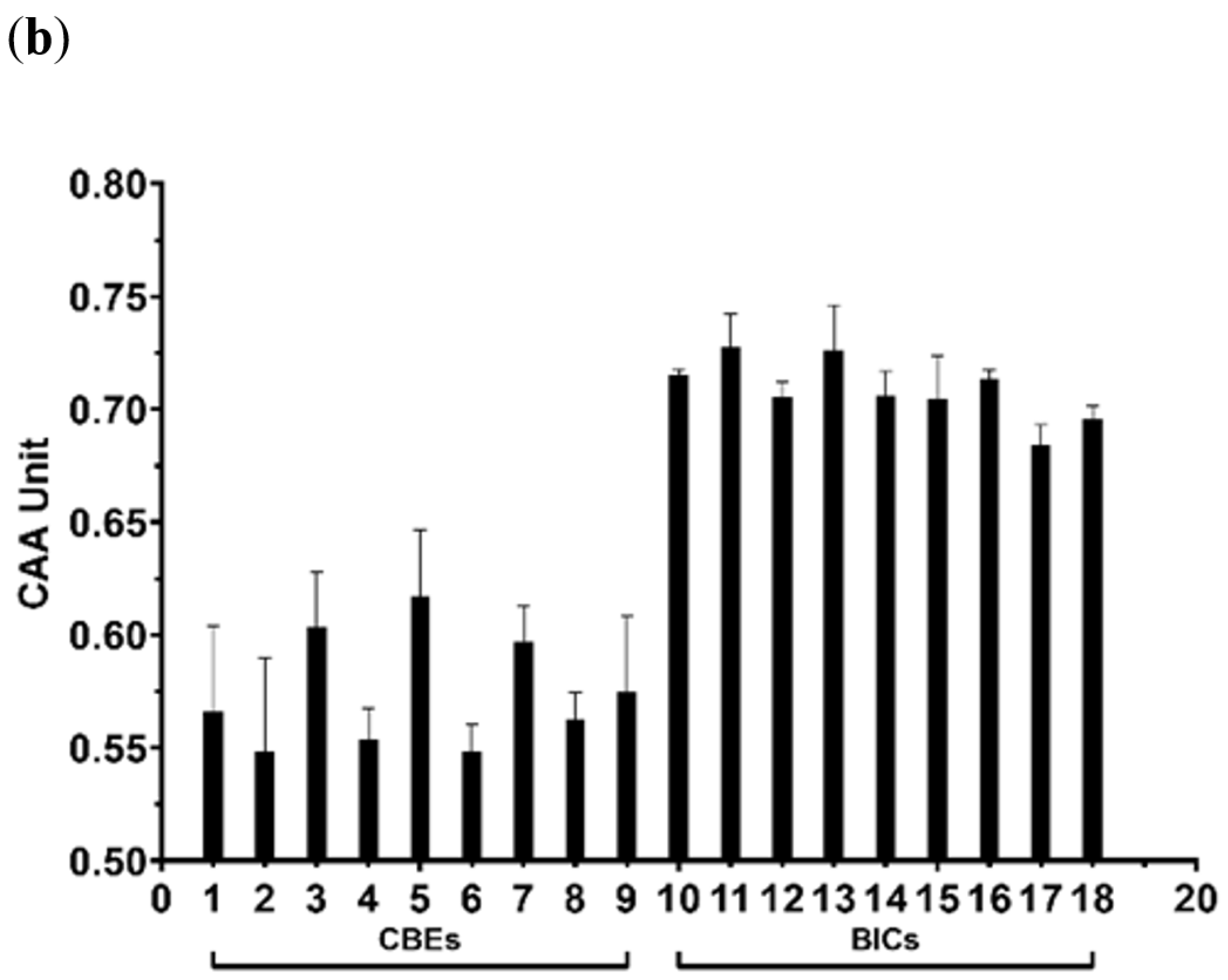
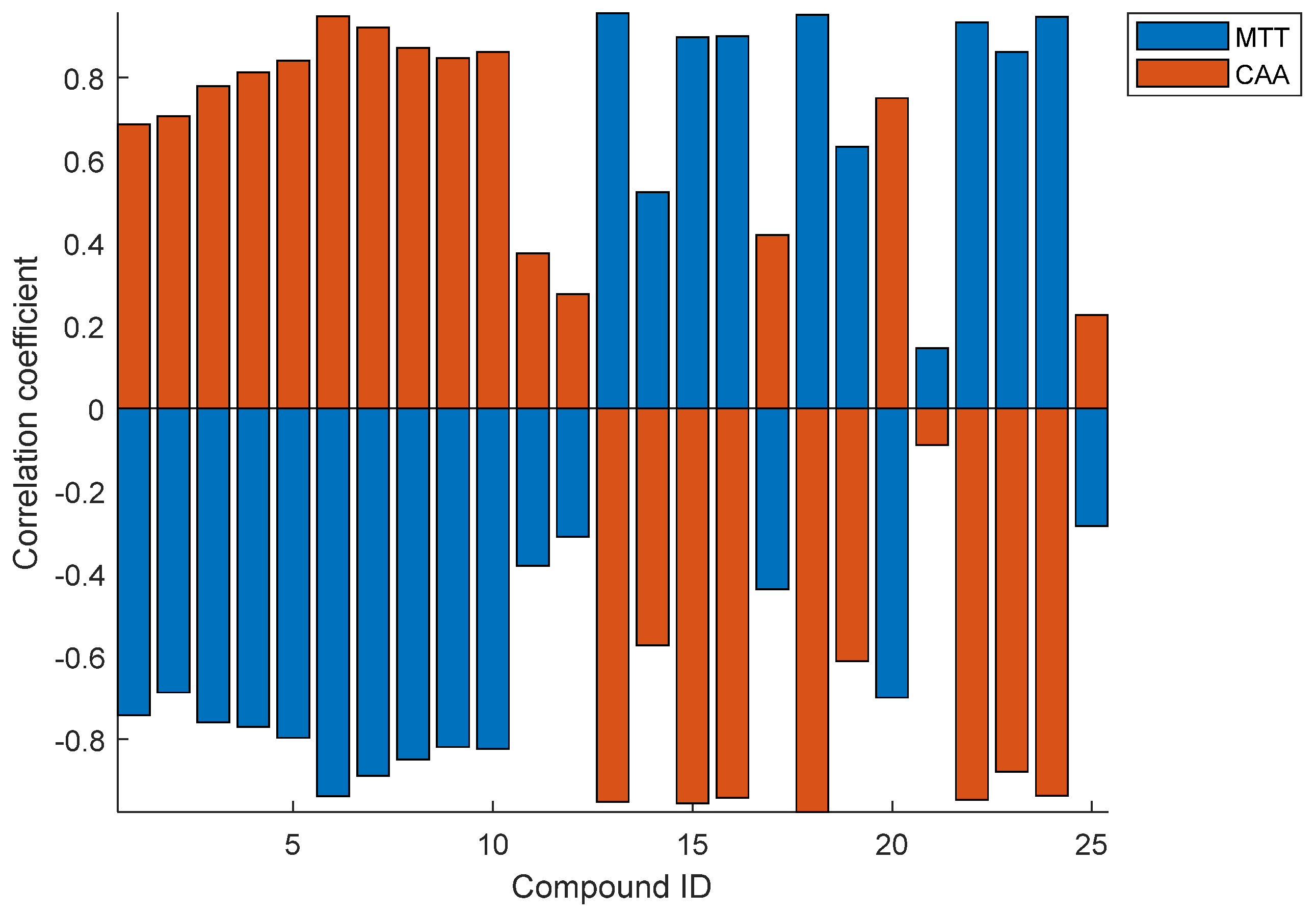
3.4. Cell Toxicity and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erskine, E.; Subasi, B.G.; Vahapoglu, B.; Capanoglu, E. Coffee phenolics and their interaction with other food phenolics: Antagonistic and synergistic effects. ACS Omega 2022, 7, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Mai, T.L.; Pham, T.T.T.; Binh, D. Supply chain coordination in sustainable agribusiness development: An investigation from coffee exporters. J. Agribus. Dev. Emerg. Econ. 2023, ahead-of-print. [Google Scholar] [CrossRef]
- Pu, Y.; Zaidin, N.; Zhu, Y. How do e-brand experience and in-store experience influence the brand loyalty of novel coffee brands in China? Exploring the roles of customer satisfaction and self-brand congruity. Sustainability 2023, 15, 1096. [Google Scholar] [CrossRef]
- Shim, J.H.; Kim, J. An analysis of Chinese coffee market and its implications for Korean coffee industry. J. Humanit. Soc. Sci. 2018, 9, 341–354. [Google Scholar] [CrossRef]
- International Coffee Organization. International Coffee Organization—Coffee Market Report. Coffee Prices Close 2022 on a Stable Note, Coalescing at Around 160 US Cents/lb. Retrieved from ICO Coffee Market Report. 2022. Available online: https://www.ico.org/documents/cy2022-23/cmr-1222-e.pdf (accessed on 1 September 2024).
- Mestanza, M.; Mori-Culqui, P.L.; Chavez, S.G. Changes of polyphenols and antioxidants of Arabica coffee varieties during roasting. Front. Nutr. 2023, 10, 1078701. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.K.; Lee, K.-G. Effect of reversed coffee grinding and roasting process on physicochemical properties including volatile compound profiles. Innov. Food Sci. Emerg. Technol. 2017, 44, 97–102. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, B.; Lu, W. Recent research advancements of coffee quality detection: Targeted analyses vs. nontargeted fingerprinting and related issues. J. Food Qual. 2023, 2023, 1–19. [Google Scholar] [CrossRef]
- Asamenew, G.; Kim, H.W.; Lee, M.K.; Lee, S.H.; Lee, S.; Cha, Y.S.; Lee, S.H.; Yoo, S.M.; Kim, J.B. Comprehensive characterization of hydroxycinnamoyl derivatives in green and roasted coffee beans: A new group of methyl hydroxycinnamoyl quinate. Food Chem.-X 2019, 2, 100033. [Google Scholar] [CrossRef]
- How Does Normalisation Work in Progenesis QI? Available online: https://www.nonlinear.com/progenesis/qi/v3.0/faq/how-normalisation-works.aspx (accessed on 21 November 2024).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8 (Suppl. S1), 3–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Xue, W.; Kennepohl, P.; Kitts, D.D. Interactions between major chlorogenic acid isomers and chemical changes in coffee brew that affect antioxidant activities. Food Chem. 2016, 213, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Guillou, C.; Berrueta, L.A. Liquid chromatography coupled with ultraviolet absorbance detection, electrospray ionization, collision-induced dissociation and tandem mass spectrometry on a triple quadrupole for the on-line characterization of polyphenols and methylxanthines in green coffee beans. Rapid Commun. Mass Spectrom. 2009, 23, 363–383. [Google Scholar] [CrossRef]
- Badmos, S.; Lee, S.H.; Kuhnert, N. Comparison and quantification of chlorogenic acids for differentiation of green Robusta and Arabica coffee beans. Food Res. Int. 2019, 126, 108544. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Frank, O.; Blumberg, S.; Krümpel, G.; Hofmann, T. Structure determination of 3-O-caffeoyl-epi-γ-quinide, an orphan bitter lactone in roasted coffee. J. Agric. Food Chem. 2008, 56, 9581–9585. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Wang, X.; Lim, L.T.; Fu, Y. Review of analytical methods to detect adulteration in coffee. J. AOAC Int. 2020, 103, 295–305. [Google Scholar] [CrossRef]
- Campa, C.; Doulbeau, S.; Dussert, S.; Hamon, S.; Noirot, M. Qualitative relationship between caffeine and chlorogenic acid contents among wild Coffea species. Food Chem. 2005, 93, 135–139. [Google Scholar] [CrossRef]
- Sinisi, V.; Stevaert, A.; Berti, F.; Forzato, C.; Benedetti, F.; Navarini, L.; Camps, A.; Persoons, L.; Vermeire, K. Chlorogenic compounds from coffee beans exert activity against respiratory viruses. Planta Med. 2017, 83, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Harrington, P. Statistical validation of classification and calibration models using bootstrapped Latin partitions. TrAC Trends Anal. Chem. 2006, 25, 1112–1124. [Google Scholar] [CrossRef]
| Peak ID. | RT (min) | Compound Abbreviation/Type | Formula | Exp. Mass [M–H]− | Accuracy (ppm) | Fragments Identified by UPLC-QTOF-MS |
|---|---|---|---|---|---|---|
| 1 | 0.54 | 1-CQA | C16H18O9 | 353.0873 | 1.7 | *135[caf-H-COO]−, 173[qui-H-H2O]−, *179[caf-H]−, *191[qui-H]− |
| 2 | 0.72 | 3-CQA | C16H18O9 | 353.0873 | 5.9 | *135[caf-H-COO]−, 179[caf-H]−, *191[qui-H]− |
| 3 | 0.86 | 5-CQA | C16H18O9 | 353.0873 | 1.7 | *135[caf-H-COO]−, *161[caf-H-H2O]−, *191[qui-H]− |
| 4 | 1.22 | 4-CQA | C16H18O9 | 353.0873 | 0.3 | *133[caf-H-H2O-CO]−, *135[caf-H-COO]−, 173[qui-H-H2O]−, *179[caf-H]−, *191[qui-H]− |
| 5 | 1.35 | 3-CQM | C17H20O9 | 367.1029 | 3.5 | 134[caf-2H-COO]−, *135[caf-H-COO]−, *173[qui-H-H2O]−, 179[caf-H]−, *191[qui-H]−, 193[M+H2O-qui-H]−, *353[M-H-CH3]−, |
| 6 | 1.59 | 3-FQA | C17H20O9 | 367.1029 | 4.9 | *134[fer-H-CH3-COO]−, 173[qui-H-H2O]−, *175[fer-H-H2O]−, *191[qui-H]−, 193[fer-H]− |
| 7 | 2.02 | 5-FQA | C17H20O9 | 367.1029 | 6 | *134[fer-H-CH3-COO]−, *173[qui-H-H2O]−, *191[qui-H]−, *735[2M-H]− |
| 8 | 2.21 | 4-CQM | C17H20O9 | 367.1029 | 4.6 | *134[caf-2H-COO]−, 161[caf-H-H2O]−, *173[qui-H-H2O]−, 179[caf-H]−, 191[qui-H]−, |
| 9 | 2.46 | 1,3-di-CQA | C25H24O12 | 515.1190 | 2.5 | *161[caf-H-H2O]−, 173[qui-H-H2O]−, *191[qui-H]− |
| 10 | 2.58 | 4-FQA | C17H20O9 | 367.1029 | 1.9 | *134[fer-H-CH3-COO]−, 173[qui-H-H2O]−, *175[fer-H-H2O]−, 191[qui-H]−, |
| 11 | 3.9 | 1,4-di-CQA | C24H26O11 | 515.1190 | 0.4 | 135[caf-H-COO]−, *161[caf-H-H2O]−, 173[qui-H-H2O]−, 179[caf-H]−, *191[qui-H]− |
| 12 | 4.13 | 3,4-di-CQA | C25H24O12 | 515.1190 | 1.9 | 135[caf-H-COO]−, *173[qui-H-H2O]−, *191[qui-H]− |
| 13 | 5.76 | 3-O-caffeoyl-epi-γ-quinide (3-C-epi-γ-Q)/CQL | C16H16O8 | 335.0767 | 5.7 | *135[caf-H-COO]−, *161[caf-H-H2O]−, 173[qui-H-H2O]−, 179[caf-H]−, *191[qui-H]− |
| 14 | 6.42 | 3,5-di-CQA | C25H24O12 | 515.1190 | 0.4 | *161[caf-H-H2O]−, 173[qui-H-H2O]−, 179[caf-H]−, *191[qui-H]−, *353[M+H2O-caf-H]− |
| 15 | 6.54 | 1,5-di-CQA | C25H24O12 | 515.1190 | 3.5 | *161[caf-H-H2O]−, 173[qui-H-H2O]−, 179[caf-H]−, *191[qui-H]−, 353[M+H2O-caf-H]− |
| 16 | 6.61 | 3-O-caffeoyl-γ-quinide (3-C-γ-Q)/CQL | C16H16O8 | 335.0767 | 0.6 | *133[caf-H-H2O-CO]−, *135[caf-H-COO]−, *161[caf-H-H2O]−, *173[qui-H-H2O]−, 191[qui-H]− |
| 17 | 6.70 | 4,5-di-CQA | C25H24O12 | 515.1190 | 0.6 | 135[caf-H-COO]−, 173[qui-H-H2O]−, 179[caf-H]−, *191[qui-H]−, *353[M+H2O-caf-H]− |
| 18 | 6.81 | 4-O-caffeoyl-muco-γ-quinide (4-C-muco-γ-Q)/CQL | C16H16O8 | 335.0767 | 3.0 | 133[caf-H-H2O-CO]−, *135[caf-H-COO]−, *161[caf-H-H2O]−, 179[caf-H]−, |
| 19 | 7.04 | 4-O-caffeoyl-γ-quinide (4-C-γ-Q)/CQL | C16H16O8 | 335.0767 | 4.0 | 133[caf-H-H2O-CO]−, 135[caf-H-COO]−, *161[caf-H-H2O]−, 173[qui-H-H2O]−, 179[caf-H]−, 191[qui-H]− |
| 20 | 7.41 | 3C-5FQA/C-FQA | C26H26O12 | 529.1346 | 1.5 | 161[caf-H-H2O]−, *173[qui-H-H2O]−, *179[caf-H]−, *191[qui-H]−, *193[fer-H]− |
| 21 | 7.56 | caffeoyl-N-tryptophan (CTry)/HNT | C20H18N2O5 | 365.1137 | 3.6 | 135[caf-H-COO]−, 161[caf-H-H2O]−, 203[Try-H]−, |
| 22 | 7.89 | p-coumaroyl-N-tryptophan (pCoTry) | C20H18N2O4 | 349.1188 | 18.0 | 173[qui-H-H2O]−, *191[qui-H]−, *203[Try-H]− |
| 23 | 7.97 | Isomers of 3-O-feruloyl-γ-quinide (3F-epi-γ-Q)/FQL | C17H18O8 | 349.0923 | 4.9 | 173[qui-H-H2O]−, 175[fer-H-H2O]−, 191[qui-H]− |
| 24 | 9.73 | 3,4-O-dicaffeoyl-γ-quinide (3,4-di-C-γ-Q)/di-CQL | C25H22O11 | 497.1084 | 0.4 | 161[caf-H-H2O]−, 173[qui-H-H2O]−, 179[caf-H]−, 191[qui-H]−, *335 |
| 25 | 10.45 | 4,5-O-dicaffeoyl-muco-γ-quinide (4,5-di-C-muco-γ-Q)/di-CQL | C25H22O11 | 497.1084 | 3.0 | 161[caf-H-H2O]−, 173[qui-H-H2O]−, 179[caf-H]−, 191[qui-H]−, *335 |
| Class | Name and Abbreviation | References |
|---|---|---|
| CGAs | 1/3/5/4-O-caffeoylquinic acid (1-CQA, 3-CQA, 5-CQA, 4-CQA) | [9,16,17] |
| CGAs | 3/5/4-O-feruloylquinic acid (3-FQA, 5-FQA, 4-FQA) | [9,16,17,18] |
| CGAs | 1,3/1,4/3,4/3,5/1,5/4,5-di-O-caffeoylquinic acid (1,3-di-CQA, 1,4-di-CQA, 3,4-di-CQA, 3,5-di-CQA, 1,5-di-CQA, 4,5-di-CQA) | [9,17,18,19] |
| CGAs | 3-O-feruloyl, 4-O-caffeoylquinic acid (3F,4CQA) | [9,16,17,18] |
| CGA derivatives | 4/5-O-caffeoylquinic acid methyl (4-CQM, 5-CQM) | [9,17] |
| Cinnamoyl quinides | Isomers of 3-O-caffeoyl-γ-quinide (3-C-γ-Q, 3C-epi-γ-Q) | [9,20] |
| Cinnamoyl quinides | Isomers of 3-O-feruloyl-γ-quinide (3F-epi-γ-Q) | [9,20] |
| Cinnamoyl quinides | 3,4/4,5-di-O-caffeoylquinide (di-CQL) | [9] |
| Cinnamoyl-amino acid conjugates | Caffeoyl-N-tryptophan (CTry) | [9,17] |
| Cinnamoyl-amino acid conjugates | p-coumaroyl-N-tryptophan (pCoTry) | [9,17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yu, W.; Niu, Y.; Li, W.; Lu, W.; Yu, L. Chemometric Classification and Bioactivity Correlation of Black Instant Coffee and Coffee Bean Extract by Chlorogenic Acid Profiling. Foods 2024, 13, 4016. https://doi.org/10.3390/foods13244016
Chen Y, Yu W, Niu Y, Li W, Lu W, Yu L. Chemometric Classification and Bioactivity Correlation of Black Instant Coffee and Coffee Bean Extract by Chlorogenic Acid Profiling. Foods. 2024; 13(24):4016. https://doi.org/10.3390/foods13244016
Chicago/Turabian StyleChen, Yumei, Wei Yu, Yuge Niu, Wenchen Li, Weiying Lu, and Liangli (Lucy) Yu. 2024. "Chemometric Classification and Bioactivity Correlation of Black Instant Coffee and Coffee Bean Extract by Chlorogenic Acid Profiling" Foods 13, no. 24: 4016. https://doi.org/10.3390/foods13244016
APA StyleChen, Y., Yu, W., Niu, Y., Li, W., Lu, W., & Yu, L. (2024). Chemometric Classification and Bioactivity Correlation of Black Instant Coffee and Coffee Bean Extract by Chlorogenic Acid Profiling. Foods, 13(24), 4016. https://doi.org/10.3390/foods13244016





