Extraction Methods and Characterization of β-Glucans from Yeast Lees of Wines Produced Using Different Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Winery Yeast Lees
2.2. Analysis of Winery Yeast Lees
2.2.1. Extraction and Analysis of β-Glucans
- Ultrasound-assisted alkali–acidic extraction of β-glucans
- B.
- Ultrasound-assisted autolysis extraction of β-glucans
2.2.2. Physico-Chemical Parameters of Winery Yeast Lees
2.2.3. Total Phenolic Compounds
2.2.4. Anthocyanin Content
2.2.5. Mineral Content
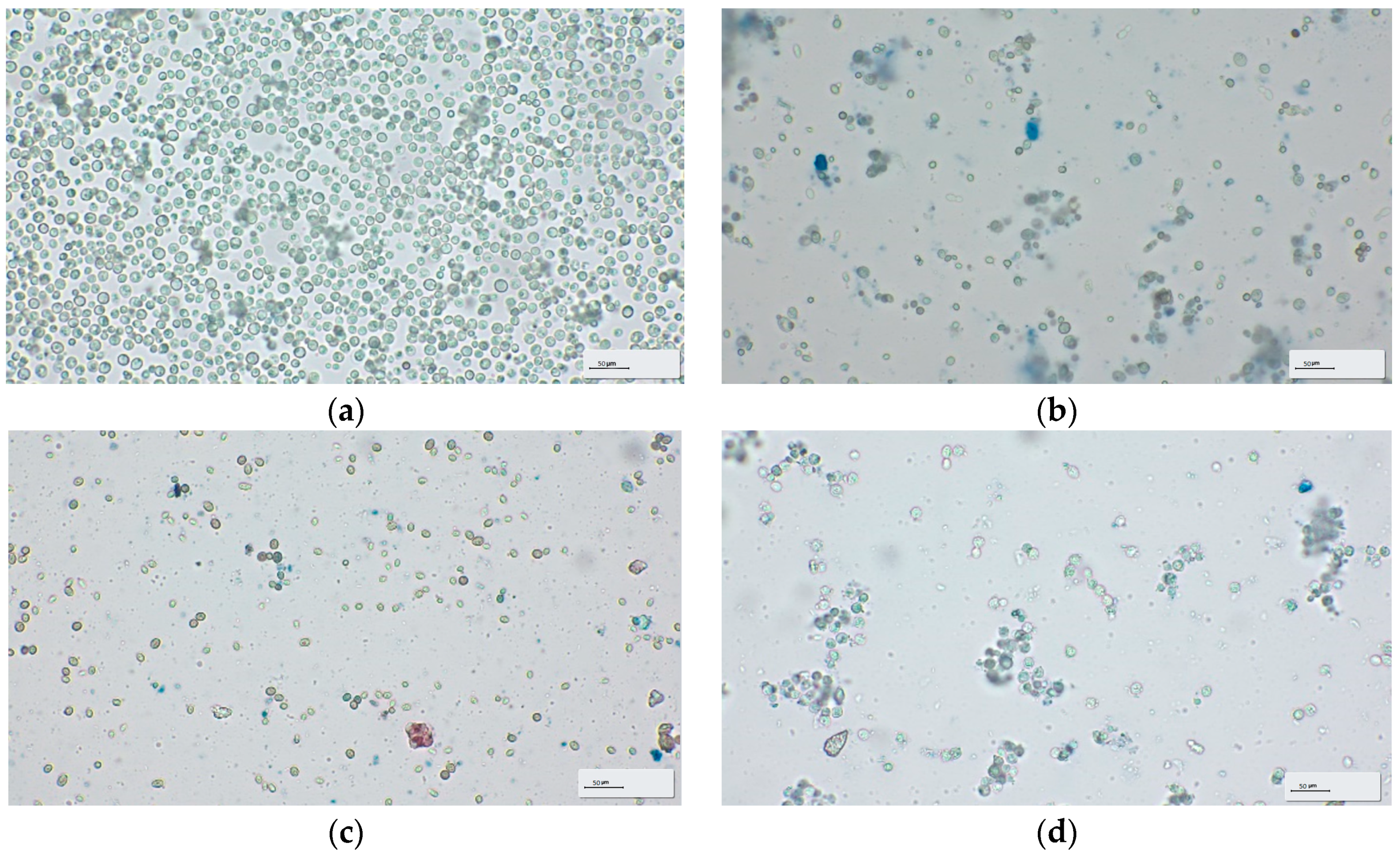
2.2.6. Microscopic Analyses of Winery Yeast Lees
2.2.7. Calculation of Extracted β-Glucan Yield
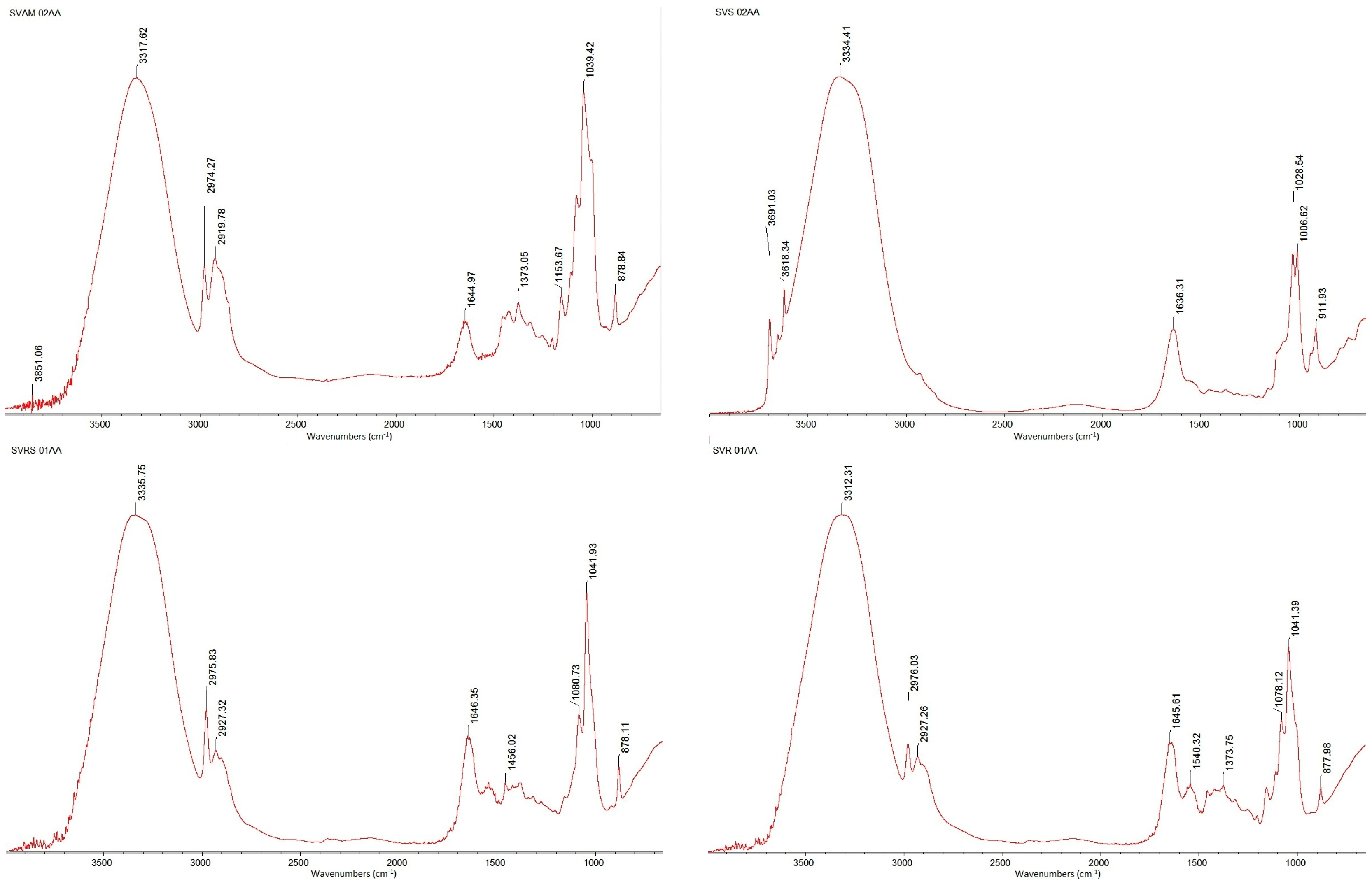
2.2.8. Fourier Transform Infrared Spectroscopy (FTIR) of Extracted β-Glucans
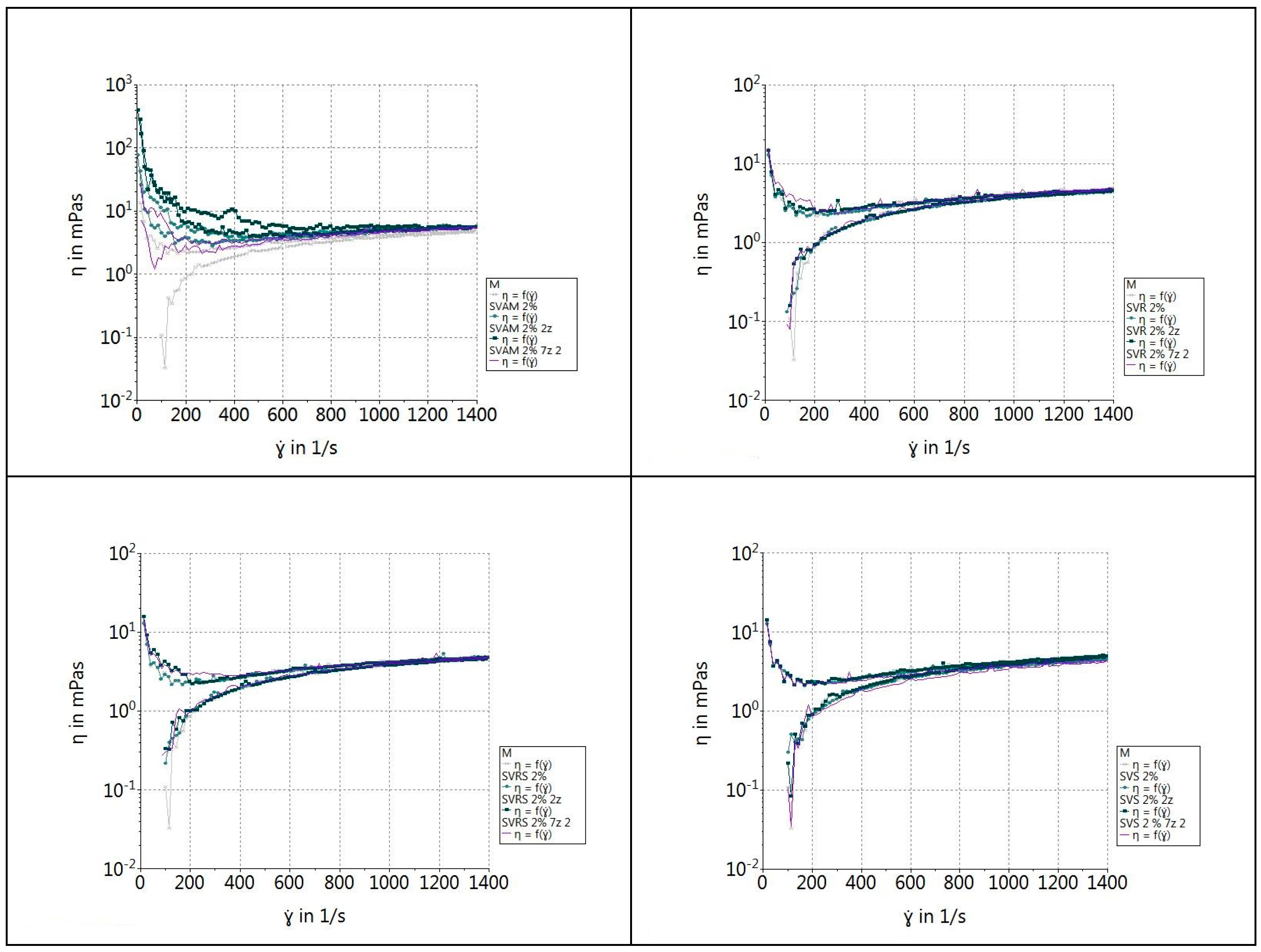
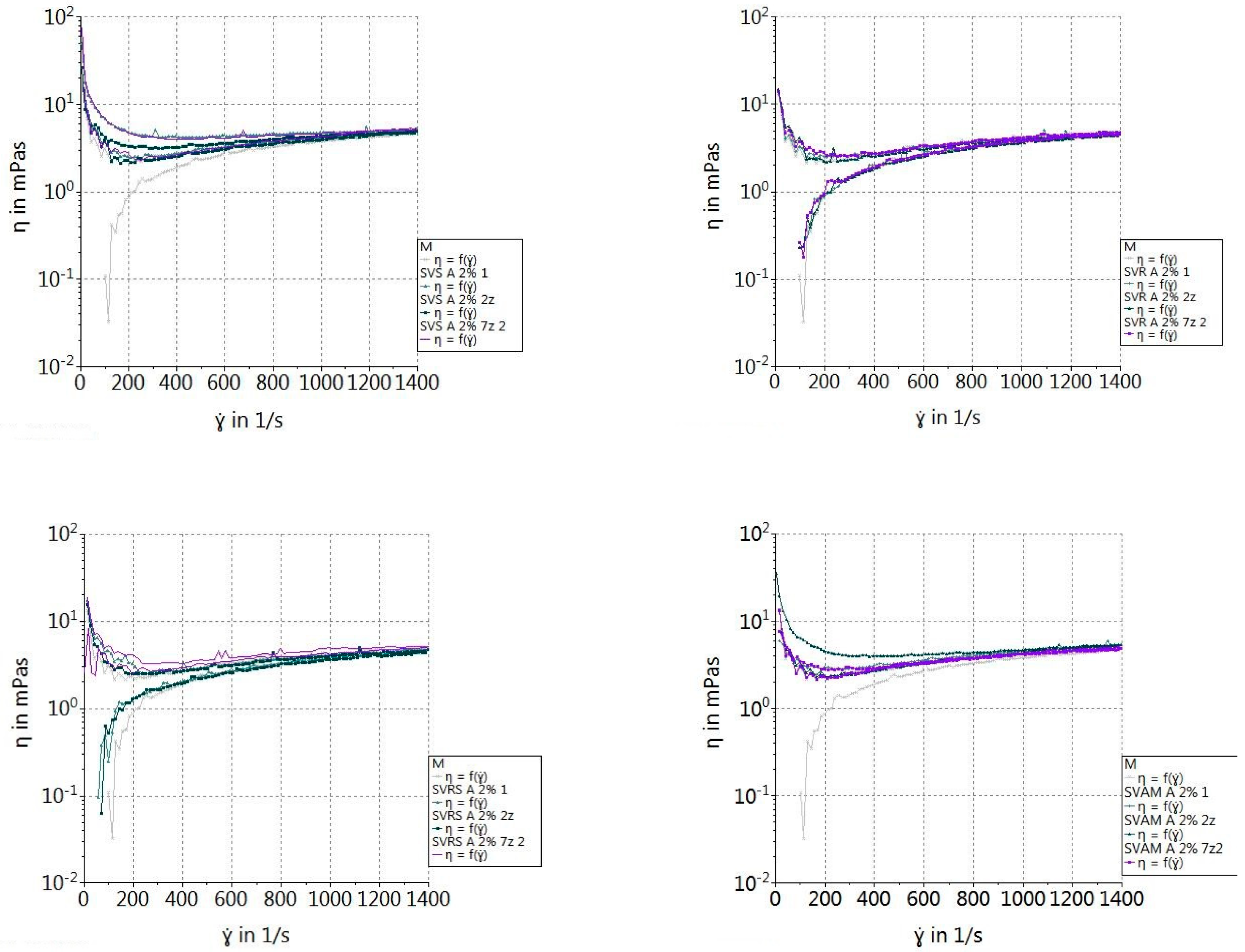
2.2.9. Rheological Properties of β-Glucan Suspensions
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Characteristics and Mineral Content of Wine Lees
3.2. Microscopy of Winery Yeast Lees
3.3. Yield of Extracted β-Glucans
3.4. FTIR Spectroscopy
3.5. Rheological Properties of β-Glucan Suspensions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A Review on Wastewater Sludge Valorisation and Its Challenges in the Context of Circular Economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Borta, A.-M.; Sturza, R. Ways of application of the circular bioeconomy in the wine industry. J. Eng. Sci. 2024, 30, 124–146. [Google Scholar] [CrossRef]
- Leder, N.; Kumar, M.; Rodrigues, V.S. Influential Factors for Value Creation within the Circular Economy: Framework for Waste Valorisation. Resour. Conserv. Recycl. 2020, 158, 104804. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stoica, F.; Rațu, R.N.; Stănciuc, N.; Bahrim, G.E.; Râpeanu, G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-Products from a Circular Bioeconomy Perspective: A Review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef]
- Kokkinomagoulos, E.; Kandylis, P. Sustainable Exploitation of By-Products of Vitivinicultural Origin in Winemaking. Proceedings 2020, 67, 5. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine STATE OF THE WORLD VINE AND WINE SECTOR IN 2023. Available online: https://www.oiv.int/sites/default/files/2024-04/OIV_STATE_OF_THE_WORLD_VINE_AND_WINE_SECTOR_IN_2023.pdf (accessed on 31 October 2024).
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Physicochemical and Nutritional Characterization of Winemaking Lees: A New Food Ingredient. Agronomy 2020, 10, 996. [Google Scholar] [CrossRef]
- Varelas, V.; Tataridis, P.; Liouni, M.; Nerantzis, E.T. Valorization of Winery Spent Yeast Waste Biomass as a New Source for the Production of β-Glucan. Waste Biomass Valor 2016, 7, 807–817. [Google Scholar] [CrossRef]
- Karslioğlu, F.; Ertunç, S.; Yilmazer Hitit, Z.; Akay, B. Investigation of Extraction Method Effect on Yeast Beta Glucan Production. Eurasian J. Biol. Chem. Sci. 2021, 4, 51–55. [Google Scholar] [CrossRef]
- Magnani, M.; Calliari, C.M.; de Macedo, F.C.; Mori, M.P.; de Syllos Cólus, I.M.; Castro-Gomez, R.J.H. Optimized Methodology for Extraction of (1 → 3)(1 → 6)-β-d-Glucan from Saccharomyces cerevisiae and in Vitro Evaluation of the Cytotoxicity and Genotoxicity of the Corresponding Carboxymethyl Derivative. Carbohydr. Polym. 2009, 78, 658–665. [Google Scholar] [CrossRef]
- Mahmoud Amer, E.; Saber, S.H.; Abo Markeb, A.; Elkhawaga, A.A.; Mekhemer, I.M.A.; Zohri, A.-N.A.; Abujamel, T.S.; Harakeh, S.; Abd-Allah, E.A. Enhancement of β-Glucan Biological Activity Using a Modified Acid-Base Extraction Method from Saccharomyces cerevisiae. Molecules 2021, 26, 2113. [Google Scholar] [CrossRef]
- Pham, K.N.; Nhut, N.D.; Cuong, N.M. New Method for Preparing Purity β-D-Glucans (Beta-Glucan) from Baker’s Yeast (Saccharomyces cerevisiae). Sci. Technol. Dev. J. 2020, 23, 678–683. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yang, P.; Jiang, W. Effect of Alkali Treatment Combined with High Pressure on Extraction Efficiency of β-d-Glucan from Spent Brewer’s Yeast. Waste Biomass Valor 2019, 10, 1131–1140. [Google Scholar] [CrossRef]
- Thammakiti, S.; Suphantharika, M.; Phaesuwan, T.; Verduyn, C. Preparation of Spent Brewer’s Yeast Β-glucans for Potential Applications in the Food Industry. Int. J. Food Sci. Technol. 2004, 39, 21–29. [Google Scholar] [CrossRef]
- Suphantharika, M. Preparation of Spent Brewer’s Yeast β-Glucans with a Potential Application as an Immunostimulant for Black Tiger Shrimp, Penaeus monodon. Bioresour. Technol. 2003, 88, 55–60. [Google Scholar] [CrossRef]
- Rozmierska, J.; Stecka, K.M.; Kotyrba, D.; Piasecka-Jóźwiak, K. Preparation of Sedimented Wine Yeast Derived Products for Potential Application in Food and Feed Industry. Waste Biomass Valor 2019, 10, 455–463. [Google Scholar] [CrossRef]
- Chiselița, N.; Chiselița, O.; Efremova, N.; Beșliu, A. Valorization of the Red Wine Yeast Waste. Pol. J. Environ. Stud. 2024, 33, 1623–1630. [Google Scholar] [CrossRef]
- Vacarciuc, L.; Breahna, E.; Bogatii, E. Filiera vitivinicolă a Republicii Moldova în contextul alinierii la cerințele UE. In Proceedings of the International Scientific Conference “European Integration Through the Straightening of Education, Re-search, Innovations in Eastern Partnership Countries”, Chisinau, Moldova, 16–17 May 2022. [Google Scholar]
- Bai, Z.; Jin, B.; Li, Y.; Chen, J.; Li, Z. Utilization of Winery Wastes for Trichoderma Viride Biocontrol Agent Production by Solid State Fermentation. J. Environ. Sci. 2008, 20, 353–358. [Google Scholar] [CrossRef]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and Future Strategies for Wine Yeast Lees Valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Porras-Agüera, J.A.; Moreno-García, J.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. First Proteomic Approach to Identify Cell Death Biomarkers in Wine Yeasts during Sparkling Wine Production. Microorganisms 2019, 7, 542. [Google Scholar] [CrossRef]
- 2-11-2022. Method OIV-MA-VI-07: R2000 “Determination of Ash Content in Vinegars”. Available online: https://www.oiv.int/standards/compendium-of-international-methods-of-analysis-for-vinegars/wine-vinegars/methods-of-analysis-for-vinegars/determination-of-ash-content-%28type-ii%29 (accessed on 29 July 2024).
- Dey, P.M.; Harborne, J.B. Methods in Plant Biochemistry. In Carbohydrats; Academic Press: Cambridge, MA, USA, 1993; Volume 2, ISBN 0-12-461012-9. [Google Scholar]
- Breil, C.; Abert Vian, M.; Zemb, T.; Kunz, W.; Chemat, F. “Bligh and Dyer” and Folch Methods for Solid–Liquid–Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents. Int. J. Mol. Sci. 2017, 18, 708. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Measurement of Total Phenolics and Tannins Using Folin-Ciocalteu Method. In Quantification of Tannins in Tree and Shrub Foliage; Springer: Dordrecht, The Netherlands, 2003; pp. 49–51. ISBN 978-90-481-6428-8. [Google Scholar]
- Lima, A.D.J.B.; Corrêa, A.D.; Saczk, A.A.; Martins, M.P.; Castilho, R.O. Anthocyanins, Pigment Stability and Antioxidant Activity in Jabuticaba [Myrciaria Cauliflora (Mart.) O. Berg]. Rev. Bras. Frutic. 2011, 33, 877–887. [Google Scholar] [CrossRef]
- SW-846 Test Method 3015A: Microwave Assisted Acid Digestion of Aqueous Samples and Extracts; 2007. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/3015a.pdf (accessed on 28 July 2024).
- Avramia, I. Cercetari Privind Extratia de B-Glucani Din Drojdia de Bere Reziduala Si Valorificarea Lor in Filme Bioactive; Performatica: Iasi, Romania, 2022. [Google Scholar]
- Petravic-Tominac, V.; Zechner-Krpan, V.; Berkovi, K.; Galovi, P.; Herceg, Z. Rheological Properties, Water-Holding and Oil-Binding Capacities of Particulate b-Glucans Isolated from Spent Brewer’s Yeast by Three Different Procedures. Food Technol. Biotechnol. 2011, 49, 56–64. [Google Scholar]
- Pengkumsri, N.; Sivamaruthi, B.S.; Sirilun, S.; Peerajan, S.; Kesika, P.; Chaiyasut, K.; Chaiyasut, C. Extraction of β-Glucan from Saccharomyces cerevisiae: Comparison of Different Extraction Methods and in Vivo Assessment of Immunomodulatory Effect in Mice. Food Sci. Technol 2016, 37, 124–130. [Google Scholar] [CrossRef]
- Meini, M.-R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of Phenolic Antioxidants from Syrah Grape Pomace through the Optimization of an Enzymatic Extraction Process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef]
- Kok, D.; Bal, E. Compositional Differences in Phenolic Compounds and Anthocyanin Contents of Some Table and Wine Grape (V. vinifera L.) Varieties from Turkey. JO—Oxid. Commun. 2017, 40, 648. [Google Scholar]
- Živković, N.; Čakar, U.; Petrović, A. Effects of Spontaneous and Inoculated Fermentation on the Total Phenolic Content and Antioxidant Activity of Cabernet Sauvignon Wines and Fermented Pomace. Food Feed Res. 2024, 51, 119–129. [Google Scholar] [CrossRef]
- Castangia, I.; Aroffu, M.; Fulgheri, F.; Abi Rached, R.; Corrias, F.; Sarais, G.; Bacchetta, G.; Argiolas, F.; Pinna, M.B.; Murru, M.; et al. From Field to Waste Valorization: A Preliminary Study Exploring the Impact of the Wine Supply Chain on the Phenolic Profile of Three Sardinian Pomace Extracts. Foods 2024, 13, 1414. [Google Scholar] [CrossRef]
- Sherman, E.; Yvon, M.; Grab, F.; Zarate, E.; Green, S.; Bang, K.W.; Pinu, F.R. Total Lipids and Fatty Acids in Major New Zealand Grape Varieties during Ripening, Prolonged Pomace Contacts and Ethanolic Extractions Mimicking Fermentation. Fermentation 2023, 9, 357. [Google Scholar] [CrossRef]
- Buzzanca, C.; Mauro, M.; Vazzana, M.; Todaro, A.; Arizza, V.; Lucarini, M.; Durazzo, A.; Di Stefano, V. Analysis of the Amino Acid Profile of Red and White Graphs Winery By-Products from Western Sicily. Meas. Food 2024, 14, 100174. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Kang, Y.; Chen, D.; Chen, G.; Zeng, X.; Wang, J. Yeast Mannoproteins Are Expected to Be a Novel Potential Functional Food for Attenuation of Obesity and Modulation of Gut Microbiota. Front. Nutr. 2022, 9, 1019344. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Avila, P.E.; Vasquez-Murrieta, M.S.; Cortés-Camargo, S.; Hernández-Botello, M.T.; Ramos-Monroy, O.; López-Cortéz, M.D.S. Wine Polyphenol Content during the Fermentation of Vitis vinifera CS Grapes and Its Relationship with the Presence of Minerals in the Resulting Wine. Appl. Sci. 2023, 13, 8314. [Google Scholar] [CrossRef]
- Biss, A.J.; Ellis, R.H. Minerality in Wine: Textual Analysis of Chablis Premier Cru Tasting Notes. Aust. J. Grape Wine Res. 2024, 2024, 4299446. [Google Scholar] [CrossRef]
- Shimizu, H.; Akamatsu, F.; Kamada, A.; Koyama, K.; Iwashita, K.; Goto-Yamamoto, N. Variation in the Mineral Composition of Wine Produced Using Different Winemaking Techniques. J. Biosci. Bioeng. 2020, 130, 166–172. [Google Scholar] [CrossRef]
- Núñez, L.; Serratosa, M.P.; Godoy, A.; Fariña, L.; Dellacassa, E.; Moyano, L. Comparison of Physicochemical Properties, Amino Acids, Mineral Elements, Total Phenolic Compounds, and Antioxidant Capacity of Cuban Fruit and Rice Wines. Food Sci. Nutr. 2021, 9, 3673–3682. [Google Scholar] [CrossRef]
- Chen, K.; Xue, H.; Shi, Q.; Zhang, F.; Ma, Q.; Sun, J.; Liu, Y.; Tang, Y.; Wang, W. Geographical Identification of Chinese Wine Based on Chemometrics Combined with Mineral Elements, Volatile Components and Untargeted Metabonomics. Food Chem. X 2024, 22, 101412. [Google Scholar] [CrossRef]
- Gao, F.; Hao, X.; Zeng, G.; Guan, L.; Wu, H.; Zhang, L.; Wei, R.; Wang, H.; Li, H. Identification of the Geographical Origin of Ecolly (Vitis vinifera L.) Grapes and Wines from Different Chinese Regions by ICP-MS Coupled with Chemometrics. J. Food Compos. Anal. 2022, 105, 104248. [Google Scholar] [CrossRef]
- Nardi, T. Microbial Resources as a Tool for Enhancing Sustainability in Winemaking. Microorganisms 2020, 8, 507. [Google Scholar] [CrossRef]
- Husain, R.; Vikram, N.; Pandey, S.; Yadav, G.; Bose, S.K.; Ahamad, A.; Shamim, M.; Kumar, K.G.; Khan, N.A.; Zuhaib, M.; et al. Microorganisms: An Eco-Friendly Tools for the Waste Management and Environmental Safety. In Biotechnological Innovations for Environmental Bioremediation; Arora, S., Kumar, A., Ogita, S., Yau, Y.-Y., Eds.; Springer Nature: Singapore, 2022; pp. 949–981. ISBN 9789811690006. [Google Scholar]
- Ayogu, P.; Martins, V.; Gerós, H. Grape Berry Native Yeast Microbiota: Advancing Trends in the Development of Sustainable Vineyard Pathogen Biocontrol Strategies. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Johnston-Monje, D.; Vergara, L.I.; Lopez-Mejia, J.; White, J.F. Plant Microbiomes as Contributors to Agricultural Terroir. Front. Agron. 2023, 5, 1216520. [Google Scholar] [CrossRef]
- Pinto, C.; Pinho, D.; Cardoso, R.; Custódio, V.; Fernandes, J.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Wine Fermentation Microbiome: A Landscape from Different Portuguese Wine Appellations. Front. Microbiol. 2015, 6, 905. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Feng, S.; Bi, P.; Han, J.; Li, S.; Liu, X.; Zhang, Z.; Long, F.; Guo, J. Simultaneous Inoculation of Non-Saccharomyces Yeast and Lactic Acid Bacteria for Aromatic Kiwifruit Wine Production. Food Microbiol. 2024, 123, 104589. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, K.; Mas, A.; Rozès, N.; Jara, C.; Portillo, M.D.C. The Impact of the Inoculation of Different Pied de Cuve on the Chemical and Organoleptic Profiles of Wines. Microorganisms 2024, 12, 1655. [Google Scholar] [CrossRef]
- Su, Y.; Dong, Q.; Chen, Y.; Wang, R.; Jiang, J.; Qin, Y.; Song, Y.; Liu, Y. Impact of Sequential Inoculation Timing on the Quality of Wine Fermented by Indigenous Lachancea thermotolerans and Saccharomyces cerevisiae. LWT 2024, 204, 116438. [Google Scholar] [CrossRef]
- Zhu, L.-X.; Hui, W.; Zhang, M.-M.; Shi, Y.; Xiang, X.-F.; Lan, Y.-B.; Zhang, R.-L. Aromatic and Chemical Differences between Msalais Wines Produced at Traditional Craft Workshops and Modern Plants. J. Food Compos. Anal. 2023, 116, 105029. [Google Scholar] [CrossRef]
- Choudhary, S.; Garg, V.K. Contribution of Microbial Mechanism and Artificial Intelligence in Wine Production. In Proceedings of the 2024 11th International Conference on Computing for Sustainable Global Development (INDIACom), New Delhi, India, 28 February–1 March 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 247–251. [Google Scholar]
- Torres-Guardado, R.; Esteve-Zarzoso, B.; Reguant, C.; Bordons, A. Microbial Interactions in Alcoholic Beverages. Int. Microbiol. 2022, 25, 1–15. [Google Scholar] [CrossRef]
- L. Binati, R.; Ferremi Leali, N.; Avesani, M.; Salvetti, E.; Felis, G.E.; Monti, F.; Torriani, S. Application of FTIR Microspectroscopy in Oenology: Shedding Light on Cell Wall Composition of Saccharomyces cerevisiae Strains. Food Bioprocess Technol. 2024, 17, 1596–1609. [Google Scholar] [CrossRef]
- Novák, M.; Synytsya, A.; Gedeon, O.; Slepička, P.; Procházka, V.; Synytsya, A.; Blahovec, J.; Hejlová, A.; Čopíková, J. Yeast β(1-3),(1-6)-d-Glucan Films: Preparation and Characterization of Some Structural and Physical Properties. Carbohydr. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]
- Nie, M.; Luo, J.; Xiao, M.; Chen, J.; Bao, K.; Zhang, W.; Chen, J.; Li, B. Structural Differences between Fusarium Strains Investigated by FT-IR Spectroscopy. Biochemistry 2007, 72, 61–67. [Google Scholar] [CrossRef]
- Chang, J.; Li, W.; Liu, Q.; Zhou, Y.; Chen, X.; Lyu, Q.; Liu, G. Preparation, Properties, and Structural Characterization of β-Glucan/Pullulan Blend Films. Int. J. Biol. Macromol. 2019, 140, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.S.; Jespersen, B.M.; Møller, B.L.; Lærke, H.N.; Larsen, F.H.; Engelsen, S.B. Comparative Spectroscopic and Rheological Studies on Crude and Purified Soluble Barley and Oat β-Glucan Preparations. Food Res. Int. 2010, 43, 2417–2424. [Google Scholar] [CrossRef]
- Badshah, S.L.; Riaz, A.; Muhammad, A.; Tel Çayan, G.; Çayan, F.; Emin Duru, M.; Ahmad, N.; Emwas, A.-H.; Jaremko, M. Isolation, Characterization, and Medicinal Potential of Polysaccharides of Morchella Esculenta. Molecules 2021, 26, 1459. [Google Scholar] [CrossRef]
- Sousa, P.; Tavares-Valente, D.; Pereira, C.F.; Pinto-Ribeiro, I.; Azevedo-Silva, J.; Madureira, R.; Ramos, Ó.L.; Pintado, M.; Fernandes, J.; Amorim, M. Circular Economyeast: Saccharomyces cerevisiae as a Sustainable Source of Glucans and Its Safety for Skincare Application. Int. J. Biol. Macromol. 2024, 265, 130933. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.C.; Clerigo, M.M.; Paano, A.M.C. Extraction, Isolation and MALDI-QTOF MS/MS Analysis of β- d -Glucan from the Fruiting Bodies of Daedalea Quercina. Int. J. Biol. Macromol. 2016, 93, 226–234. [Google Scholar] [CrossRef]
- Hromádková, Z.; Ebringerová, A.; Sasinková, V.; Šandula, J.; Hříbalová, V.; Omelková, J. Influence of the Drying Method on the Physical Properties and Immunomodulatory Activity of the Particulate (1→3)-β-d-Glucan from Saccharomyces cerevisiae. Carbohydr. Polym. 2003, 51, 9–15. [Google Scholar] [CrossRef]
- Alzorqi, I.; Sudheer, S.; Lu, T.-J.; Manickam, S. Ultrasonically Extracted β-d-Glucan from Artificially Cultivated Mushroom, Characteristic Properties and Antioxidant Activity. Ultrason. Sonochem. 2017, 35, 531–540. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Pinto, M.; Coelho, E.; Nunes, A.; Brandão, T.; Coimbra, M.A. Valuation of Brewers Spent Yeast Polysaccharides: A Structural Characterization Approach. Carbohydr. Polym. 2015, 116, 215–222. [Google Scholar] [CrossRef]
- Lante, A.; Canazza, E.; Tessari, P. Beta-Glucans of Cereals: Functional and Technological Properties. Nutrients 2023, 15, 2124. [Google Scholar] [CrossRef]
| Winery Yeast Lees | Abbreviation | Grape Variety | Harvest Year | Manufacturer | Recovery Stage |
|---|---|---|---|---|---|
| Semi-dry white wine | SVAM | Muscat | 2023 | Chateau Vartely winery | Collected after the wine was stored |
| Dry red wine | SVRS | Shiraz | 2023 | Purcari winery | Collected after the wine was stored |
| Sweet white wine (Ice wine) | SVR | Traminer and Muscat | 2023 | Poiana winery | Collected after fermentation |
| White sparkling wine | SVS | Chardonnay, Pinot Blanc and Pinot Noir | 2022 | Purcari winery | Collected after disgorging the sparkling wine |
| Nr. | Parameters | Wine Lees | |||
|---|---|---|---|---|---|
| Semi-Dry White Wine (SVAM) | Sweet White Wine (SVR) | Dry Red Wine (SVRS) | White Sparkling Wine (SVS) | ||
| Physico-chemical characteristics | |||||
| 1 | Ash, % | 0.04 ± 0.11 a | 0.03 ± 0.26 b | 0.04 ± 0.26 a | 0.04 ± 0.14 a |
| 2 | Dry matter, % | 24.16 ± 0.14 a | 21.09 ± 0.12 b | 26.09 ± 0.17 b | 12.22 ± 0.04 c |
| 3 | Carbohydrates, % SU | 21.16 ± 0.65 b | 24.61 ± 0.66 c | 19.15 ± 0.09 a | 20.31 ± 0.65 a |
| 4 | Lipids, % SU | 8.11 ± 0.49 a | 11.09 ± 0.95 b | 4.71 ± 0.21 c | 8.13 ± 0.96 a |
| 5 | Proteins, % SU | 41.12 ± 0.87 a | 32.62 ± 0.19 c | 45.98 ± 0.23 b | 42.42 ± 0.43 a |
| 6 | Total phenolic substances, mg GAE/kg | 162.12 ± 0.84 b | 192.59 ± 1.04 a | 3350.17 ± 2.42 c | 142.19 ± 0.77 b |
| 7 | Total anthocyanins, mg GAE/kg fw | 227.48 ± 1.14 a | 277.12 ± 0.94 a | 1311.08 ± 0.92 c | 269.98 ± 0.49 b |
| Mineral content, mg/g | |||||
| 1 | K | 59.1 ± 0.14 c | 67.9 ± 0.11 b | 99.7 ± 0.10 a | 93.2 ± 0.04 a |
| 2 | Na | 0.1 ± 0.41 b | 0.3 ± 0.04 b | 0.8 ± 0.24 c | 0.4 ± 0.11 a |
| 3 | Mg | 0.4 ± 0.16 b | 0.6 ± 0.23 c | 1.4 ± 0.04 a | 1.1 ± 0.21 a |
| 4 | P | 3.2 ± 0.04 b | 4.6 ± 0.29 a | 15.1 ± 0.07 c | 4.1 ± 0.43 a |
| 5 | Ca | 2.6 ± 0.11 a | 1.9 ± 0.19 b | 6.9 ± 0.17 c | 2.7 ± 0.07 a |
| 6 | S | 1.1 ± 0.21 a | 2.5 ± 0.41 b | 3.9 ± 031 c | 1.9 ± 0,15 a |
| Sample | Yield of β-Glucan Compounds, % | Sample | Yield of β-Glucan Compounds, % | |||
|---|---|---|---|---|---|---|
| Acid–base method assisted with ultrasound | ||||||
| Semi-dry white wine | 1 M NaOH, 25 kHz | SVAM 01AA | 9.65 ± 0.30 a | 1 M NaOH, 45 kHz | SVAM 11AA | 3.47 ± 0.66 b |
| Dry red wine | SVRS 01AA | 14.38 ± 0.45 a | SVRS 11AA | 16.39 ± 0.39 a | ||
| Sweet white wine | SVR 01AA | 5.99 ± 0.16 a | SVR 11AA | 5.17 ± 0.19 a | ||
| Sparkling white wine | SVS 01AA | 14.33 ± 0.22 b | SVS 11AA | 5.27 ± 0.25 a | ||
| Semi-dry white wine | 1.5 M NaOH, 25 kHz | SVAM 02AA | 19.76 ± 0.58 a | 1.5 M NaOH, 45 kHz | SVAM 11AA | 5.72 ± 0.44 b |
| Dry red wine | SVRS 02AA | 18.98 ± 0.29 b | SVRS 11AA | 9.49 ± 0.29 a | ||
| Sweet white wine | SVR 02AA | 9.99 ± 0.30 a | SVR 11AA | 6.70 ± 0.10 a | ||
| Sparkling white wine | SVS 02AA | 12.68 ± 0.12 b | SVS 11AA | 5.11 ± 0.17 c | ||
| Semi-dry white wine | 2 M NaOH, 25 kHz | SVAM 03AA | 5.89 ± 0.56 a | 2 M NaOH, 45 kHz | SVAM 12AA | 5.89 ± 0.33 a |
| Dry red wine | SVRS 03AA | 10.64 ± 0.37 a | SVRS 12AA | 11.36 ± 0.16 b | ||
| Sweet white wine | SVR 03AA | 9.65 ± 0.21 a | SVR 12AA | 6.23 ± 0.24 b | ||
| Sparkling white wine | SVS 03AA | 14.38 ± 0.15 a | SVS 12AA | 8.07 ± 0.08 c | ||
| Autolysis assisted with ultrasound method | ||||||
| Semi-dry white wine | Autolysis, 25 kHz | SVAM 01A | 37.28 ± 0.29 a | Autolysis, 45 kHz | SVAM 11A | 39.36 ± 0.19 b |
| Dry red wine | SVRS 01A | 26.59 ± 0.0.6 a | SVRS 11A | 30.91 ± 0.07 b | ||
| Sweet white wine | SVR 01A | 21.97 ± 0.07 a | SVR 11A | 21.03 ± 0.20 a | ||
| Sparkling white wine | SVS 01A | 18.95 ± 0.49 a | SVS 11A | 37.06 ± 0.06 c | ||
| Extraction without the use of ultrasound | ||||||
| Semi-dry white wine | SVAM AA | 2.77 ± 0.14 c | SVAM 01A | 37.62 ± 0.20 a | ||
| Dry red wine | SVRS AA | 13.80 ± 0.05 b | SVRS 01A | 26.59 ± 0.04 c | ||
| Sweet white wine | 2 M NaOH | SVR AA | 6.46 ± 0.22 c | Autolysis | SVR 01A | 21.90 ± 0.17 a |
| Sparkling white wine | SVS AA | 17.30 ± 0.30 c | SVS 01A | 41.34 ± 0.31 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chioru, A.; Chirsanova, A.; Dabija, A.; Avrămia, I.; Boiştean, A.; Chetrariu, A. Extraction Methods and Characterization of β-Glucans from Yeast Lees of Wines Produced Using Different Technologies. Foods 2024, 13, 3982. https://doi.org/10.3390/foods13243982
Chioru A, Chirsanova A, Dabija A, Avrămia I, Boiştean A, Chetrariu A. Extraction Methods and Characterization of β-Glucans from Yeast Lees of Wines Produced Using Different Technologies. Foods. 2024; 13(24):3982. https://doi.org/10.3390/foods13243982
Chicago/Turabian StyleChioru, Ana, Aurica Chirsanova, Adriana Dabija, Ionuț Avrămia, Alina Boiştean, and Ancuța Chetrariu. 2024. "Extraction Methods and Characterization of β-Glucans from Yeast Lees of Wines Produced Using Different Technologies" Foods 13, no. 24: 3982. https://doi.org/10.3390/foods13243982
APA StyleChioru, A., Chirsanova, A., Dabija, A., Avrămia, I., Boiştean, A., & Chetrariu, A. (2024). Extraction Methods and Characterization of β-Glucans from Yeast Lees of Wines Produced Using Different Technologies. Foods, 13(24), 3982. https://doi.org/10.3390/foods13243982









