Ultrasonic Extraction of Polysaccharides from Dendrobium officinale Leaf: Kinetics, In Vitro Activities, and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Pretreatment
2.2. Ultrasonic Water Extraction of DOLP
2.3. Development of Kinetic Model
2.4. Analysis of the Content of DOLP, Total Flavone, Polyphenol, and Protein
2.5. Preparation of Samples of DOL Extracts and DOLP
2.5.1. Samples of DOL Extracts
2.5.2. Sample of DOLP
2.6. In Vitro Activity Assay of DOL Extracts and DOLP
2.6.1. In Vitro Antioxidant Activity Assay
DPPH Radical Scavenging Activity Assay
ABTS Radical Scavenging Activity Assay
OH Radical Scavenging Activity Assay
Ferrous Reducing Power Assay
2.6.2. In Vitro α-Amylase Inhibitory Activity Assay
2.6.3. In Vitro α-Glucosidase Inhibitory Activity Assay
2.7. UV and FT−IR Spectrum Analysis
2.8. Monosaccharide Composition Analysis
2.9. Statistical Analysis
3. Results
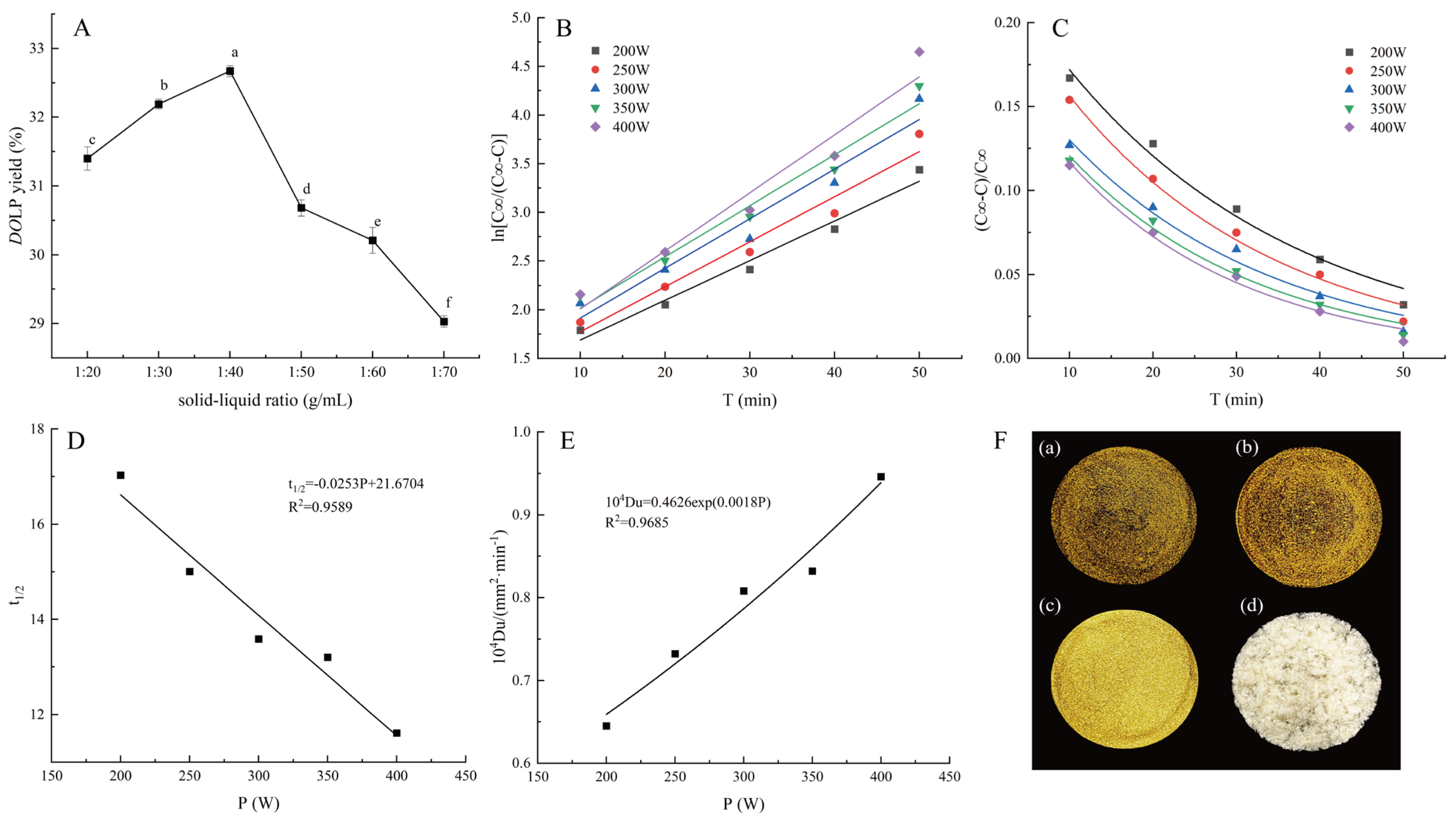
3.1. Kinetics of Ultrasonic Extraction of DOLP
3.1.1. The Effect of Solid–Liquid Ratio on the DOLP Yield
3.1.2. The Effect of Ultrasonic Power and Time on the DOLP Concentration
3.1.3. Rate Constant Determination
3.1.4. Relative Extraction Remaining Rate Determination
3.1.5. Half-Life Determination
3.1.6. Diffusion Coefficient (Du) Determination
3.2. Samples of DOL Extracts and DOLP as Well as Changes After Purification
3.3. In Vitro Antioxidant Activity of DOL Extracts and DOLP
3.4. α-Amylase and α-Glucosidase Inhibitory Activity
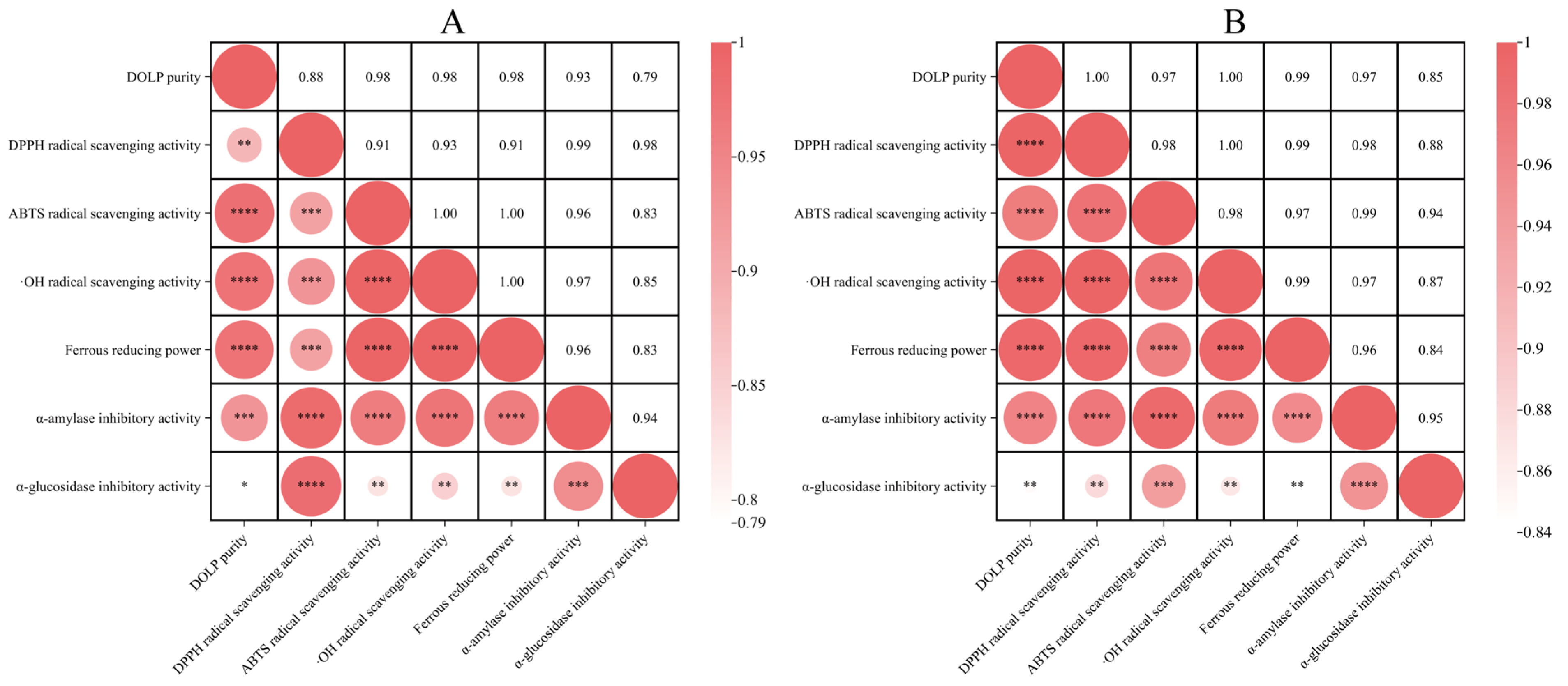
3.5. Pearson Correlation Analysis
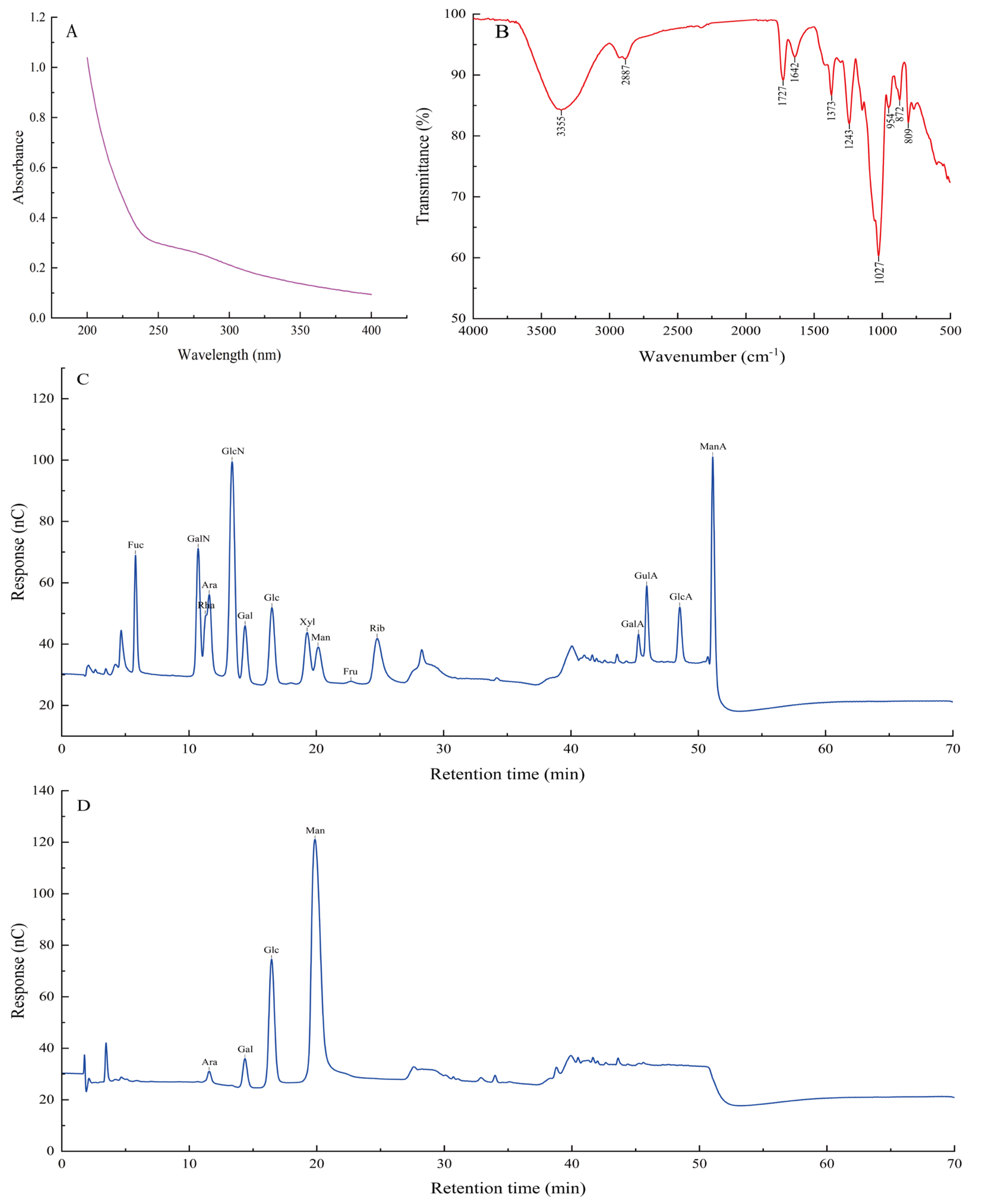
3.6. UV and FT−IR Spectrum Analysis
3.7. Monosaccharide Composition Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Liu, S.; Wang, Z.; Zhang, G. Ultrasonic-assisted extraction of polysaccharide from Dendrobium officinale: Kinetics, thermodynamics and optimization. Biochem. Eng. 2022, 177, 108227. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, characterization and biological activity of polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xing, Y.; Wang, Y.; Ren, X.; Zhang, D.; Dai, J.; Dong, Y. Dendrobium officinale polysaccharide prevents diabetes via the regulation of gut microbiota in prediabetic mice. Foods 2023, 12, 2310. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.; Xu, Y.; Lo, K.; Zheng, H.; Hu, H.; Lin, Y. Study on the polar extracts of Dendrobium nobile, D. officinale, D. loddigesii, and Flickingeria fimbriata: Metabolite identification, content evaluation, and bioactivity assay. Molecules 2018, 23, 1185. [Google Scholar] [CrossRef]
- He, Y.; Li, L.; Chang, H.; Cai, B.; Gao, H.; Chen, G.; Yan, Y. Research progress on extraction, purification, structure and biological activity of Dendrobium officinale polysaccharides. Front. Nutr. 2022, 9, 965073. [Google Scholar] [CrossRef]
- Mu, Y.; Cheng, L.; Gong, X.; Ma, J.; Zhang, S.; Mu, Y.; Zhao, C. Simultaneous determination of nine phenolic compounds in imitation wild Dendrobium officinale samples using ultrahigh-performance liquid chromatography-tandem mass spectrometry. Front. Nutr. 2023, 10, 1129953. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Wang, K.; Cao, X. Structural characterisation and bioactivity of polysaccharides isolated from fermented Dendrobium officinale. J. Sci. Food. Agric. 2022, 102, 280–290. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Ren, X. Inhibitory effect of Dendrobium officinale polysaccharide on human gastric cancer cell xenografts in nude mice. Food Sci. Technol. 2017, 38, 78–83. [Google Scholar] [CrossRef]
- Chan, C.F.; Wu, C.T.; Huang, W.Y.; Lin, W.S.; Wu, H.W.; Huang, T.K.; Lin, Y.S. Antioxidation and melanogenesis inhibition of various Dendrobium tosaense extracts. Molecules 2018, 23, 1810. [Google Scholar] [CrossRef]
- Xie, S.Z.; Liu, B.; Zhang, D.D.; Zha, X.Q.; Pan, L.H.; Luo, J.P. Intestinal immunomodulating activity and structural characterization of a new polysaccharide from stems of Dendrobium officinale. Food Funct. 2016, 7, 2789–2799. [Google Scholar] [CrossRef]
- Chen, H.; Shi, X.; Zhang, L.; Yao, L.; Cen, L.; Li, L.; Wei, C. Ultrasonic extraction process of polysaccharides from Dendrobium nobile Lindl.: Optimization, physicochemical properties and anti-inflammatory activity. Foods 2022, 11, 2957. [Google Scholar] [CrossRef] [PubMed]
- Jie, X.; Feng, Y.; Jiahao, F.; Ganggui, L.; Jiani, Y.; Zhongyu, X.; Zongsuo, L. Comprehensive chemical profiling of two Dendrobium species and identification of anti-hepatoma active constituents from Dendrobium chrysotoxum by network pharmacology. BMC Complement. Med. Ther. 2023, 23, 217. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shang, Z.Z.; Li, Q.M.; Zha, X.Q.; Wu, D.L.; Yu, N.J.; Luo, J.P. Structural features and anti-gastric cancer activity of polysaccharides from stem, root, leaf and flower of cultivated Dendrobium huoshanense. Int. J. Biol. Macromol. 2020, 143, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Liu, J.; Liang, J.; Si, J.; Wu, S. Dendrobium officinale leaves as a new antioxidant source. J. Funct. Foods. 2017, 37, 400–415. [Google Scholar] [CrossRef]
- Cai, G.; Dong, H.; Liu, S.; Wu, W.; Yang, H. Comparative Evaluation of the Physiochemical Properties, and Antioxidant and Hypoglycemic Activities of Dendrobium officinale Leaves Processed Using Different Drying Techniques. Antioxidants 2023, 12, 1911. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Huang, W.; Nie, S. Metabolism amelioration of Dendrobium officinale polysaccharide on type II diabetic rats. Food Hydrocoll. 2020, 102, 105582. [Google Scholar] [CrossRef]
- Xiong, J.; Fang, J.; Chen, D.; Xu, H. Physicochemical property changes of Dendrobium officinale leaf polysaccharide LDOP-A and it promotes GLP-1 secretion in NCI-H716 cells by simulated saliva-gastrointestinal digestion. Food Sci. Nutr. 2023, 11, 2686–2696. [Google Scholar] [CrossRef]
- Aoi, W.; Iwasa, M.; Marunaka, Y. Metabolic functions of flavonoids: From human epidemiology to molecular mechanism. Neuropeptides 2021, 88, 102163. [Google Scholar] [CrossRef]
- Cao, H.; Ji, Y.; Li, S.; Lu, L.; Tian, M.; Yang, W.; Li, H. Extensive metabolic profiles of leaves and stems from the medicinal plant Dendrobium officinale Kimura et Migo. Metabolites 2019, 9, 215. [Google Scholar] [CrossRef]
- Yang, K.; Lu, T.; Zhan, L.; Zhou, C.; Zhang, N.; Lei, S.; Chen, S. Physicochemical characterization of polysaccharide from the leaf of Dendrobium officinale and effect on LPS induced damage in GES-1 cell. J. Biol. Macromol. 2020, 149, 320–330. [Google Scholar] [CrossRef]
- Xie, H.; Fang, J.; Farag, M.A.; Li, Z.; Sun, P.; Shao, P. Dendrobium officinale leaf polysaccharides regulation of immune response and gut microbiota composition in cyclophosphamide-treated mice. Food Chem. X 2022, 13, 100235. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Tian, W.; Chen, H.; Yang, Y.; Xu, Y.; Chen, Y.; Du, B. Structural characterization and immunoregulatory activity of polysaccharides from Dendrobium officinale leaves. J. Food Biochem. 2022, 46, e14023. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, M.; Yan, H.; Luo, L.; Fang, X.; He, L.; Liu, H. Purification, structural characterization, and immunomodulatory activity of two polysaccharides from Portulaca oleracea L. Int. J. Biol. Macromol. 2024, 264, 130508. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Liang, Q.; He, X.; Du, X.; Liu, Y.; Liu, Y.; Fang, C. Hypoglycemic effects of dendrobium officinale leaves. Front. Pharmacol. 2023, 14, 1163028. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shao, C.; Huang, P.; Yu, D.; Yang, J.; Wan, H.; He, Y. Optimization, characterization of Astragalus polysaccharides, and evaluation of anti-inflammation effect in primary cultured astrocytes via HMGB1/RAGE/NF-κB/NLRP3 signal pathway. Ind. Crop. Prod. 2023, 197, 116594. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Li, Q.; Liu, C.; Han, J.; Zhu, L.; Liu, H. Rheological properties and chain conformation of soy hull water-soluble polysaccharide fractions obtained by gradient alcohol precipitation. Food Hydrocoll. 2019, 91, 34–39. [Google Scholar] [CrossRef]
- Yang, B.; Luo, Y.; Wu, Q.; Yang, Q.; Kan, J. Hovenia dulcis polysaccharides: Influence of multi-frequency ultrasonic extraction on structure, functional properties, and biological activities. Int. J. Biol. Macromol. 2020, 148, 1010–1020. [Google Scholar] [CrossRef]
- Hu, X.; Xu, F.; Li, J.; Li, J.; Mo, C.; Zhao, M.; Wang, L. Ultrasonic-assisted extraction of polysaccharides from coix seeds: Optimization, purification, and In Vitro digestibility. Food Chem. 2022, 374, 131636. [Google Scholar] [CrossRef]
- Sun, C.; Wang, G.; Sun, J.; Yin, J.; Huang, J.; Li, Z.; Qu, W. A New Method of Extracting Polygonatum sibiricum Polysaccharide with Antioxidant Function: Ultrasound-Assisted Extraction-Deep Eutectic Solvents Method. Foods 2023, 12, 3438. [Google Scholar] [CrossRef]
- Al-Ajalein, A.H.A.S.; Shafie, M.H.; Yap, P.G.; Kassim, M.A.; Naharudin, I.; Wong, T.W.; Gan, C.Y. Microwave-assisted extraction of polysaccharide from Cinnamomum cassia with anti-hyperpigmentation properties: Optimization and characterization studies. Int. J. Biol. Macromol. 2023, 226, 321–335. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Qi, S.; Fan, M.; Zheng, S.; Huang, Q.; Lu, X. Ultrasonic-microwave-assisted extraction for enhancing antioxidant activity of Dictyophora indusiata polysaccharides: The difference mechanisms between single and combined assisted extraction. Ultrason. Sonochem. 2023, 95, 106356. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yan, X.; Liang, J.; Li, S.; He, H.; Xiong, Q.; Huang, S. Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohydr. Polym. 2018, 198, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chen, H.; Ma, J.; Zhang, Y.; Chen, H.; Wei, T.; Li, J. Enzyme-assisted extraction, characterization, and in vitro antioxidant activity of polysaccharides from Potentilla anserina L. Front. Nutr. 2023, 10, 1216572. [Google Scholar] [CrossRef]
- Li, J.; Tao, W.; Zhou, W.; Xing, J.; Luo, M.; Lu, S.; Yang, Y. Dendrobium officinale leaf polysaccharide has a dual effect of alleviating the syndromes of immunosuppressed mice and modulating immune system of normal mice. J. Funct. Foods. 2024, 113, 105974. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ma, X.; Zhang, K.; Li, S.; Wang, X.; Zhu, X. Study on the kinetic model, thermodynamic and physicochemical properties of Glycyrrhiza polysaccharide by ultrasonic assisted extraction. Ultrason. Sonochem. 2019, 51, 249–257. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wang, C.S.; Wei, M.C. A green approach for the extraction and characterization of oridonin and ursolic and oleanolic acids from Rabdosia rubescens and its kinetic behavior. Food Chem. 2020, 319, 126582. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation In Vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT-Food Sci. Technol. 2021, 150, 111932. [Google Scholar] [CrossRef]
- Yu, K.; Huang, X.; Yu, Z.; Chen, C.; Li, P.; Wu, D.; Du, C. Application of steam explosion pretreatment for accelerating the phenolics extraction from pomegranate peel: Mechanism and modeling. J. Food Eng. 2023, 357, 111629. [Google Scholar] [CrossRef]
- Yang, Y.L.; Guan, E.Q.; Zhang, T.J.; Xu, F.; Li, M.M.; Bian, K. Behavior of wheat flour dough at different pretreated temperatures through rheological characteristics and molecular interactions of proteins. Food Chem. 2023, 404, 134188. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, S.; Sun, W.; Yang, Y.; Xu, Y.; Tang, Y.; Zhang, Y. Study on the mechanisms by which pumpkin polysaccharides regulate abnormal glucose and lipid metabolism in diabetic mice under oxidative stress. Int. J. Biol. Macromol. 2024, 270, 132249. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Kou, R.; Huang, X.; Wang, N.; Di, D.; Wang, H.; Liu, J. Separation of polysaccharides from Lycium barbarum L. by high-speed countercurrent chromatography with aqueous two-phase system. Int. J. Biol. Macromol. 2024, 256, 128282. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Huang, W.; Suo, J.; Chen, X.; Ding, K.; Zhang, H. Antioxidant and anti-inflammatory activities of an anti-diabetic polysaccharide extracted from Gynostemma pentaphyllum herb. Int. J. Biol. Macromol. 2020, 145, 484–491. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Liang, X.; Zheng, X.; Shu, Z.; Sun, Q.; Li, N. Antioxidant and antibacterial activities of a polysaccharide produced by Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 2023, 233, 123628. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, J.; Chen, Y.; Ma, Y.; Yang, Q.; Fan, Y.; Liao, W. Extraction, structural characterization and antioxidant activity of turmeric polysaccharides. LWT-Food Sci. Technol. 2022, 154, 112805. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, A.; Benjakul, S. α-amylase inhibitory activity of chitooligosaccharide from shrimp shell chitosan and its epigallocatechin gallate conjugate: Kinetics, fluorescence quenching and structure–activity relationship. Food Chem. 2023, 403, 134456. [Google Scholar] [CrossRef]
- Wang, B.H.; Cao, J.J.; Zhang, B.; Chen, H.Q. Structural characterization, physicochemical properties and α-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohydr. Polym. 2019, 211, 227–236. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Huang, G.; Huang, H. Ultrasound-assisted extraction, analysis and antioxidant activity of polysaccharide from the rinds of Garcinia mangostana L. Ultrason. Sonochem. 2023, 97, 106474. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Chen, B.; Hou, Y.; Wen, Y.; Gan, L.; Wu, R. Ultrasonic assisted extraction, characterization and gut microbiota-dependent anti-obesity effect of polysaccharide from Pericarpium Citri Reticulatae ‘Chachiensis’. Ultrason. Sonochem. 2023, 95, 106383. [Google Scholar] [CrossRef]
- Wang, K.; Guo, J.; Cheng, J.; Zhao, X.; Ma, B.; Yang, X.; Shao, H. Ultrasound-assisted extraction of polysaccharide from spent Lentinus edodes substrate: Process optimization, precipitation, structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 191, 1038–1045. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Chen, R.; Li, Y.; Dong, H.; Liu, Z.; Li, S.; Yang, S.; Li, X. Optimization of ultrasonic extraction process of polysaccharides from Ornithogalum Caudatum Ait and evaluation of its biological activities. Ultrason. Sonochem. 2012, 19, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Lu, C.; Luo, J.; Zha, X. A new kinetic model of ultrasound-assisted extraction of polysaccharides from Chinese chive. Food Chem. 2016, 212, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jiang, X.; Li, Q.; Wang, J.; Lv, M.; Liu, J. Preparation of Phosphorylated Auricularia cornea var. Li. Polysaccharide Liposome Gel and Analysis of Its In Vitro Antioxidant Activity. Foods 2024, 13, 335. [Google Scholar] [CrossRef]
- Pang, X.; Wang, H.; Guan, C.; Chen, Q.; Cui, X.; Zhang, X. Impact of Molecular Weight Variations in Dendrobium officinale Polysaccharides on Antioxidant Activity and Anti-Obesity in Caenorhabditis elegans. Foods 2024, 13, 1040. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, W.; Li, X.; Chen, X.; Wang, Y.; Huang, G.; Jia, Z. Enzyme-Assisted Ultrasonic Extraction and Antioxidant Activities of Polysaccharides from Schizochytrium limacinum Meal. Foods 2024, 13, 880. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Wang, H.; Shen, B.; He, X.; Gu, W.; Wu, Q. Extraction optimization, isolation, preliminary structural characterization and antioxidant activities of the cell wall polysaccharides in the petioles and pedicels of Chinese herbal medicine Qian (Euryale ferox Salisb.). Int. J. Biol. Macromol. 2014, 64, 458–467. [Google Scholar] [CrossRef]
- Fan, J.; Wu, Z.; Zhao, T.; Sun, Y.; Ye, H.; Xu, R.; Zeng, X. Characterization, antioxidant and hepatoprotective activities of polysaccharides from Ilex latifolia Thunb. Carbohydr. Polym. 2014, 101, 990–997. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Qu, Z.; Xie, B. Antioxidant activities of different fractions of polysaccharide conjugates from green tea (Camellia Sinensis). Food Chem. 2008, 106, 559–563. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Andrae-Marobela, K.; Okatch, H.; Xiao, J. Tea polysaccharides as food antioxidants: An old woman’s tale? Food Chem. 2013, 138, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Thondre, P.S.; Ryan, L.; Henry, C.J.K. Barley β-glucan extracts as rich sources of polyphenols and antioxidants. Food Chem. 2011, 126, 72–77. [Google Scholar] [CrossRef]
- Nie, S.P.; Xie, M.Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 2011, 25, 144–149. [Google Scholar] [CrossRef]
- Renard, C.M.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Lin, C.L.; Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int. J. Biol. Macromol. 2009, 45, 146–151. [Google Scholar] [CrossRef]
- Barber, E.; Houghton, M.J.; Williamson, G. Flavonoids as human intestinal α-glucosidase inhibitors. Foods 2021, 10, 1939. [Google Scholar] [CrossRef]
- Gong, H.; Gan, X.; Qin, B.; Chen, J.; Zhao, Y.; Qiu, B.; Wang, H. Structural characteristics of steamed Polygonatum cyrtonema polysaccharide and its bioactivity on colitis via improving the intestinal barrier and modifying the gut microbiota. Carbohydr. Polym. 2024, 327, 121669. [Google Scholar] [CrossRef]

| Ultrasonic Power/W | |||||
|---|---|---|---|---|---|
| Ultrasonic Time/min | 200 | 250 | 300 | 350 | 400 |
| 10 | 7.552 ± 0.074 q | 7.874 ± 0.081 p | 8.180 ± 0.121 o | 8.424 ± 0.058 lm | 8.501 ± 0.069 kl |
| 20 | 7.904 ± 0.061 p | 8.310 ± 0.057 mn | 8.524 ± 0.061 kl | 8.769 ± 0.053 j | 8.891 ± 0.040 i |
| 30 | 8.256 ± 0.057 no | 8.608 ± 0.113 k | 8.753 ± 0.105 j | 9.052 ± 0.040 gh | 9.144 ± 0.060 fg |
| 40 | 8.528 ± 0.047 kl | 8.838 ± 0.087 ij | 9.021 ± 0.048 h | 9.243 ± 0.058 def | 9.342 ± 0.070 cd |
| 50 | 8.776 ± 0.046 ij | 9.098 ± 0.128 gh | 9.220 ± 0.074 ef | 9.419 ± 0.069 bc | 9.519 ± 0.013 ab |
| 60 | 9.067 ± 0.080 gh | 9.304 ± 0.105 cde | 9.365 ± 0.166 c | 9.549 ± 0.115 a | 9.610 ± 0.117 a |
| Ultrasonic Powers/W | Regression Equation | R2 | k/min−1 |
|---|---|---|---|
| 200 | ln[C∞/(C∞ − C)] = 0.0407t + 1.2826 | 0.9757 | 0.0407 |
| 250 | ln[C∞/(C∞ − C)] = 0.0462t + 1.3125 | 0.9629 | 0.0462 |
| 300 | ln[C∞/(C∞ − C)] = 0.0510t + 1.4053 | 0.9520 | 0.0510 |
| 350 | ln[C∞/(C∞ − C)] = 0.0525t + 1.4920 | 0.9705 | 0.0525 |
| 400 | ln[C∞/(C∞ − C)] = 0.0597t + 1.4113 | 0.9775 | 0.0597 |
| Ultrasonic Powers/W | Regression Equation | R2 |
|---|---|---|
| 200 | Y = 0.2452exp(−0.0355t) | 0.9991 |
| 250 | Y = 0.2327exp(−0.0398t) | 0.9873 |
| 300 | Y = 0.1956exp(−0.0407t) | 0.9780 |
| 350 | Y = 0.1875exp(−0.0441t) | 0.9890 |
| 400 | Y = 0.1880exp(−0.0476t) | 0.9883 |
| DOLP Purity/% | Total Flavone Content (g/100 g) | Polyphenol Content (g/100 g) | Protein Content (mg/g) | |
|---|---|---|---|---|
| Unpurified (vacuum freeze drying) | 44.90 ± 0.45 b | 0.97 ± 0.03 a | 18.34 ± 0.82 a | 3.90 ± 0.04 a |
| Purifying treatments: Ethanol + papain hydrolysis combined with Sevage reagent + dialysis (vacuum freeze drying) | 74.07 ± 0.52 a | 0.27 ± 0.05 b | 8.22 ± 0.14 b | 0.37 ± 0.01 b |
| DOL Extracts by VFD | DOL Extracts by MD | DOL Extracts by HAD | DOLP | |
|---|---|---|---|---|
| α-amylase | 5.71 ± 0.02 b | 4.80 ± 0.02 a | 6.68 ± 0.08 c | 9.78 ± 0.09 d |
| α-glucosidase | 7.06 ± 0.03 c | 5.07 ± 0.04 a | 6.40 ± 0.01 b | 14.39 ± 0.21 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Yang, X.; He, S.; Duan, T.; Liang, X.; Ma, S.; Gong, J. Ultrasonic Extraction of Polysaccharides from Dendrobium officinale Leaf: Kinetics, In Vitro Activities, and Characterization. Foods 2024, 13, 3737. https://doi.org/10.3390/foods13233737
Shi X, Yang X, He S, Duan T, Liang X, Ma S, Gong J. Ultrasonic Extraction of Polysaccharides from Dendrobium officinale Leaf: Kinetics, In Vitro Activities, and Characterization. Foods. 2024; 13(23):3737. https://doi.org/10.3390/foods13233737
Chicago/Turabian StyleShi, Xuerong, Xuzhong Yang, Shaotong He, Ting Duan, Xin Liang, Shuzhen Ma, and Jijun Gong. 2024. "Ultrasonic Extraction of Polysaccharides from Dendrobium officinale Leaf: Kinetics, In Vitro Activities, and Characterization" Foods 13, no. 23: 3737. https://doi.org/10.3390/foods13233737
APA StyleShi, X., Yang, X., He, S., Duan, T., Liang, X., Ma, S., & Gong, J. (2024). Ultrasonic Extraction of Polysaccharides from Dendrobium officinale Leaf: Kinetics, In Vitro Activities, and Characterization. Foods, 13(23), 3737. https://doi.org/10.3390/foods13233737





