Preparation, Characterization, Stability and In Vitro Release of a Pea Protein Fibril-Based Iron Fortificant via Self-Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
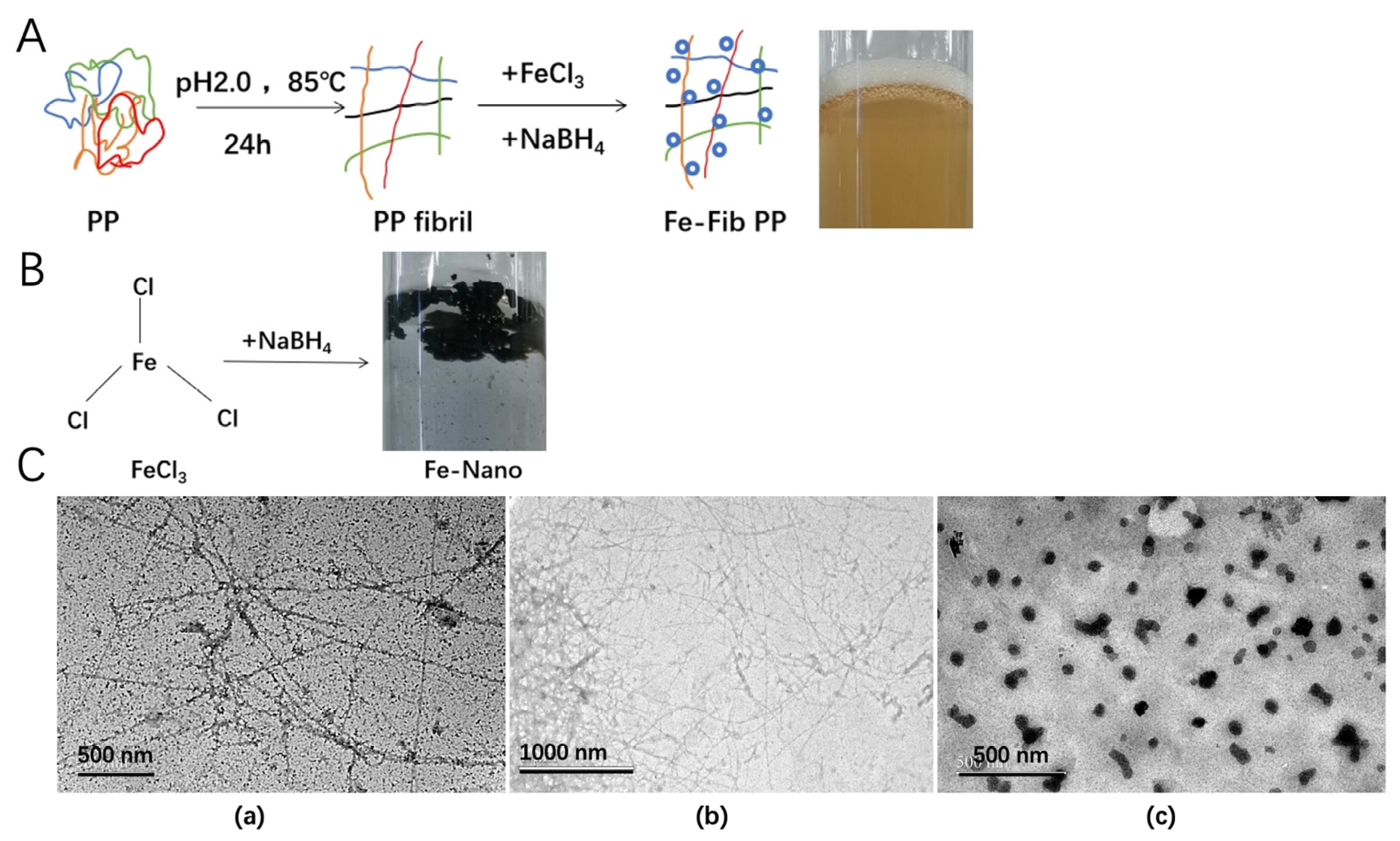
2.2. PP Fibril Preparation
2.3. Preparation of Iron–Pea Protein Fibril (Fe-Fib PP) Nanocomposites
2.4. Thioflavin T (ThT) Fluorescence Analysis
2.5. SDS-PAGE
2.6. Atomic Force Microscopy (AFM)
2.7. Free Sulfhydryl Content
2.8. Antioxidant Activity
2.8.1. Reducing Power
2.8.2. ABTS+ Scavenging Activity
2.8.3. Reducing Effect on Iron Ions by PP Fibrils
2.9. Transmission Electron Microscopy (TEM)
2.10. Stability Evaluation
2.11. X-Ray Photoelectron Spectroscopy (XPS)
2.12. Bioaccessibility
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Pea Protein Fibrils
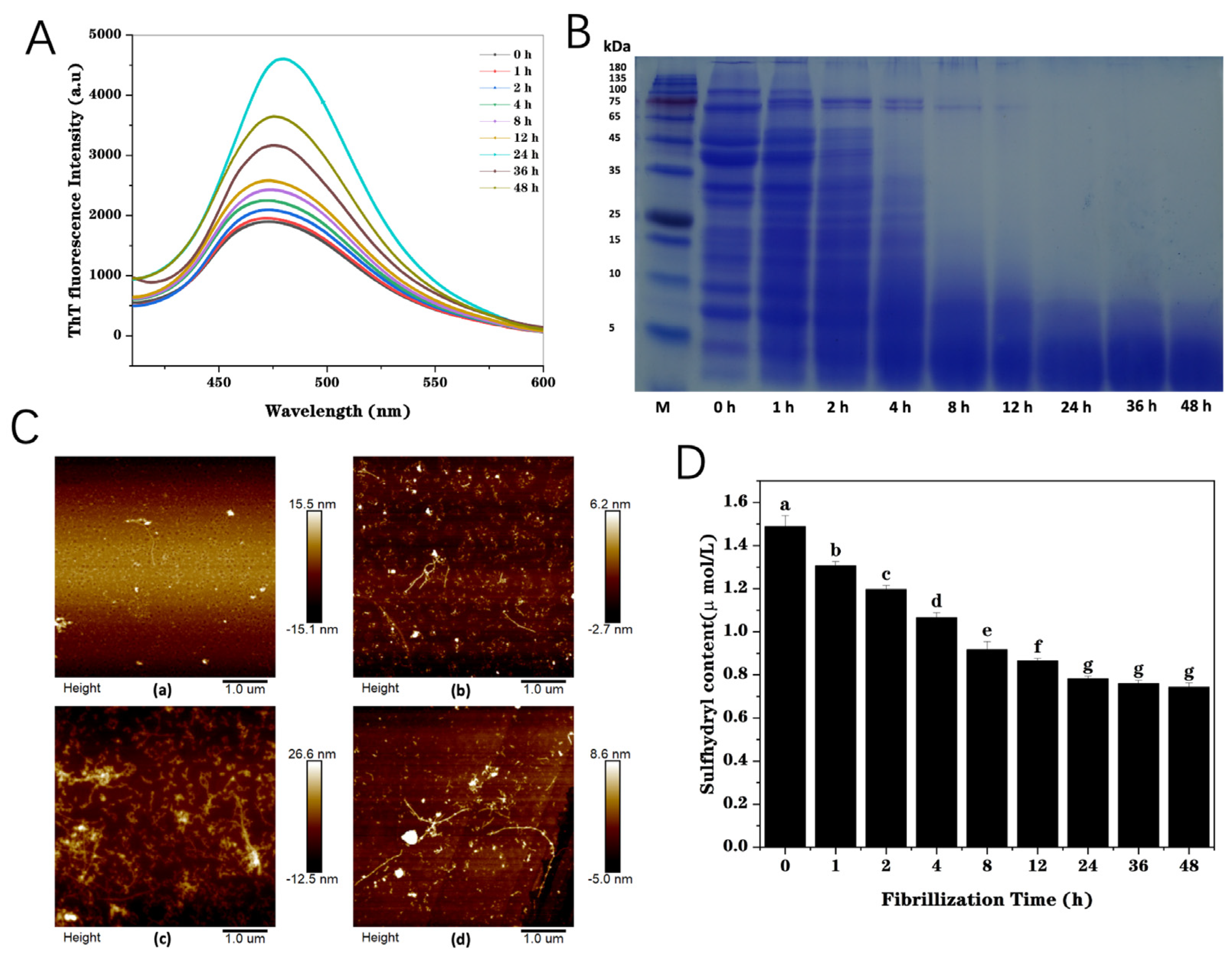
3.1.1. ThT Fluorescence
3.1.2. SDS-PAGE
3.1.3. Atomic Force Microscopy (AFM)
3.1.4. Free Sulfhydryl Content
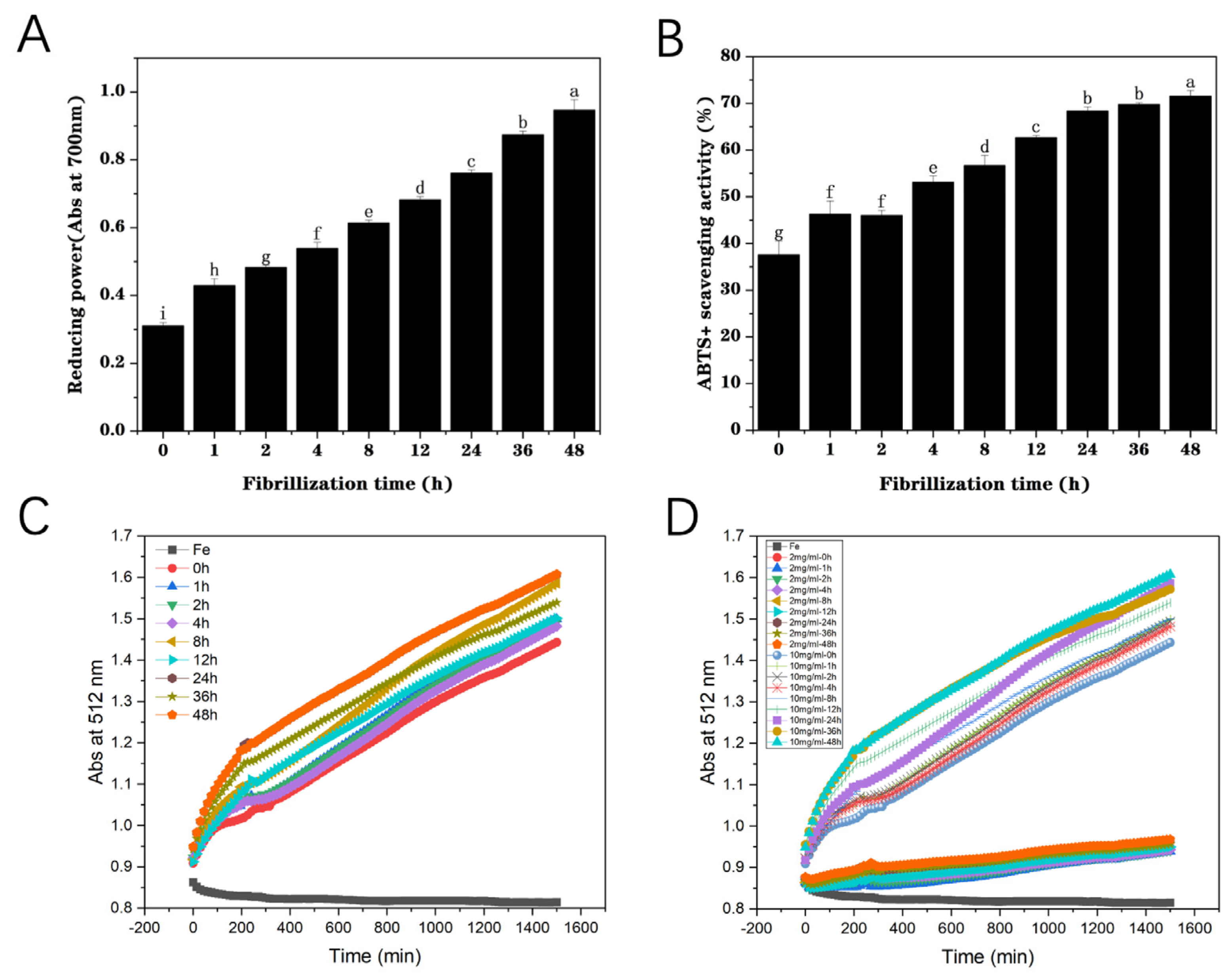
3.1.5. Reducing Power
3.1.6. ABTS+ Scavenging Activity Assay
3.2. Reducing Effect of Fibrillization on Iron Ions
3.3. Characterization and Morphology of Fe-Fib PP
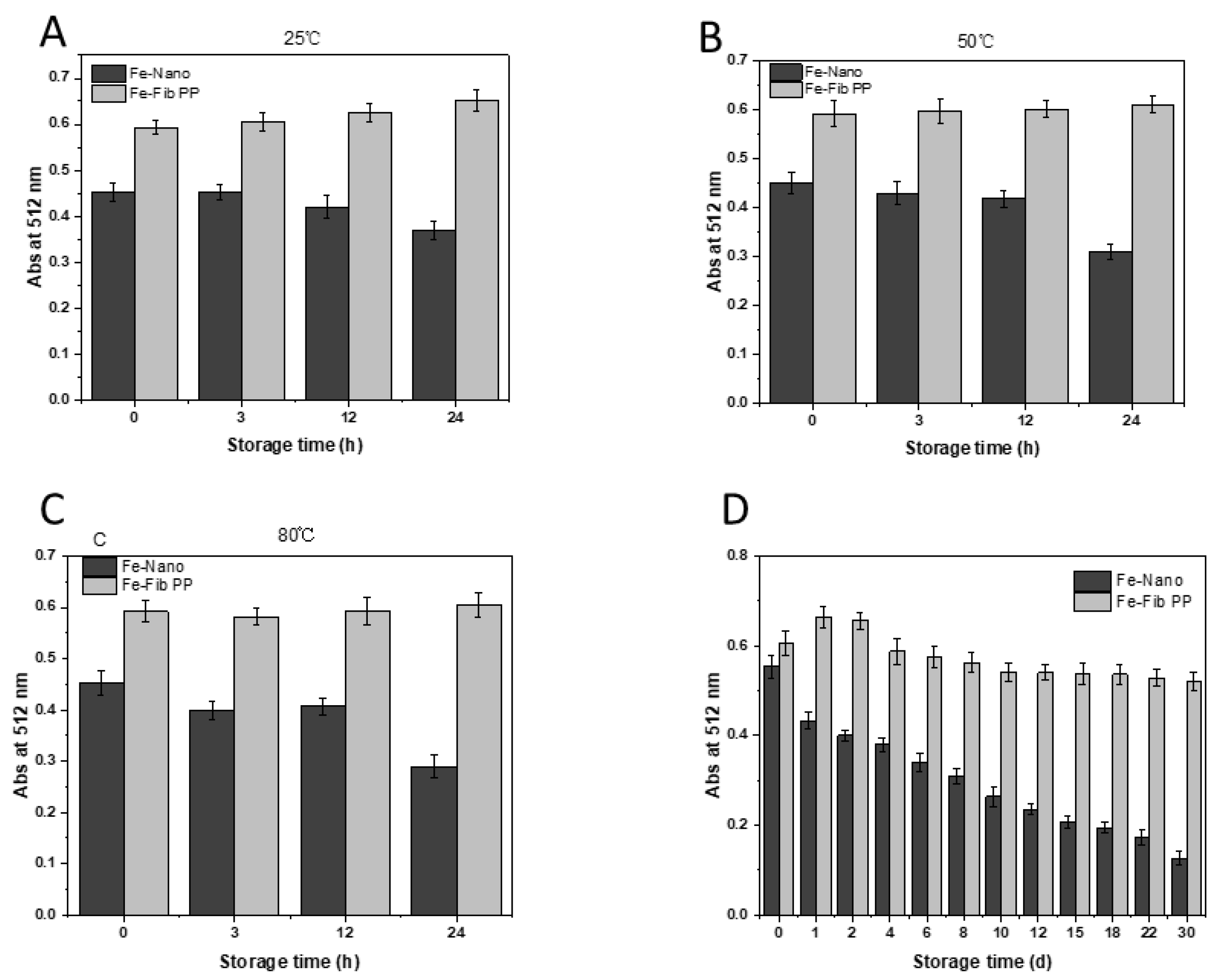
3.4. Stability of Iron–Pea Protein-Isolated Fibril (Fe-Fib PP) Nanocomposites and Iron Nanoparticles (Fe–Nano)
3.5. XPS
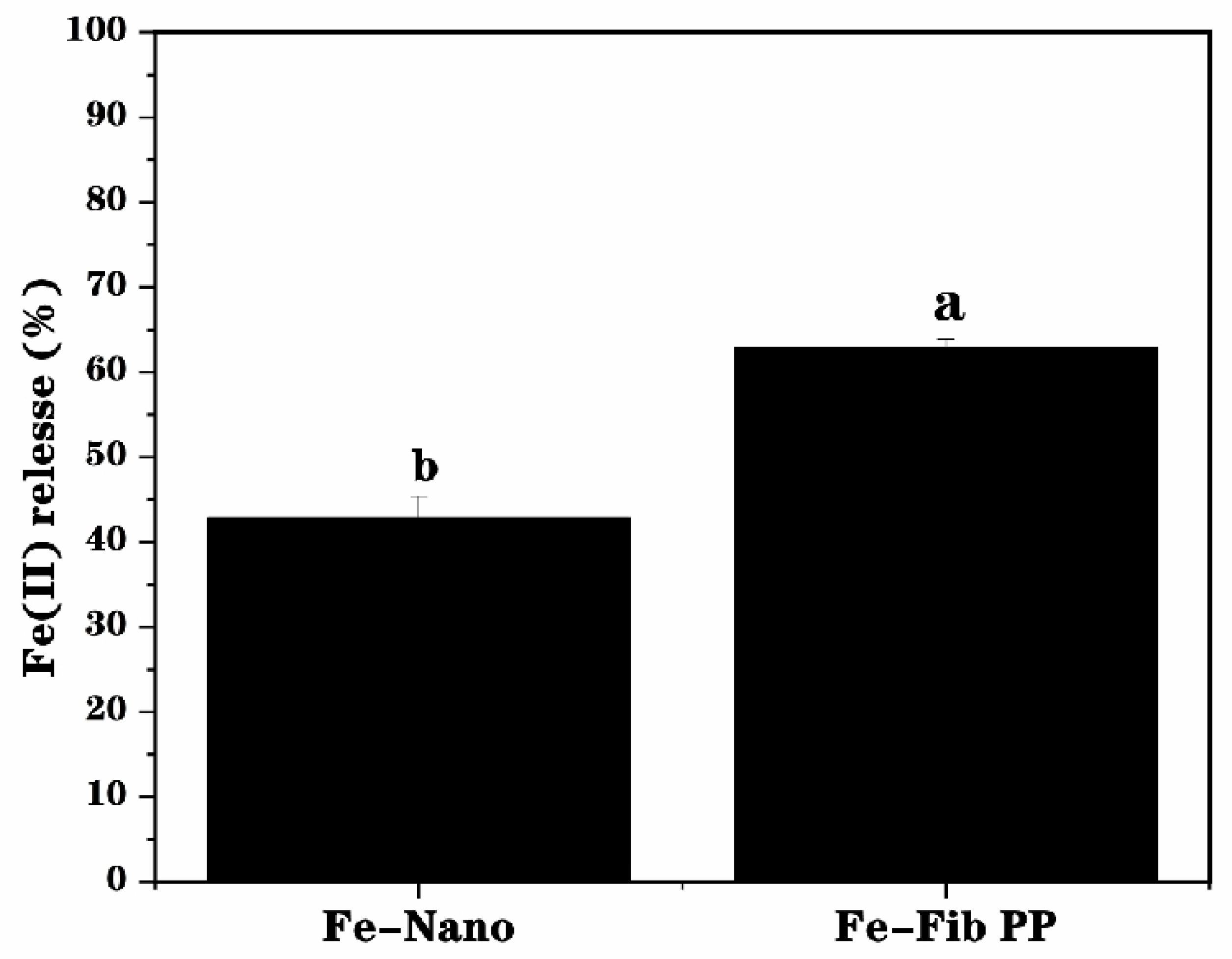
3.6. Bioaccessibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marks, P.W. Anemia: Clinical Approach. In Concise Guide to Hematology; Lazarus, H.M., Schmaier, A.H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 21–27. [Google Scholar]
- Asrie, F. Prevalence of anemia and its associated factors among pregnant women receiving antenatal care at Aymiba Health Center, northwest Ethiopia. J. Blood Med. 2017, 8, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Athira, S.; Mann, B.; Sharma, R.; Pothuraju, R.; Bajaj, R.K. Preparation and characterization of iron-chelating peptides from whey protein: An alternative approach for chemical iron fortification. Food Res. Int. 2021, 141, 110133. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.S.; Fayed, H.M.; Ismail, A.M.; Hakeem, G.L.A. Assessment of peripheral blood lymphocyte subsets in children with iron deficiency anemia. BMC Pediatr. 2018, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Shubham, K.; Anukiruthika, T.; Dutta, S.; Kashyap, A.V.; Moses, J.A.; Anandharamakrishnan, C. Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci. 2020, 99, 58–75. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Brown, E.B. The Absorption of Iron. Am. J. Clin. Nutr. 1963, 12, 205–213. [Google Scholar] [CrossRef]
- Shen, Y.; Posavec, L.; Bolisetty, S.; Hilty, F.M.; Nyström, G.; Kohlbrecher, J.; Hilbe, M.; Rossi, A.; Baumgartner, J.; Zimmermann, M.B.; et al. Amyloid fibril systems reduce, stabilize and deliver bioavailable nanosized iron. Nat. Nanotechnol. 2017, 12, 642–647. [Google Scholar] [CrossRef]
- Xiang, N.; Wu, S.; Wei, Z.; Shao, P.; Sun, P. Characterization of iron reducibility of soy protein amyloid fibrils and their applications in iron fortification. Food Chem. 2021, 353, 129420. [Google Scholar] [CrossRef]
- Loveday, S.M.; Su, J.; Rao, M.A.; Anema, S.G.; Singh, H. Whey protein nanofibrils: Kinetic, rheological and morphological effects of group IA and IIA cations. Int. Dairy. J. 2012, 26, 133–140. [Google Scholar] [CrossRef]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003, 78 (Suppl. S3), 660s–663s. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Chen, X.; Wen, Z.; Fan, Y. Improving the functionality of pea protein with laccase-catalyzed crosslinking mediated by chlorogenic acid. Food Chem. 2024, 433, 137344. [Google Scholar] [CrossRef] [PubMed]
- Munialo, C.D.; Martin, A.H.; van der Linden, E.; de Jongh, H.H.J. Fibril Formation from Pea Protein and Subsequent Gel Formation. J. Agric. Food Chem. 2014, 62, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; He, Q.; Peng, G.; Fan, Y. Improved water solubility, chemical stability, antioxidant and anticancer activity of resveratrol via nanoencapsulation with pea protein nanofibrils. Food Chem. 2022, 377, 131942. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ma, Y.; Ngadi, M.O. Binding of curcumin to β-lactoglobulin and its effect on antioxidant characteristics of curcumin. Food Chem. 2013, 141, 1504–1511. [Google Scholar] [CrossRef]
- Chen, X.; Yi, J.; Wen, Z.; Fan, Y. Ultrasonic pretreatment and epigallocatechin gallate incorporation enhance the formation, apparent viscosity, and antioxidant activity of pea protein amyloid-like fibrils. Food Hydrocoll. 2024, 149, 109630. [Google Scholar] [CrossRef]
- Nilsson, M.R. Techniques to study amyloid fibril formation in vitro. Methods 2004, 34, 151–160. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, G.; Pang, X.; Wen, Z.; Yi, J. Physicochemical, emulsifying, and interfacial properties of different whey protein aggregates obtained by thermal treatment. LWT 2021, 149, 111904. [Google Scholar] [CrossRef]
- Ou, S.Y.; Kwok, K.C.; Wang, Y.; Bao, H.Y. An improved method to determine SH and -S-S- group content in soymilk protein. Food Chem. 2004, 88, 317–320. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar] [CrossRef]
- Liu, Y.X.; Gao, L.Y.; Yi, J.; Fan, Y.T.; Wu, X.L.; Zhang, Y.Z. α-Lactalbumin and chitosan core-shell nanoparticles: Resveratrol loading, protection, and antioxidant activity. Food Funct. 2020, 11, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric determination of iron(II) with 1,10-phenanthroline in the presence of large amounts of iron(III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.L.; Zhang, Y.Q.; Huang, G.R.; Liu, J.; Slavin, M.; Yu, L.L. Zein-caseinate composite nanoparticles for bioactive delivery using curcumin as a probe compound. Food Hydrocoll. 2018, 83, 25–35. [Google Scholar] [CrossRef]
- Riek, R.; Eisenberg, D.S. The activities of amyloids from a structural perspective. Nature 2016, 539, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, Q. Assembly of iron-bound ovotransferrin amyloid fibrils. Food Hydrocoll. 2019, 89, 579–589. [Google Scholar] [CrossRef]
- Tang, C.-H.; Wang, S.-S.; Huang, Q. Improvement of heat-induced fibril assembly of soy β-conglycinin (7S Globulins) at pH 2.0 through electrostatic screening. Food Res. Int. 2012, 46, 229–236. [Google Scholar] [CrossRef]
- Akkermans, C.; van der Goot, A.J.; Venema, P.; van der Linden, E.; Boom, R.M. Formation of fibrillar whey protein aggregates: Influence of heat and shear treatment, and resulting rheology. Food Hydrocoll. 2008, 22, 1315–1325. [Google Scholar] [CrossRef]
- Koyoro, H.; Powers, J. Functional properties of pea globulin fractions. Cereal Chem. 1987, 64, 97–101. [Google Scholar]
- Alavi, F.; Emam-Djomeh, Z.; Mohammadian, M.; Salami, M.; Moosavi-Movahedi, A.A. Physico-chemical and foaming properties of nanofibrillated egg white protein and its functionality in meringue batter. Food Hydrocoll. 2020, 101, 105554. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, F. The reciprocal interaction between polyphenols and other dietary compounds: Impact on bioavailability, antioxidant capacity and other physico-chemical and nutritional parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Technological functionality and biological properties of food protein nanofibrils formed by heating at acidic condition. Trends Food Sci. 2018, 75, 115–128. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Tong, X.H.; Qi, B.K.; Wang, Z.J.; Li, Y.; Sui, X.N.; Jiang, L.Z. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Kumari, A.; Chauhan, A.K. Iron nanoparticles as a promising compound for food fortification in iron deficiency anemia: A review. J. Food Sci. Technol. 2022, 59, 3319–3335. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Li, X.-Q.; Cao, J.; Zhang, W.-X.; Wang, H.P. Characterization of zero-valent iron nanoparticles. Adv. Colloid. Interface Sci. 2006, 120, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Akkermans, C.; Venema, P.; van der Goot, A.J.; Gruppen, H.; Bakx, E.J.; Boom, R.M.; van der Linden, E. Peptides are Building Blocks of Heat-Induced Fibrillar Protein Aggregates of β-Lactoglobulin Formed at pH 2. Biomacromolecules 2008, 9, 1474–1479. [Google Scholar] [CrossRef]
- Akasapu, K.; Ojah, N.; Gupta, A.K.; Choudhury, A.J.; Mishra, P. An innovative approach for iron fortification of rice using cold plasma. Food Res. Int. 2020, 136, 109599. [Google Scholar] [CrossRef]
- Wardhani, D.H.; Ulya, H.N.; Rahmawati, A.; Sugiarto, T.V.K.; Kumoro, A.C.; Aryanti, N. Preparation of degraded alginate as a pH-dependent release matrix for spray-dried iron and its encapsulation performances. Food Biosci. 2021, 41, 101002. [Google Scholar] [CrossRef]
- Cheng, J.; Kenaan, A.; Zhao, D.; Qi, D.Z.; Song, J. Photo-polymerizable ferrous sulfate liposomes as vehicles for iron fortification of food. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102286. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
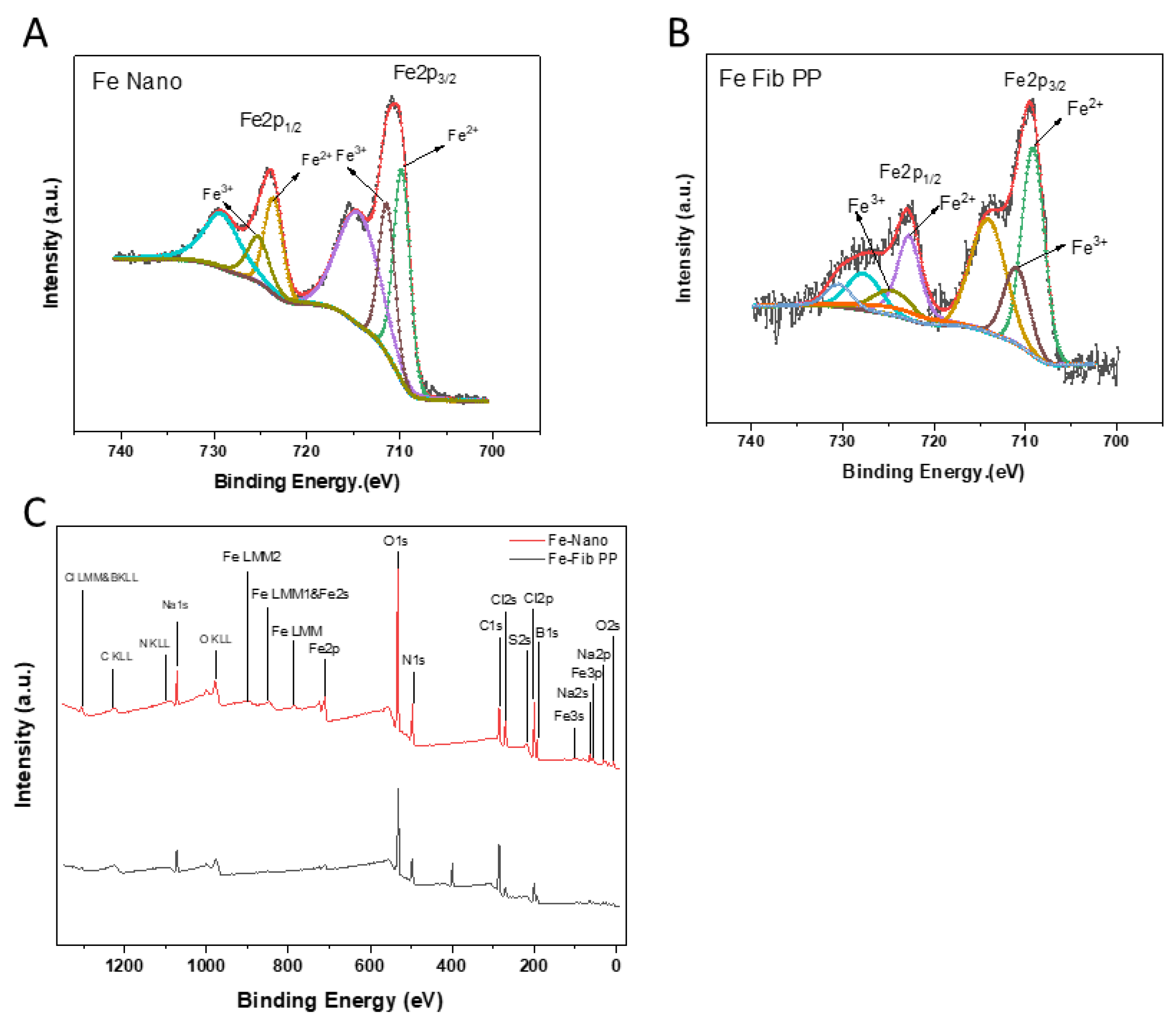
| Peak Area (%) | Fe–Nano | Fe-Fib PP |
|---|---|---|
| Fe (II) | 56.05 ± 0.50% | 70.75 ± 0.65% |
| Fe (III) | 43.95 ± 0.75% | 29.25 ± 0.30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yi, J.; Wen, Z.; Fan, Y. Preparation, Characterization, Stability and In Vitro Release of a Pea Protein Fibril-Based Iron Fortificant via Self-Assembly. Foods 2024, 13, 3558. https://doi.org/10.3390/foods13223558
Chen X, Yi J, Wen Z, Fan Y. Preparation, Characterization, Stability and In Vitro Release of a Pea Protein Fibril-Based Iron Fortificant via Self-Assembly. Foods. 2024; 13(22):3558. https://doi.org/10.3390/foods13223558
Chicago/Turabian StyleChen, Xiaoting, Jiang Yi, Zhen Wen, and Yuting Fan. 2024. "Preparation, Characterization, Stability and In Vitro Release of a Pea Protein Fibril-Based Iron Fortificant via Self-Assembly" Foods 13, no. 22: 3558. https://doi.org/10.3390/foods13223558
APA StyleChen, X., Yi, J., Wen, Z., & Fan, Y. (2024). Preparation, Characterization, Stability and In Vitro Release of a Pea Protein Fibril-Based Iron Fortificant via Self-Assembly. Foods, 13(22), 3558. https://doi.org/10.3390/foods13223558






