Unraveling the Impact of Aspergillus sojae—A Food-Grade Fungus—On Phytoalexins, Phenolic Acids, and the Antioxidant and Antidiabetic Activity of Different Legumes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Induction of Legume Seeds by Food-Grade Fungus A. sojae
Fungal Cultures and Fungal Inoculations of Legume Seeds
2.3. Extraction of A. sojae-Induced and Non-Induced Legumes
2.4. UPLC-DAD Quantification of Phytoalexins
2.5. Total Phenolic Content and Total Flavonoid Content
2.6. UPLC-ESI-QTOF-MS/MS Analysis of Phenolic Acids in Legume Extracts
2.7. Antioxidant Capacity of A. sojae-Induced and Non-Induced Legumes
2.7.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.7.2. DPPH Radical Scavenging Activity
2.7.3. ABTS Radical Cation-Based Assay
2.8. Antidiabetic Assays
2.8.1. α-Glucosidase Enzymatic Inhibition
2.8.2. α-Amylase Inhibition
2.9. Pancreatic Lipase Inhibition
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Phytoalexins in A. sojae-Induced Legumes
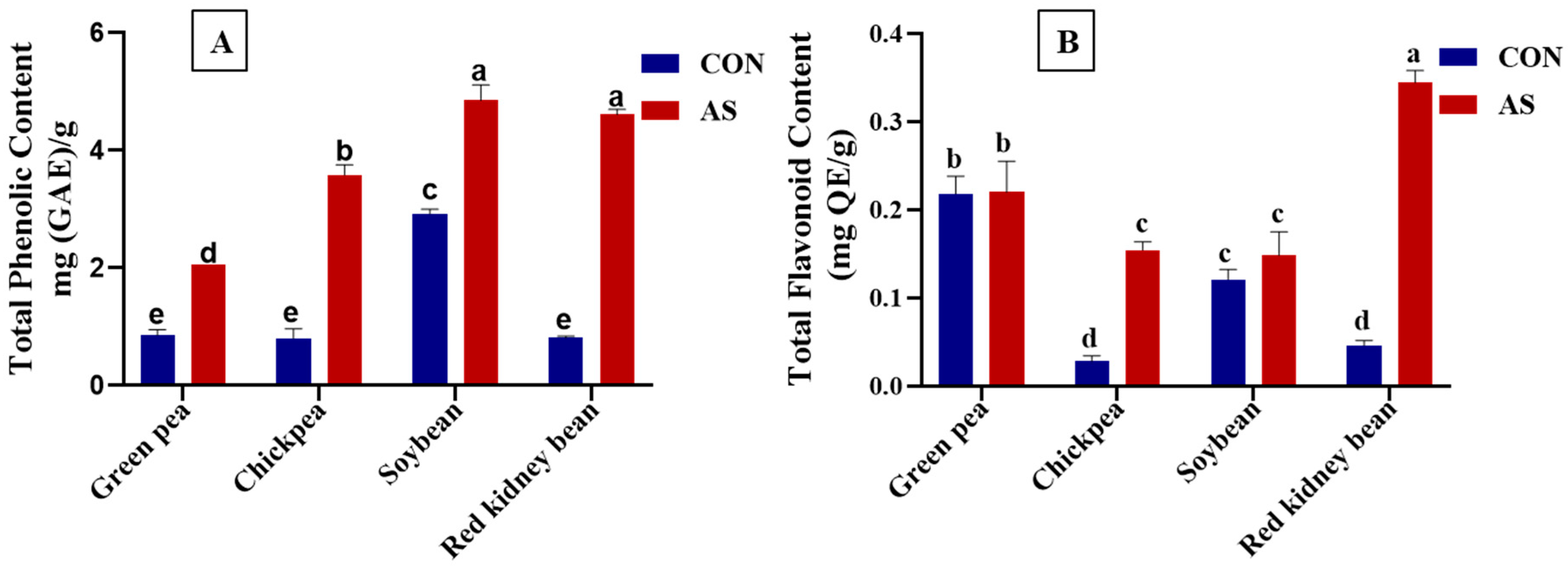
3.2. Total Phenolics and Total Flavonoid Content
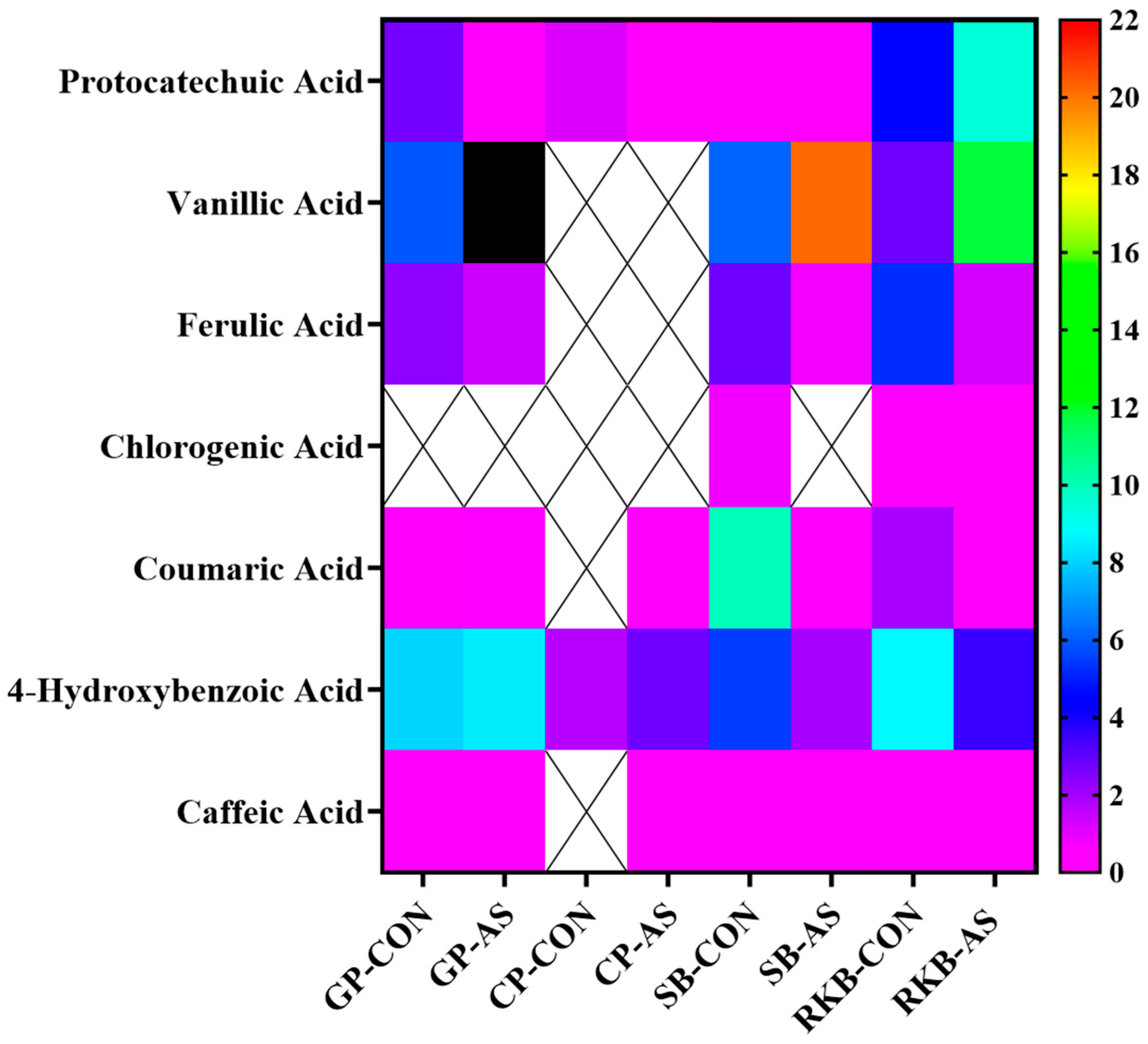
3.3. UPLC-ESI-QTOF-MS/MS Characterization of Phenolic Acids in Legumes
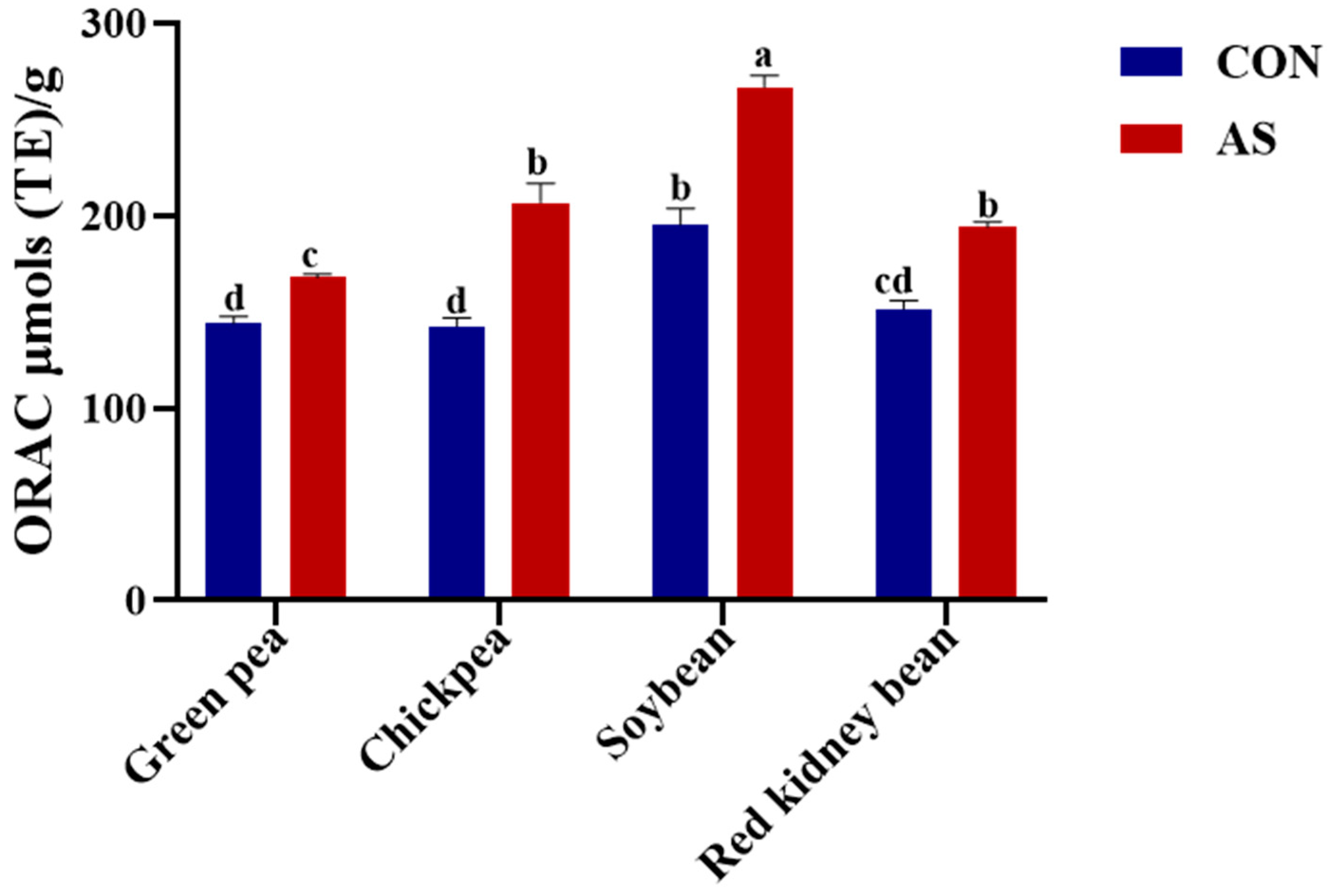
3.4. Antioxidant Activity
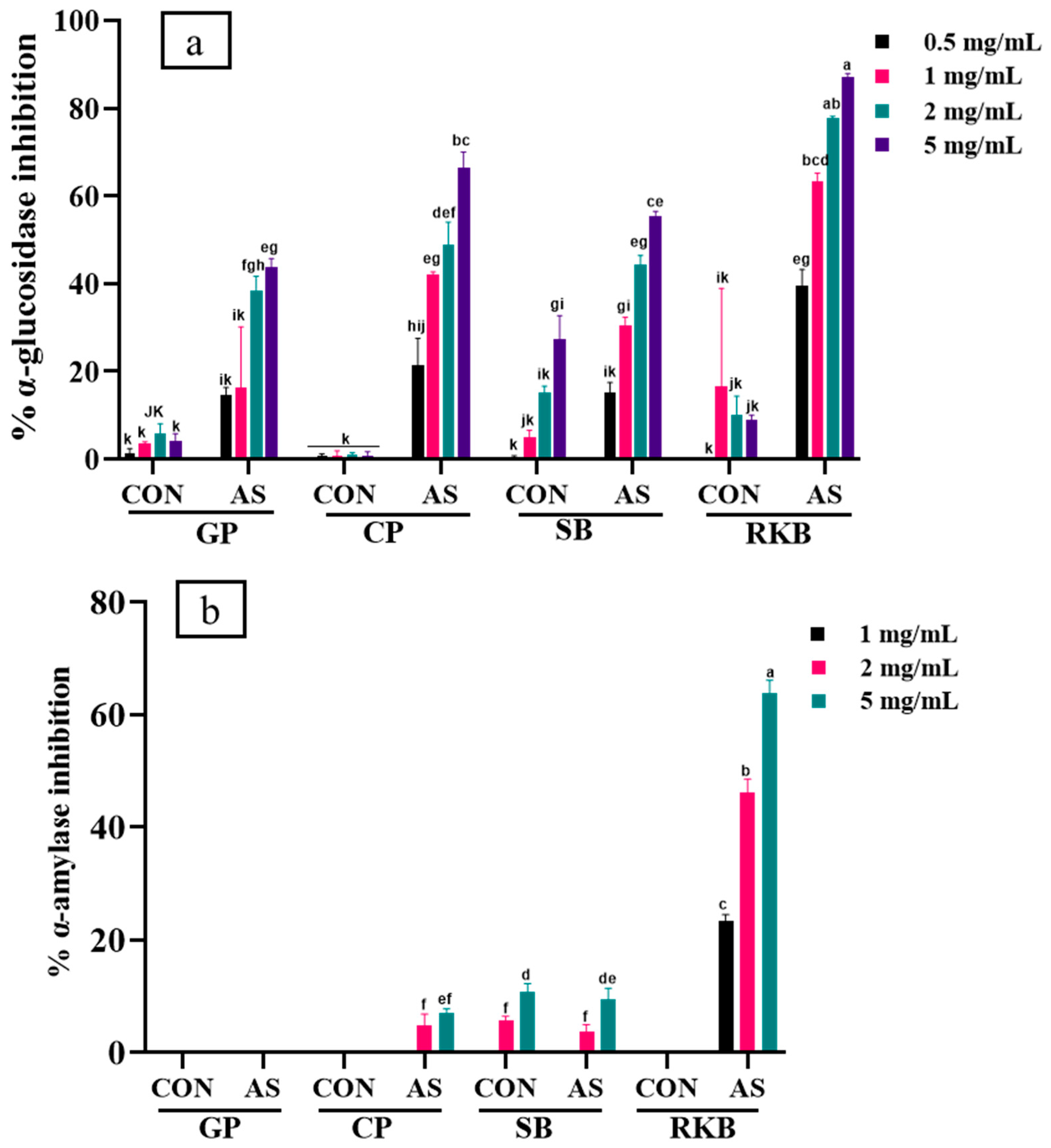
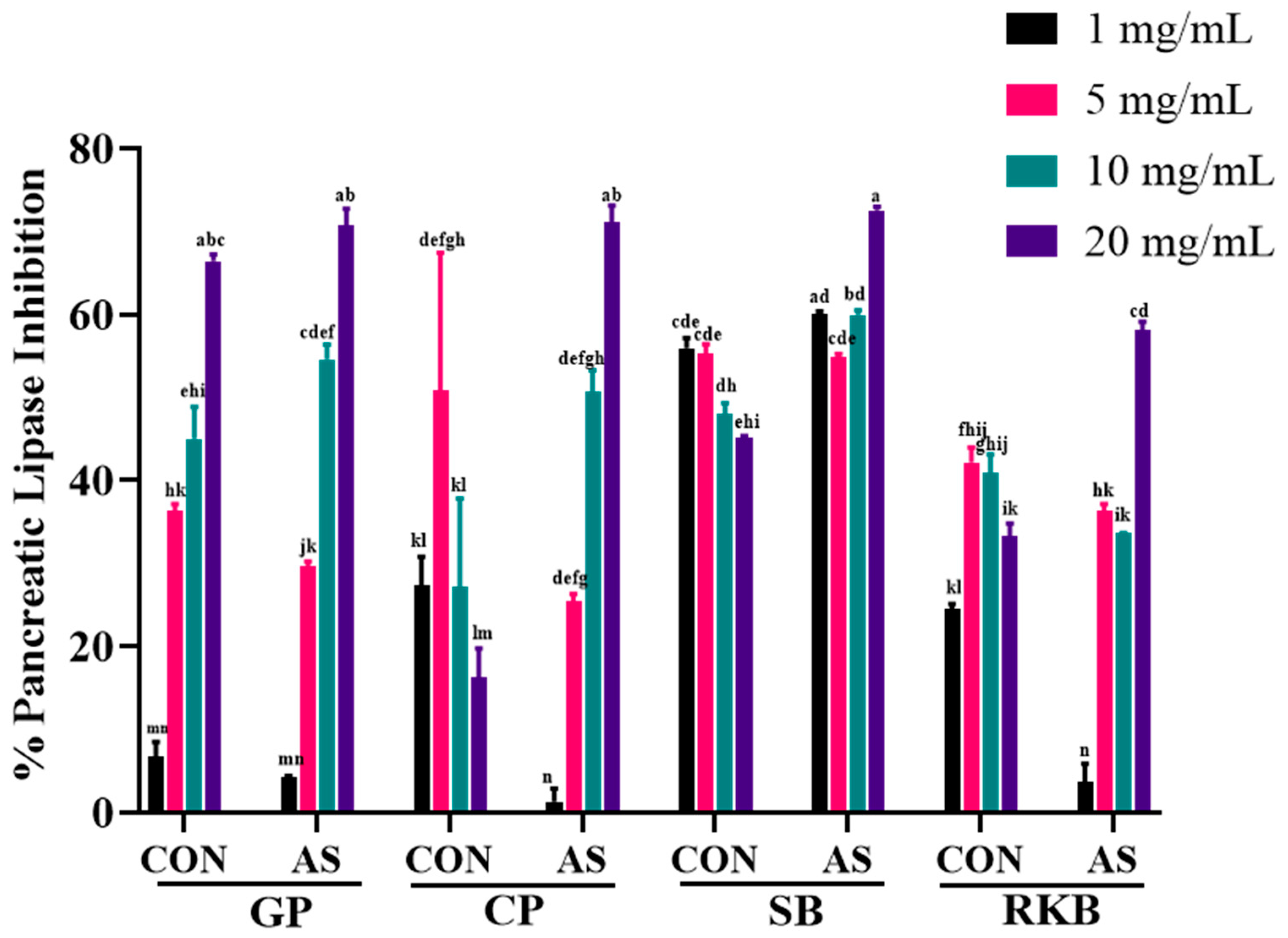
3.5. Enzyme Inhibition of A. sojae-Induced and Non-Induced Legumes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.M.; Lim, J.; Lee, J.J.; Hurh, B.-S.; Lee, I. Characterization of Aspergillus sojae isolated from meju, Korean traditional fermented soybean brick. J. Microbiol. Biotechnol. 2017, 27, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, X.; Hu, F.; Fu, J.; Zhang, Z.; Liu, Z.; Wang, B.; He, R.; Ma, H.; Ho, C.T. The latest advances on soy sauce research in the past decade: Emphasis on the advances in China. Food Res. Int. 2023, 173, 113407. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, D.K.; Jadhav, S.J.; Kadam, S.S.; Chavan, J.K. Chemical, biochemical, and biological significance of polyphenols in cereals and legumes. Crit. Rev. Food Sci. Nutr. 1982, 17, 277–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Q.; Gan, R.-Y.; Ge, Y.-Y.; Zhang, D.; Corke, H. Polyphenols in common beans (Phaseolus vulgaris L.): Chemistry, analysis, and factors affecting composition. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1518–1539. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar] [CrossRef]
- Aisyah, S.; Gruppen, H.; Andini, S.; Bettonvil, M.; Severing, E.; Vincken, J.P. Variation in accumulation of isoflavonoids in Phaseoleae seedlings elicited by Rhizopus. Food Chem. 2016, 196, 694–701. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Walk, T.E.; Arias, R.S.; Massa, A.N.; Orner, V.A.; Lamb, M.C. Transformation of major peanut (Arachis hypogaea) stilbenoid phytoalexins caused by selected microorganisms. J. Agric. Food Chem. 2022, 70, 1101–1110. [Google Scholar] [CrossRef]
- Zhuang, W.B.; Li, Y.H.; Shu, X.C.; Pu, Y.T.; Wang, X.J.; Wang, T.; Wang, Z. The classification, molecular structure and biological biosynthesis of flavonoids, and their roles in biotic and abiotic stresses. Molecules 2023, 28, 3599. [Google Scholar] [CrossRef]
- Dhull, S.B.; Punia, S.; Kidwai, M.K.; Kaur, M.; Chawla, P.; Purewal, S.S.; Sangwan, M.; Palthania, S. Solid-state fermentation of lentil (Lens culinaris L.) with Aspergillus awamori: Effect on phenolic compounds, mineral content, and their bioavailability. Legume Sci. 2020, 2, e37. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Neff, S.A.; Gloer, J.B. New stilbenoids from peanut (Arachis Hypogaea) seeds challenged by an Aspergillus caelatus strain. J. Agric. Food Chem. 2009, 57, 62–68. [Google Scholar] [CrossRef]
- Burow, M.E.; Boue, S.M.; Collins-Burow, B.M.; Melnik, L.I.; Duong, B.N.; Carter-Wientjes, C.H.; Li, S.; Wiese, T.E.; Cleveland, T.E.; McLachlan, J.A. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J. Clin. Endocrinol. Metab. 2001, 86, 1750–1758. [Google Scholar] [PubMed]
- Didinger, C.; Thompson, H.J. The role of pulses in improving human health: A review. Legume Sci. 2022, 4, e147. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich lentils and their health promoting effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Pegg, R.B. Legumes as a source of natural antioxidants. Eur. J. Lipid Sci. Technol. 2008, 110, 865–878. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, S.K.; Wang, H.; Cai, M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 2014, 152, 462–466. [Google Scholar] [CrossRef]
- Sreerama, Y.N.; Takahashi, Y.; Yamaki, K. Phenolic antioxidants in some Vigna species of legumes and their distinct inhibitory effects on α-glucosidase and pancreatic lipase activities. J. Food Sci. 2012, 77, C927–C933. [Google Scholar] [CrossRef]
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. Health implications of obesity. Am. J. Clin. Nutr. 1991, 53, 1595S–1603S. [Google Scholar] [CrossRef]
- Resnick, H.E.; Valsania, P.; Halter, J.B.; Lin, X. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J. Epidemiol. Community Health 2000, 54, 596–602. [Google Scholar] [CrossRef]
- Johansen, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc. Diabetol. 2005, 4, 5. [Google Scholar] [CrossRef]
- Singh, R.; Devi, S.; Gollen, R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: Larger-than-life. Diabetes Metab. Res. Rev. 2015, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Tucci, S.A.; Boyland, E.J.; Halford, J.C.G. The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: A review of current and emerging therapeutic agents. Diabetes Metab. Syndr. Obes. 2010, 3, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Rhabasa-Lhoret, R.; Chiasson, J.L. α-Glucosidase inhibitors. In International Textbook of Diabetes Mellitus, 3rd ed.; Defronzo, R.A., Ferannini, E., Keen, H., Zimmet, P., Eds.; Wiley: Chichester, UK, 2004; Volume 1, pp. 673–685. [Google Scholar]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of two a-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 2005, 54, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Goke, B.; Fuder, H.; Wieckhorst, G.; Theiss, U.; Stridde, E.; Littke, T.; Kleist, P.; Arnold, R.; Lücker, P.W. Voglibose (AO-128) is an efficient alpha-glucosidase inhibitor and mobilizes the endogenous GLP-1 reserve. Digestion 1995, 56, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions: A critical review. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef]
- Chiou, S.-Y.; Lai, J.-Y.; Liao, J.-A.; Sung, J.-M.; Lin, S.-D. In vitro inhibition of lipase, α-amylase, α-glucosidase, and angiotensin-converting enzyme by defatted rice bran extracts of red-pericarp rice mutant. Cereal Chem. 2018, 95, 167–176. [Google Scholar] [CrossRef]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef]
- Gu, Y.; Hurst, W.J.; Stuart, D.A.; Lambert, J.D. Inhibition of key digestive enzymes by cocoa extracts and procyanidins. J. Agric. Food Chem. 2011, 59, 5305–5311. [Google Scholar] [CrossRef]
- Slanc, P.; Doljak, B.; Kreft, S.; Lunder, M.; Janes, D.; Strukelj, B. Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytother. Res. 2009, 23, 874–877. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef]
- Boue, S.M.; Burow, M.E.; Wiese, T.E.; Shih, B.Y.; Elliott, S.; Carter-Wientjes, C.H.; McLachlan, J.A.; Bhatnagar, D. Estrogenic and antiestrogenic activities of phytoalexins from red kidney bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2011, 59, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Boue, S.M.; Carter, C.H.; Ehrlich, K.C.; Cleveland, T.E. Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J. Agric. Food Chem. 2000, 48, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Mani, J.S.; Naiker, M. Development and validation of a 96-well microplate assay for the measurement of total phenolic content in ginger extracts. Food Anal. Methods 2022, 15, 413–420. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N.; Boland Nazar, A.R. Total phenolic, total flavonoids, antioxidant and antimicrobial activities of scrophularia striata boiss extracts. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 15–19. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Ksouri, W.M.; Cluzet, S.; Krisa, S.; Richard, T.; Ksouri, R. Assessment of Antioxidant Activity and Neuroprotective Capacity on PC12 Cell Line of Frankenia thymifolia and Related Phenolic LC-MS/MS Identification. Evid. Based Complement. Alternat. Med. eCAM 2016, 2016, 2843463. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.I.; Ghaedian, R.; Shetty, K. Fermentation of milk and soymilk by Lactobacillus bulgarius and Lactobacillus acidophilus enhances functionality for potential dietary management of hyperglycemia and hypertension. Food Biotechnol. 2007, 21, 217–236. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Blando, F.; Orfila, C.; Marshall, L.J.; Boesch, C. Chromogenic assay is more efficient in identifying α-amylase inhibitory properties of anthocyanin-rich samples when compared to the 3,5-dinitrosalicylic acid (DNS) assay. Molecules 2023, 28, 6399. [Google Scholar] [CrossRef]
- Podsedek, A.; Majewsk, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Luo, S.; Gill, H.; Dias, D.A.; Li, M.; Hung, A.; Nguyen, L.T.; Lenon, G.B. The inhibitory effects of an eight-herb formula (RCM-107) on pancreatic lipase: Enzymatic, HTLC profiling and in silico approaches. Heliyon 2019, 5, e02453. [Google Scholar] [CrossRef] [PubMed]
- Abe, C.; Zhang, Y.; Takao, K.; Sasaki, K.; Ochiai, K.; Matsui, T. Visualization analysis of glyceollin production in germinating soybeans by matrix-assisted laser desorption/ionization mass spectrometric imaging technique. J. Agric. Food Chem. 2021, 69, 7057–7063. [Google Scholar] [CrossRef] [PubMed]
- Darvill, A.G.; Albersheim, P. Phytoalexins and their elicitors: A defense against microbial infections in plants. Annu. Rev. Plant Biol. 1984, 35, 243–275. [Google Scholar] [CrossRef]
- Graham, T.L.; Kim, J.E.; Graham, M.Y. Role of Constitutive Isoflavone Conjugates in the Accumulation of Glyceollin in Soybean Infected with Phytophthora megasperma. Mol. Plant Microbe Interact. 1989, 3, 157–166. [Google Scholar] [CrossRef]
- Paxton, J.D. Biosynthesis and accumulation of legume phytoalexins. In Mycotoxins and Phytoalexins; Sharma, R.P., Salunkhe, D.K., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 485–499. [Google Scholar]
- Yamamoto, T.; Sakamoto, C.; Tachiwana, H.; Kumabe, M.; Matsui, T.; Yamashita, T.; Shinagawa, M.; Ochiai, K.; Saitoh, N.; Nakao, M. Endocrine therapy-resistant breast cancer model cells are inhibited by soybean glyceollin I through Eleanor non-coding RNA. Sci. Rep. 2018, 8, 15202. [Google Scholar] [CrossRef]
- John, K.M.M.; Jung, E.S.; Lee, S.; Kim, J.-S.; Lee, C.-H. Primary and secondary metabolites variation of soybean contaminated with Aspergillus sojae. Food Res. Int. 2013, 54, 487–494. [Google Scholar] [CrossRef]
- Simons, R.; Vincken, J.-P.; Roidos, N.; Bovee, T.F.H.; Iersel, M.V.; Verbruggen, M.A.; Gruppen, H. Increasing soy isoflavonoid content and diversity by simultaneous malting and challenging by a fungus to modulate estrogenicity. J. Agric. Food Chem. 2011, 59, 6748–6758. [Google Scholar] [CrossRef]
- Botero, L.; Vizcaíno, S.; Quiñones, W.; Echeverri, F.; Gil, J.; Durango, D. Increased accumulation of isoflavonoids in common bean (Phaseolus vulgaris L.) tissues treated with 1-oxo-indane-4-carboxylic acid derivatives. Biotechnol. Rep. 2021, 29, e00601. [Google Scholar] [CrossRef]
- Hadwiger, L.A.; Tanaka, K.A. Simple and rapid assay for measuring phytoalexin pisatin, an indicator of plant defense response in pea (Pisum sativum L.). Bio Protoc. 2017, 7, e2362. [Google Scholar] [CrossRef]
- Schwochau, M.E.; Hadwiger, L.A. Stimulation of pisatin production in Pisum sativum by actinomycin D and other compounds. Arch. Biochem. Biophys. 1968, 126, 731–733. [Google Scholar] [CrossRef]
- Wu, Q.; VanEtten, H.D. Introduction of plant and fungal genes into pea (Pisum sativum L.) hairy roots reduces their ability to produce pisatin and affects their response to a fungal pathogen. Mol. Plant Microbe Interact. 2004, 17, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, L.; Feng, S.; Liu, Y.; He, G.; Yioe, Y.; Liu, S.Q.; Huang, D. Germination dramatically increases isoflavonoid content and diversity in chickpea (Cicer arietinum L.) seeds. J. Agric. Food Chem. 2012, 60, 8606–8615. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.; Tiemann, K.; Wittkampf, U.; Bless, W.; Hinderer, W.; Barz, W. Elicitor-induced metabolic changes in cell cultures of chickpea (Cicer Arietinum L.) cultivars resistant and susceptible to Ascochyta rabiei: I. Investigations of enzyme activities involved in isoflavone and pterocarpan phytoalexin Biosynthesis. Planta 1990, 182, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Arman, M. LC-ESI-MS characterisation of phytoalexins induced in chickpea and pea tissues in response to a biotic elicitor of Hypnea musciformis (red algae). Nat. Prod. Res. 2011, 25, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kovinich, N. Regulation of phytoalexin biosynthesis for agriculture and human health. Phytochem. Rev. 2021, 20, 483–505. [Google Scholar] [CrossRef]
- Cruickshank, I.A.M.; Perrin, D.R. Isolation of a phytoalexin from Pisum sativum L. Nature 1960, 187, 799–800. [Google Scholar] [CrossRef]
- Celoy, R.M.; VanEtten, H.D. (+)-Pisatin biosynthesis: From (−) enantiomeric intermediates via an achiral 7,2′-dihydroxy-4′,5′-methylenedioxyisoflav-3-ene. Phytochemistry 2014, 98, 120–127. [Google Scholar] [CrossRef]
- Sharma, K.R.; Giri, G. Quantification of phenolic and flavonoid content, antioxidant activity, and proximate composition of some legume seeds grown in Nepal. Int. J. Food Sci. 2022, 29, 4629290. [Google Scholar] [CrossRef]
- Elhamid, M.A.A.; Mandour, A.E.S.; Ismail, T.A.; Al-Zohairy, A.M.; Almowallad, S.; Alqahtani, L.S.; Osman, A. Powerful antioxidants and cytotoxic activities of the methanol extracts from eight soybean cultivars. Molecules 2022, 27, 2895. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Cui, S.W.; Li, Y.; Xu, S.; Wu, Y.; Wang, J.; Bai, Z.; et al. Nutrients, phytochemicals and antioxidant activities of 26 kidney bean cultivars. Food Chem. Toxicol. 2017, 108 Pt B, 467–477. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Zhang, X.; Chen, G.-L.; Yu, J.; Yang, L.-Q.; Gao, Y.-Q. Antioxidant property and their free, soluble conjugate and insoluble-bound phenolic contents in selected beans. J. Funct. Foods 2016, 24, 359–372. [Google Scholar] [CrossRef]
- Okubo, K.; Iijima, M.; Kobayashi, Y.; Yoshikoshi, M.; Uchida, T.; Kudou, S. Components responsible for the undesirable taste of soybean seeds. Biosci. Biotechnol. Biochem. 1992, 56, 99–103. [Google Scholar] [CrossRef]
- Chung, I.-M.; Seo, S.-H.; Ahn, J.-K.; Kim, S.-H. Effect of processing, fermentation, and aging treatment to content and profile of phenolic compounds in soybean seed, soy curd and soy paste. Food Chem. 2011, 127, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Boue, S.M.; Shih, F.F.; Shih, B.Y.; Daigle, K.W.; Carter-Wientjes, C.H.; Cleveland, T.E. Effect of biotic elicitors on enrichment of antioxidant properties and induced isoflavones in soybean. J. Food Sci. 2008, 73, H43–H49. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. 2010, 61, 109–124. [Google Scholar] [CrossRef]
- Son, H.-U.; Yoon, E.-K.; Yoo, C.-Y.; Park, C.-H.; Bae, M.-A.; Kim, T.-H.; Lee, C.H.; Lee, K.W.; Seo, H.; Kim, K.-J.; et al. Effects of Synergistic Inhibition on α-glucosidase by Phytoalexins in Soybeans. Biomolecules 2019, 9, 828. [Google Scholar] [CrossRef]
- Dendup, T.; Prachyawarakorn, V.; Pansanit, A.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. α-Glucosidase inhibitory activities of isoflavanones, isoflavones, and pterocarpans from Mucuna pruriens. Planta Med. 2014, 80, 604–608. [Google Scholar] [CrossRef]
- Liu, R.; Xu, B. Inhibitory effects of phenolics and saponins from commonly consumed food legumes in China against digestive enzymes pancreatic lipase and α -glycosidase. Int. J. Food Prop. 2015, 18, 2246–2255. [Google Scholar] [CrossRef]
- Lee, S.S.; Mohd Esa, N.; Loh, S.P. Inhibitory activity of legumes against pancreatic lipase. J. Food Biochem. 2015, 39, 485–490. [Google Scholar] [CrossRef]
- Hong, J.; Choi, Y.; Lee, J.; Park, Y.J.; Lee, D.Y.; Chang, P.-S. Inhibitory characteristics of flavonoids from soybean (Glycine max [L.] Merr.) leaf against pancreatic lipase. Food Biosci. 2023, 56, 103311. [Google Scholar] [CrossRef]
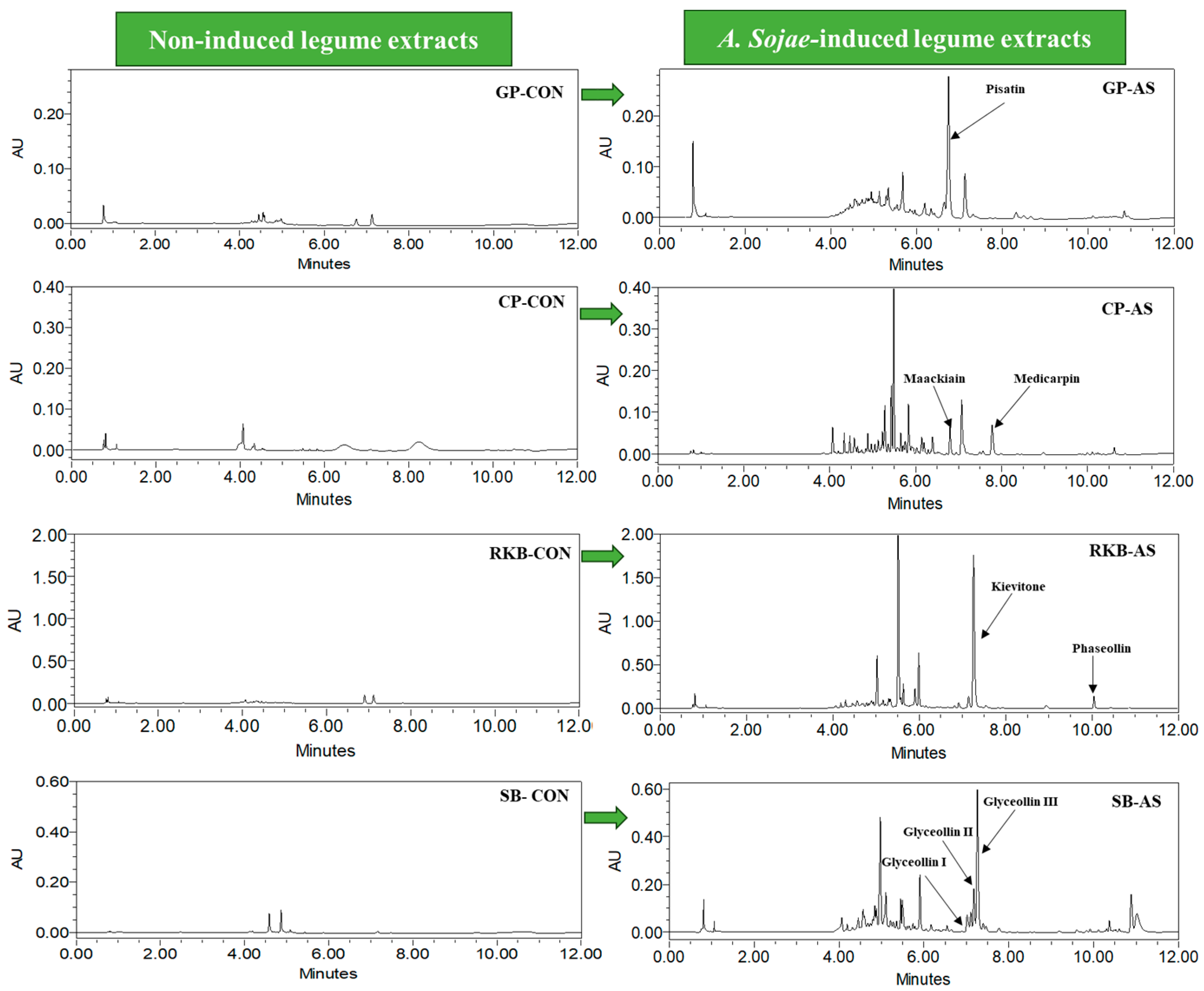
| Extracts | Phytoalexins | Content (µg/g DW) | Molecular Formula | Molecular Weight (g/mol) | Absorbance |
|---|---|---|---|---|---|
| CP-AS | Maackiain | 892.40 ± 23.98 a | C16H12O5 | 284.26 | 310 |
| Medicarpin | 675.94 ± 46.64 b | C16H14O4 | 270.27 | 285 | |
| GP-AS | Pisatin | 465.95 ± 17.79 c | C17H14O6 | 314.293 | 310 |
| SB-AS | Glyceollin III | 93.615 ± 4.636 g | C20H18O5 | 338.4 | 285 |
| Glyceollin II | 153.82 ± 8.895 f | C20H18O5 | 338.4 | 285 | |
| Glyceollin I | 441.43 ± 7.589 cd | C20H18O5 | 338.4 | 285 | |
| RKB-AS | Kievitone | 386.76 ± 23.08 d | C20H20O6 | 356.4 | 292 |
| Phaseollin | 248.59 ± 30.52 e | C20H18O4 | 322.36 | 285 |
| Phenolic Acids | Molecular Formula | RT (min) | Mode of Ionization | Molecular Weight | Target (Precursor) Ion [M − H]− (m/z) | Product Ion 1 (m/z) | Product Ion 2 (m/z) | LOQ (µg/mL) | LOD (µg/mL) | Samples |
|---|---|---|---|---|---|---|---|---|---|---|
| Protocatechuic Acid | C7H6O4 | 3.4 | [M − H]− | 154.02 | 153.0293 | 109.0354 | 0.005 | 0.002 | GP-CON, GP-AS, CP-CON, CP-AS, SB-CON, SB-AS, RKB-CON, RKB-AS | |
| Vanillic Acid | C8H8O4 | 4.40 | [M − H]− | 168.14 | 167.0442 | 108.0284 | 123.0439 | 0.100 | 0.050 | GP-CON, GP-AS, SB-CON, SB-AS, RKB-CON, RKB-AS |
| Ferulic Acid | C10H10O4 | 5.07 | [M − H]− | 194.05 | 193.0601 | 134.0442 | 178.0371 | 0.100 | 0.050 | GP-CON, GP-AS, SB-CON, SB-AS, RKB-CON, RKB-AS |
| Chlorogenic Acid | C16H18O9 | 4.10 | [M − H]− | 354.09 | 353.1069 | 191.0680 | 174.9671 | 0.005 | 0.002 | SB-CON, RKB-CON, RKB-AS |
| Coumaric Acid | C9H8O3 | 5.01 | [M − H]− | 164.04 | 163.0491 | 119.0572 | 0.003 | 0.002 | GP-CON, GP-AS, CP-AS, SB-CON, SB-AS, RKB-CON, RKB-AS | |
| 4-Hydroxybenzoic Acid | C7H6O3 | 4.01 | [M − H]− | 138.03 | 137.0310 | 93.0400 | 0.010 | 0.005 | GP-CON, GP-AS, CP-CON, CP-AS, SB-CON, SB-AS, RKB-CON, RKB-AS | |
| Caffeic Acid | C9H8O4 | 4.30 | [M − H]− | 180.04 | 179.0310 | 135.0520 | 0.002 | 0.001 | GP-CON, GP-AS, SB-CON, SB-AS, RKB-CON, RKB-AS |
| Phenolic Acids (µg/g) | Green Pea | Chickpea | Soybean | Red Kidney Bean | ||||
|---|---|---|---|---|---|---|---|---|
| CON | AS | CON | AS | CON | AS | CON | AS | |
| Protocatechuic Acid | 2.67 ± 0.26 | 0.37 ± 0.03 | 1.16 ± 0.04 | 0.10 ± 0.04 | 0.53 ± 0.20 | 0.43 ± 0.05 | 4.49 ± 0.28 | 9.44 ± 0.76 |
| Vanillic Acid | 5.87 ± 0.69 | 327.26 ± 31.86 | - | - | 6.13 ± 0.82 | 20.19 ± 0.97 | 2.76 ± 0.62 | 11.78 ± 0.69 |
| Ferulic Acid | 2.30 ± 0.43 | 1.40 ± 0.09 | - | - | 2.75 ± 0.30 | 0.78 ± 0.08 | 5.20 ± 1.12 | 01.31 ± 0.22 |
| Chlorogenic Acid | - | - | - | - | 0.81 ± 0.05 | - | 0.62 ± 0.02 | 0.56 ± 0.01 |
| Coumaric Acid | 0.58 ± 0.11 | 0.67 ± 0.07 | - | 0.10 ± 0.02 | 9.89 ± 1.23 | 0.42 ± 0.02 | 1.91 ± 0.24 | 0.40 ± 0.03 |
| 4-Hydroxybenzoic Acid | 8.05 ± 2.39 | 8.46 ± 0.16 | 1.69 ± 0.09 | 2.73 ± 0.57 | 5.46 ± 0.58 | 1.95 ± 0.12 | 8.71 ± 1.08 | 3.52 ± 0.12 |
| Caffeic Acid | 0.04 ± 0.01 | 0.05 ± 0.01 | - | - | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.10 ± 0.03 | 0.10 ± 0.01 |
| Total | 19.51 | 338.21 | 2.85 | 2.93 | 25.59 | 23.8 | 23.79 | 27.11 |
| Legumes | ABTS mg (Trolox)/g | DPPH mg (Trolox)/g | |
|---|---|---|---|
| Green pea | CON | 0.25 ± 0.040 f | 0.15 ± 0.006 g |
| AS | 3.01 ± 0.021 c | 0.39 ± 0.010 f | |
| Chickpea | CON | 0.12 ± 0.07 f | 0.09 ± 0.0008 h |
| AS | 2.78 ± 0.08 c | 0.51 ± 0.006 e | |
| Soybean | CON | 1.87 ± 0.20 d | 0.65 ± 0.02 d |
| AS | 3.76 ± 0.12 b | 0.82 ± 0.01 c | |
| Red kidney bean | CON | 1.19 ± 0.29 e | 0.92 ± 0.005 b |
| AS | 5.86 ± 0.06 a | 1.34 ± 0028 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, S.; Broussard, W.; Elliott, S.; Burow, M.E.; Boue, S.M. Unraveling the Impact of Aspergillus sojae—A Food-Grade Fungus—On Phytoalexins, Phenolic Acids, and the Antioxidant and Antidiabetic Activity of Different Legumes. Foods 2024, 13, 3533. https://doi.org/10.3390/foods13223533
Rana S, Broussard W, Elliott S, Burow ME, Boue SM. Unraveling the Impact of Aspergillus sojae—A Food-Grade Fungus—On Phytoalexins, Phenolic Acids, and the Antioxidant and Antidiabetic Activity of Different Legumes. Foods. 2024; 13(22):3533. https://doi.org/10.3390/foods13223533
Chicago/Turabian StyleRana, Shalika, William Broussard, Steven Elliott, Matthew E. Burow, and Stephen M. Boue. 2024. "Unraveling the Impact of Aspergillus sojae—A Food-Grade Fungus—On Phytoalexins, Phenolic Acids, and the Antioxidant and Antidiabetic Activity of Different Legumes" Foods 13, no. 22: 3533. https://doi.org/10.3390/foods13223533
APA StyleRana, S., Broussard, W., Elliott, S., Burow, M. E., & Boue, S. M. (2024). Unraveling the Impact of Aspergillus sojae—A Food-Grade Fungus—On Phytoalexins, Phenolic Acids, and the Antioxidant and Antidiabetic Activity of Different Legumes. Foods, 13(22), 3533. https://doi.org/10.3390/foods13223533







