Abstract
Mycotoxins are toxic compounds produced by fungi such as Aspergillus, Penicillium, and Fusarium, contaminating various food crops and posing severe risks to food safety and human health. This review discusses mycotoxins‘ origins, significance, and impact, particularly in relation to cancer risk. Major mycotoxins like aflatoxins, ochratoxins, fumonisins, zearalenone, and patulin are examined, along with their sources and affected foods. The carcinogenic mechanisms of these toxins, including their biochemical and molecular interactions, are explored, as well as epidemiological evidence linking mycotoxin exposure to cancer in high-risk populations. The review also highlights critical methodologies for mycotoxin detection, including HPLC, GC-MS, MS, and ELISA, and the sample preparation techniques critical for accurate analysis. Strategies for controlling mycotoxin contamination, both pre- and post-harvest, are discussed, along with regulations from organizations like the FAO and WHO. Current challenges in detection sensitivity, cost, and control effectiveness are noted. Future research is needed to develop innovative analytical techniques, improve control strategies, and address the influence of climate change on mycotoxin production. Finally, global collaboration and emerging technologies are essential for advancing mycotoxin control and enhancing food safety.
1. Introduction
Mycotoxins are toxic secondary metabolites produced by various species of fungi, primarily molds, that pose significant risks to food safety and public health. These fungi, which include notable genera such as Aspergillus, Penicillium, and Fusarium, thrive on a wide range of food crops, especially under warm and humid conditions [1]. They can grow in the field, during harvest, and even during food storage, contaminating essential commodities like cereals, nuts, dried fruits, coffee, and spices. As a result, mycotoxins are a significant food safety concern, especially in regions where environmental conditions favor fungal growth and food preservation systems may be inadequate [2].
The production of mycotoxins by fungi is a natural defense mechanism, typically triggered under stressful conditions such as drought, insect damage, or improper food storage [3]. These toxic metabolites can contaminate food at any stage in the supply chain, from pre-harvest to processing and storage. Several mycotoxins are of particular concern due to their prevalence and toxicity. Aflatoxins, produced by Aspergillus species, are commonly found in peanuts, maize, and other grains and are well known for their carcinogenic properties [4]. Ochratoxins, produced by Aspergillus and Penicillium species, are often detected in cereals, coffee, and dried fruits [5]. Fumonisins, predominantly made by Fusarium species, are found in maize, while zearalenone and deoxynivalenol (DON), also produced by Fusarium, are common contaminants in wheat, barley, and corn [6].
The health risks posed by mycotoxins are significant. Some mycotoxins, such as aflatoxins, are highly carcinogenic and are directly linked to liver cancer, while others cause immunosuppression, kidney damage, and reproductive disorders [7]. Chronic exposure to low levels of mycotoxins can be detrimental to health. This challenge is enhanced in developing countries where food contamination is more prevalent and diets heavily depend on susceptible crops [8]. The health impacts are not limited to humans as livestock consuming contaminated feed may suffer, leading to economic losses in agriculture and food-producing sectors [9].
From an economic standpoint, mycotoxin contamination has far-reaching consequences. Contaminated crops may be rejected for sale, reducing yields and causing substantial financial losses for farmers and food producers [10]. Furthermore, the costs associated with mycotoxin detection, management, and control measures increase the economic burden, affecting local food security and international trade [11]. Countries with stricter food safety standards may reject imported contaminated food products, resulting in trade barriers that affect global food markets [12].
Due to mycotoxins’ health risks and economic impacts, regulatory bodies established stringent guidelines and limits for mycotoxin levels in food and animal feed [13]. International organizations like the Codex Alimentarius, in collaboration with the World Health Organization (WHO) and the Food and Agriculture Organization (FAO), set global standards aimed at minimizing mycotoxin contamination and protecting public health [14]. These regulatory efforts are critical in ensuring food safety, yet challenges remain in achieving comprehensive control across all stages of the food production process [15].
In summary, mycotoxins produced by molds that grow on various crops represent a significant concern in food safety due to their toxic effects on health, including the potential to cause cancer [16]. The economic implications of mycotoxin contamination further complicate food security and trade. Effective monitoring, control measures, and international regulatory standards are crucial in mitigating the impact of mycotoxins on public health and the global food supply chain [17].
Mycotoxins are crucial due to their significant impact on public health and food safety, particularly their association with cancer risk. Mycotoxins, such as aflatoxins, are highly potent carcinogens and are directly linked to liver cancer, among other health issues [18]. The International Agency for Research on Cancer (IARC) has classified aflatoxins as Group 1 carcinogens, indicating clear evidence of their cancer-causing potential in humans [19]. Such toxins, produced by fungi that contaminate staple food crops like maize, peanuts, and grains, can accumulate in the food chain, posing chronic health risks when consumed over time [20]. Therefore, regular food analysis and monitoring of mycotoxins are essential to prevent long-term exposure that could elevate cancer risks, particularly in vulnerable populations with limited dietary diversity [21].
The synergistic interactions between various mycotoxins, along with other environmental and dietary factors, significantly amplify their toxicity and complicate public health risks [22]. Multiple mycotoxins often co-occur in contaminated food or feed, such as aflatoxins and fumonisins in maize, where their combined presence enhances the carcinogenic potential beyond the effects of each toxin alone [23]. This synergism not only exacerbates liver cancer risk but also increases the likelihood of other adverse health outcomes, including immunosuppression and impaired growth [24]. Factors like dose and exposure levels further influence this synergism, where low doses of multiple mycotoxins can have enhanced effects than higher doses of a single one [25].
Metabolic interactions also play a role, with the metabolites of one mycotoxin potentially enhancing the toxic effects of another [26]. The immune system, often suppressed by certain mycotoxins, becomes more vulnerable to further toxic impact, while disruptions in gut microbiota and impaired detoxification processes can increase susceptibility to multiple mycotoxins. Nutritional deficiencies, particularly in essential nutrients like proteins, vitamins, and minerals, worsen these effects by reducing the body’s ability to detoxify mycotoxins, increasing vulnerability to chronic diseases, including cancer [27]. Additionally, mycotoxins can interfere with liver enzymes responsible for detoxification and induce oxidative stress, weakening the body’s defenses against toxins. Understanding these complex interactions is crucial for effective risk assessment, food safety interventions, and developing strategies to mitigate the health risks associated with multiple mycotoxin exposures [28].
In addition to cancer risks, mycotoxins present broader food safety concerns that necessitate thorough analysis. Contaminated foods can lead to a range of health issues beyond carcinogenicity, including immune suppression, gastrointestinal disorders, and reproductive problems [29]. These effects are particularly concerning in regions with poor food safety infrastructure, where contaminated foods may be widely consumed due to limited regulation or insufficient post-harvest management [30]. Ensuring food safety through analyzing mycotoxin levels helps mitigate these health risks and safeguard consumers and the global food supply chain [31]. Therefore, effective mycotoxin management is pivotal in food safety and cancer prevention and sustaining economic viability in the food industry [32].
1.1. Types of Mycotoxins
Different species of fungi produce common mycotoxins, which are a significant concern due to their toxic effects on human and animal health [33]. The most prevalent mycotoxins include aflatoxins, ochratoxins, fumonisins, zearalenone, and patulin, each with specific characteristics, sources, and health implications (Table 1) [34]. These mycotoxins contaminate a wide range of food products, making their presence a critical issue in food safety management.

Table 1.
Types of mycotoxins in foods.
Aflatoxins, ochratoxins, fumonisins, zearalenone, and patulin are the major mycotoxins of concern due to their prevalence in food products and significant health impacts. These toxins pose various risks, from carcinogenicity to reproductive and kidney disorders, emphasizing the need for rigorous monitoring and control in food production to ensure food safety.
1.2. Sources and Affected Foods of Mycotoxins
Mycotoxins contaminate various food products at multiple stages of production, from pre-harvest to post-harvest, due to fungal growth. Different fungi are responsible for producing these toxic compounds, with certain foods being more susceptible to contamination based on environmental conditions and storage practices [40]. Foods are commonly contaminated by major mycotoxins (Table 2) and the fungi responsible for their production.

Table 2.
Major mycotoxins in several food items.
Aflatoxins, ochratoxins, fumonisins, zearalenone, and patulin are the most concerning mycotoxins due to their prevalence in various food products and harmful health effects. The fungi producing these toxins thrive in specific environmental conditions, contaminating crops like maize, peanuts, cereals, dried fruits, and apples. Proper storage, handling, and monitoring of these foods are essential to reduce the risk of mycotoxin contamination and ensure food safety [45]. Table 3 provides an overview of the regulatory limits and health risk levels for various mycotoxins in food products, emphasizing the need for continuous monitoring and control to ensure food safety. Figure 1 represents the percentages for the estimated contribution of each mycotoxin to the total global mycotoxin burden based on toxicity and occurrence.

Table 3.
Toxic levels of various mycotoxins in food.
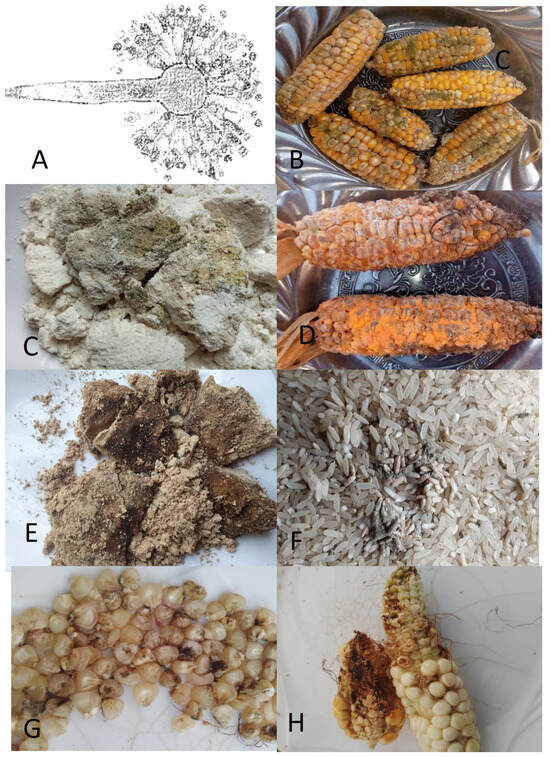
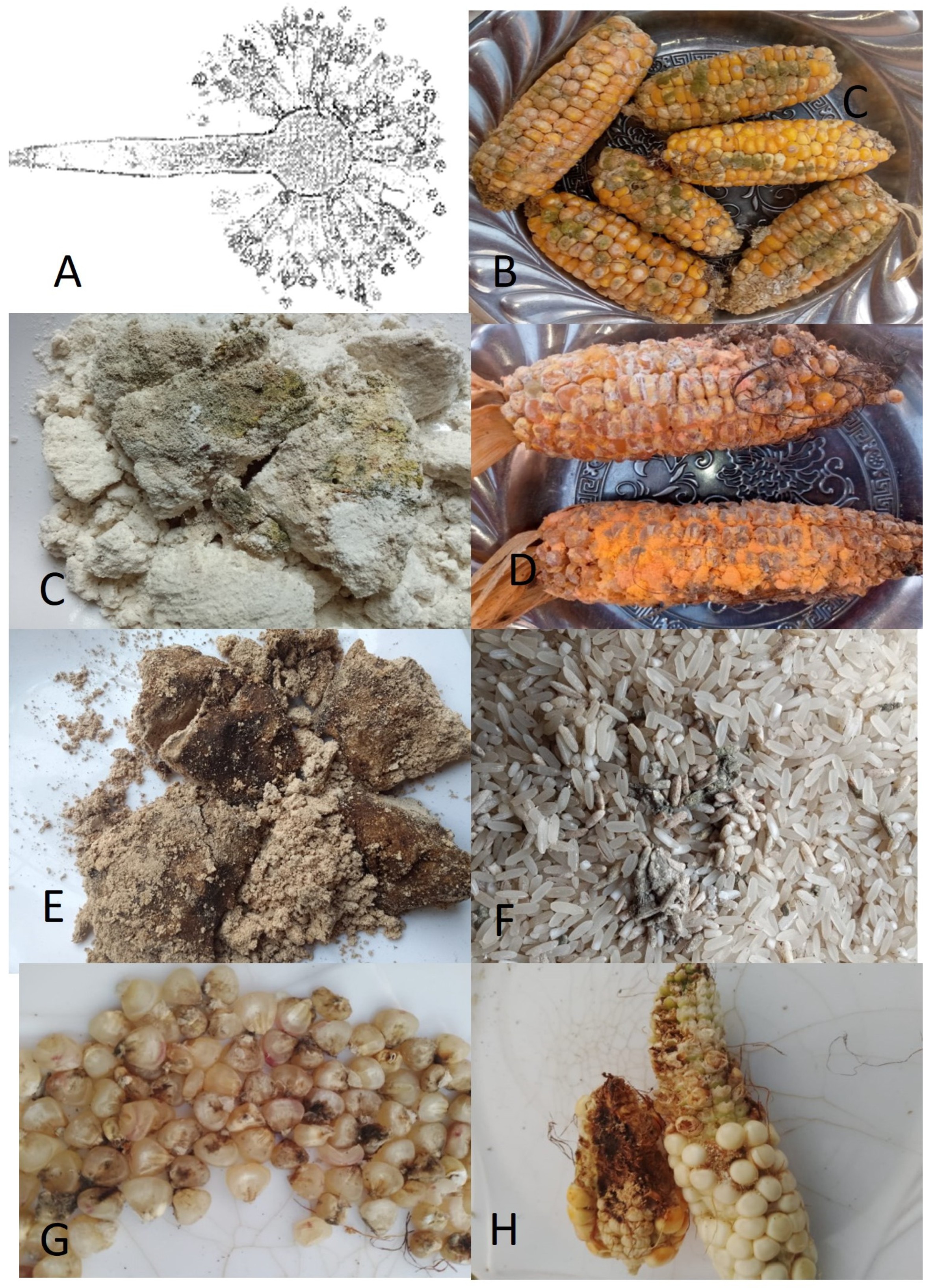
Figure 1.
Molds generate mycotoxins in foods. (A) Aspergilus fumigatus, which releases aflatoxin, (B) molds on red corn, (C) molds on maize flour, (D) molds on red corn, (E) molds on soybean flour, (F) molds in rice grains, (G) mold on white maize grains, and (H) molds on white corn. All pictures by Alice N. Mafe.
1.3. Global Toxic Levels of Mycotoxins in Foods
The global distribution of mycotoxins presents a significant concern for food safety due to their toxic effects on human and animal health. Based on global toxicity levels, aflatoxins are the most prevalent, accounting for 35% of the global toxic load. These highly toxic compounds are commonly found in crops like maize and peanuts, particularly in warm and humid climates, and are known for their carcinogenic properties [52].
Following aflatoxins, ochratoxin A and deoxynivalenol (DON) each contributes 20% to the total mycotoxin burden. Ochratoxin A is often found in cereals, coffee, and dried fruits and is associated with nephrotoxicity, while DON, commonly referred to as “vomitoxin”, occurs in grains and can cause acute gastrointestinal distress [53].
Fumonisins, which make up 15% of the global mycotoxin contamination, are prevalent in maize and are linked to esophageal cancer and neural tube defects. Lastly, zearalenone, responsible for 10% of the global burden, is an estrogenic mycotoxin found in grains that disrupt hormonal balance, particularly in livestock [34].
2. Cancer Risk Associated with Mycotoxins
Mycotoxins are widely recognized for their carcinogenic potential, with certain types posing significant cancer risks to humans. The primary mycotoxin associated with cancer risk is aflatoxin, which has been classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC). Other mycotoxins, such as ochratoxin A and fumonisin B1, are also considered potential carcinogens. These toxins contribute to cancer development through various biochemical and molecular mechanisms that lead to genetic damage, cell cycle disruption, and immune suppression [54].
2.1. Mechanisms of Carcinogenicity
Mycotoxins, particularly aflatoxins, contribute to cancer development through multiple biochemical and molecular mechanisms. These include direct DNA damage and mutagenesis, oxidative stress, cell cycle regulation, disruption, apoptosis inhibition, immune suppression, and epigenetic modifications [55]. The cumulative effects of these mechanisms (Figure 2 and Table 4) can lead to the initiation, promotion, and progression of cancer, making mycotoxins a significant concern for public health, particularly in regions with high exposure to contaminated foods. Understanding these mechanisms is crucial for developing effective strategies to reduce the cancer risk associated with mycotoxin exposure [56].
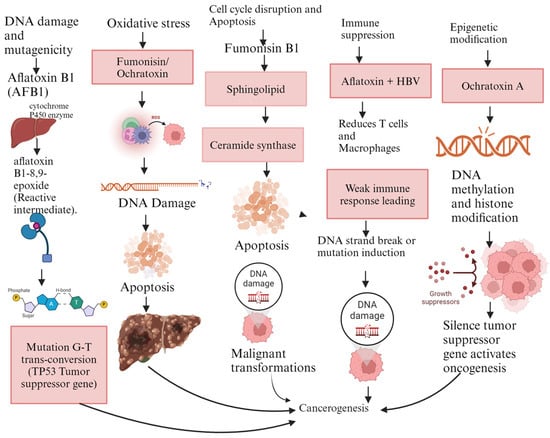
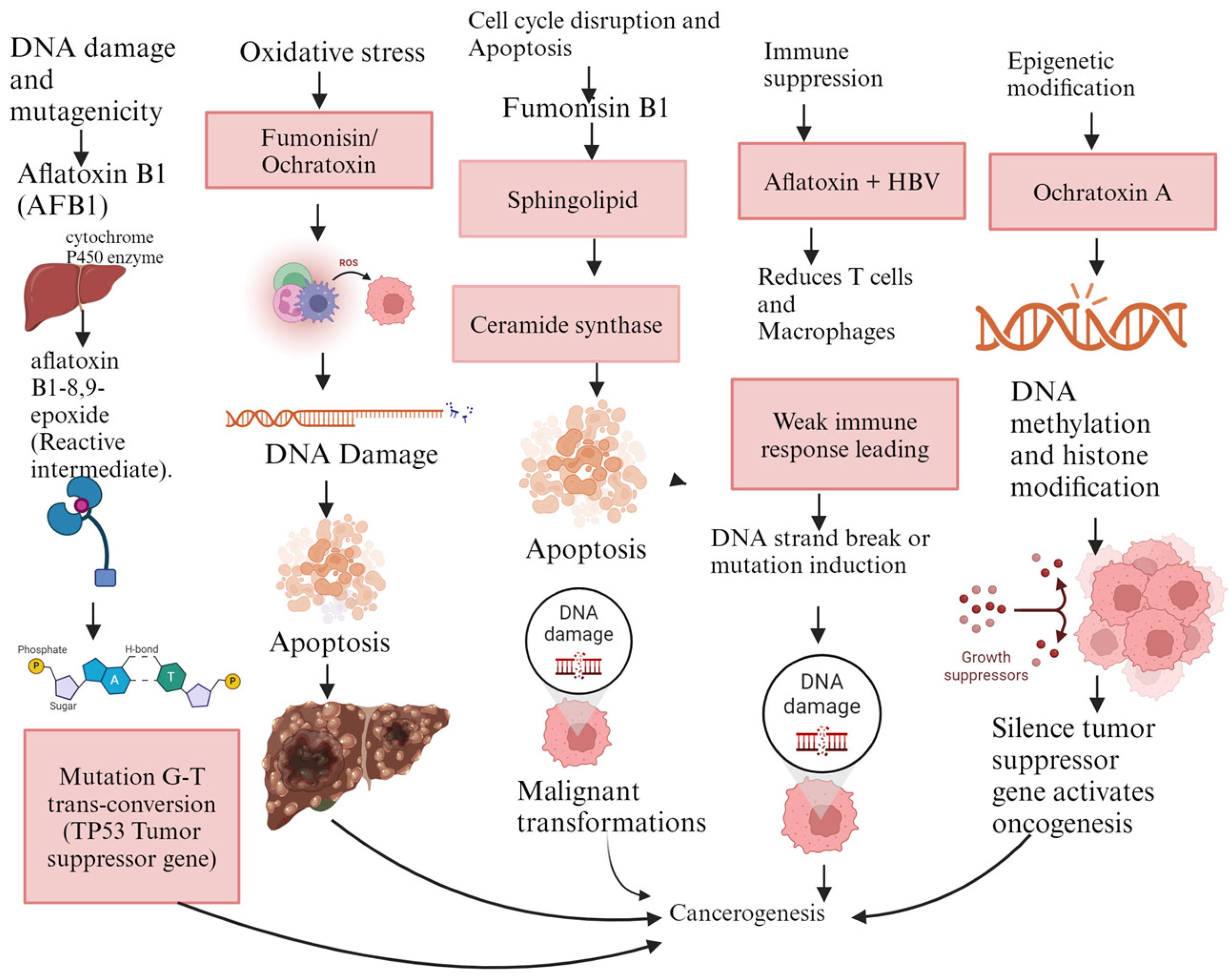
Figure 2.
Mechanisms of carcinogenicity for aflatoxin B1, fumonisins, and ochratoxin A. Legend: AFB1: aflatoxin B1, HBV: hepatitis B virus, ROS: reactive oxygen species, TP53: tumor suppressor gene. The figure was generated using BioRender.

Table 4.
Mechanisms of carcinogenicity caused by mycotoxins.
2.2. Epidemiological Evidence Linking Mycotoxins to Cancer Risk
Epidemiological studies provide strong evidence linking exposure to mycotoxins, particularly aflatoxins, to an increased risk of cancer (Table 5). Most notably, aflatoxin exposure is extensively associated with liver cancer or hepatocellular carcinoma (HCC). Ochratoxin A and fumonisins have also been implicated in developing kidney and esophageal cancers, respectively. Below is an overview of critical studies and data illustrating the correlation between mycotoxin exposure and cancer risk (Table 5), focusing on high-risk populations and regions.

Table 5.
Key studies and data illustrating the correlation between mycotoxin exposure and cancer risk for zearalenone and patulin.
2.3. Cancer Risk Associated with Mycotoxins
Epidemiological evidence strongly supports the link between mycotoxin exposure and increased cancer risk, particularly liver cancer due to aflatoxins and esophageal and kidney cancers from fumonisins [72]. Populations in regions with high contamination and limited food safety measures are at risk. This underscores the urgent need for effective monitoring, regulatory measures, and food safety interventions to reduce exposure to mycotoxins and mitigate their associated cancer risks.
2.4. Non-Cancer Risks Associated with Aflatoxins
Aflatoxins, a group of mycotoxins produced by Aspergillus species, primarily Aspergillus flavus and Aspergillus parasiticus, are known for their carcinogenic properties. However, the non-cancer risks, particularly acute toxicities and their potential to cause stunting in infants, are significant public health concerns that merit further examination [41].
2.4.1. Acute Toxicities of Aflatoxins
Aflatoxins can induce a range of acute toxic effects upon ingestion, particularly at high exposure levels. Acute aflatoxicosis is characterized by rapid-onset symptoms, which can vary depending on the dose and route of exposure. The main acute toxic effects include the following:
- Hepatotoxicity: The liver is the primary target organ for aflatoxins. Acute exposure can lead to liver damage, manifesting as jaundice, abdominal pain, and elevated liver enzymes. Severe cases can progress to liver failure, which may be fatal. The hepatotoxic effects are often attributed to the bioactivation of aflatoxins to reactive epoxide intermediates, leading to cellular damage and necrosis [73].
- Gastrointestinal Symptoms: Ingestion of contaminated food can cause gastrointestinal disturbances such as nausea, vomiting, abdominal cramps, and diarrhea. These symptoms result from direct irritation of the gastrointestinal tract and liver dysfunction.
- Immune System Suppression: Aflatoxins can impair immune function, making individuals more susceptible to infections. This is particularly concerning in infants, who already have immature immune systems. Immune suppression can lead to higher rates of morbidity and mortality from infectious diseases.
- Neurological Effects: In some cases, aflatoxin exposure has been linked to neurological symptoms, including headaches, confusion, and altered mental status. These effects may be due to hepatic encephalopathy resulting from liver dysfunction or direct neurotoxicity.
2.4.2. Stunting in Infants
The relationship between aflatoxin exposure and stunting in infants is an emerging area of research, with significant implications for child health and development. Stunting refers to impaired growth and development in children, characterized by low height-for-age. It is a critical public health issue, as it can lead to long-term consequences for physical and cognitive development [74]. The mechanisms through which aflatoxin exposure may contribute to stunting include the following:
- Nutritional Deficiencies: Aflatoxins can interfere with nutrient absorption and metabolism. They can cause malabsorption syndromes by damaging the intestinal lining, leading to nutrient deficiencies, particularly of proteins, vitamins, and minerals essential for growth. Infants exposed to aflatoxins may not receive adequate nutrition, exacerbating the risk of stunting [75].
- Chronic Inflammation: Aflatoxin exposure can provoke an inflammatory response, resulting in chronic inflammation that impairs growth. Prolonged inflammation can alter metabolic processes and hinder the body’s ability to utilize nutrients effectively, which is critical for growth and development during infancy [76].
- Impaired Immune Function: As mentioned earlier, aflatoxins can suppress the immune system. Infants who experience repeated infections due to immune compromise may have increased metabolic demands and reduced nutrient absorption, contributing to stunting. Frequent illness can also lead to increased energy expenditure, diverting resources away from growth and development [77].
- Hormonal Disruption: Aflatoxins have been shown to affect the endocrine system, potentially disrupting growth hormone pathways. Any disruption in growth hormone signaling can have significant effects on growth and development, leading to stunted growth in infants [66].
- Maternal Exposure: The effects of aflatoxins are not limited to direct exposure in infants. Pregnant and lactating women exposed to aflatoxins can transfer these toxins to their infants through placental transfer and breast milk. This transference can adversely affect the growth and development of infants, compounding the risk of stunting [78].
2.4.3. Public Health Implications
The non-cancer risks associated with aflatoxins, particularly acute toxicities and stunting in infants, highlight the urgent need for public health interventions. Effective strategies to mitigate aflatoxin exposure include the following:
- Food Safety Regulations: implementing strict regulations and monitoring systems to limit aflatoxin levels in food supplies, particularly in high-risk regions where staple crops are often contaminated [79].
- Education and Awareness: raising awareness among farmers, food processors, and consumers about the risks of aflatoxins, safe storage practices, and proper food handling techniques [80].
- Nutritional Interventions: providing nutritional support and supplementation for vulnerable populations, particularly in areas with high aflatoxin exposure, to mitigate the adverse effects of malnutrition and improve overall health outcomes.
- Research and Monitoring: continued research into the health effects of aflatoxins, particularly in children, and ongoing monitoring of aflatoxin levels in food sources will help to inform public health policies and interventions [81].
While aflatoxins are widely recognized for their carcinogenic properties, the acute toxicities and potential for stunting in infants represent significant non-cancer risks. Addressing these concerns is essential for improving child health and preventing long-term developmental consequences associated with aflatoxin exposure [82].
3. Methods of Analyzing Mycotoxins
Accurate detection and quantification of mycotoxins in food products are critical for ensuring food safety and preventing mycotoxin-related health risks, including cancer. Various analytical techniques are employed to identify and measure mycotoxins in agricultural products, processed foods, and animal feed. These methods differ in sensitivity, specificity, and complexity, allowing for qualitative and quantitative analysis across a range of mycotoxin types [83]. Table 6 describes the primary techniques used for mycotoxin analysis while Table 7 focuses on the recent publications on the immuno-detection of mycotoxins in food.

Table 6.
Primary techniques used for mycotoxin analysis.

Table 7.
Recent publications on immuno-detection of mycotoxins.
The analysis of mycotoxins in food relies on a combination of advanced techniques to ensure accurate detection and quantification. Chromatographic methods such as high-performance liquid chromatography (HPLC) and gas chromatography–mass spectrometry (GC-MS) are susceptible and precise, especially when combined with mass spectrometry [96]. Immunoassays, including enzyme-linked immunosorbent assay (ELISA) and lateral flow immunoassay (LFIA), offer rapid, cost-effective screening, making them suitable for routine testing [97]. Each method has its advantages depending on the type of mycotoxin, the complexity of the food matrix, and the desired level of accuracy, enabling comprehensive monitoring and control of mycotoxin contamination.
Sample Preparation for Mycotoxin Analysis
Sample preparation is critical in accurately detecting and quantifying mycotoxins in food. The preparation process involves several stages aimed at isolating mycotoxins from complex food matrices while maintaining the integrity and concentration of the target mycotoxins [98]. Proper sample preparation reduces interference from food components, improves extraction efficiency, and enhances the sensitivity of subsequent analytical techniques [99]. The main steps in preparing food samples for mycotoxin analysis (Table 8) are sampling, homogenization, extraction, cleanup, and concentration.

Table 8.
Main steps in preparing food samples for mycotoxin analysis.
Preparing food samples for mycotoxin analysis involves carefully executing steps to ensure accurate detection and quantification. Each stage, from sampling and homogenization to extraction, cleanup, and concentration, must be tailored to the specific type of food and mycotoxin being analyzed [102]. Proper sample preparation is essential for reducing interference, enhancing extraction efficiency, and improving analytical techniques’ reliability for mycotoxin detection.
4. Strategies for Mycotoxin Control
Controlling mycotoxin contamination is a pressing issue that requires a comprehensive approach. This approach should address pre- and post-harvest stages and incorporate effective regulatory and monitoring strategies. By implementing comprehensive control measures, we can significantly reduce the risk of mycotoxin contamination in food and feed, protecting public health and ensuring food safety [104].
4.1. Pre-Harvest Control Measures
Pre-harvest control measures focus on preventing fungal contamination and mycotoxin production before crops are harvested. These strategies involve various agricultural practices and interventions minimizing the conditions favoring fungal growth (Table 9) [62,75].

Table 9.
Strategies involve various agricultural practices and interventions minimizing the conditions favoring fungal growth.
4.2. Post-Harvest Control Measures
Post-harvest control measures are crucial for minimizing mycotoxin contamination after harvesting. Proper storage conditions, such as maintaining cool and dry environments, are essential to inhibit fungal growth and mycotoxin production. Effective processing techniques, including cleaning, sorting, and milling, help reduce mycotoxin levels by removing contaminated parts and diluting mycotoxins. Chemical treatments, like ammonization and ozone treatment, can also detoxify mycotoxins in food products. Implementing these measures is vital to ensure food safety and protect public health.
4.3. Regulatory and Monitoring Approaches
Regulatory and monitoring approaches are essential for managing mycotoxin contamination and ensuring food safety. Regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), establish guidelines and standards for maximum allowable levels of mycotoxins in food and feed [108]. These regulations protect public health by limiting exposure to mycotoxins through food and animal feed. Monitoring programs play a critical role in enforcing these standards. Regular testing of food and feed samples for mycotoxin contamination helps to identify and mitigate risks before they reach consumers. Techniques such as high-performance liquid chromatography (HPLC) and enzyme-linked immunosorbent assays (ELISAs) are commonly used to ensure compliance with regulatory limits. Additionally, government agencies and industry organizations often collaborate to conduct surveillance and train producers on best practices for managing mycotoxin risks [109].
Mycotoxin Regulation Framework
Global regulatory limits for various mycotoxins are set by different countries and international bodies, highlighting the variation in food safety standards. Aflatoxins, known for their carcinogenic effects, are regulated most strictly in the European Union (EU) at 2 ppb, while the United States (FDA) allows up to 20 ppb. Ochratoxin A, a nephrotoxic mycotoxin, has a 5 ppb limit in most regions, though it is unregulated in the U.S. [14]. For fumonisins, the limits range widely, from 1000 ppb in China to 4000 ppb in the U.S. Zearalenone, which affects hormone regulation, has limits varying from 60 ppb in China to 200 ppb in Japan, with no regulation in some countries. Lastly, deoxynivalenol (DON), known for its gastrointestinal effects, is regulated between 1000 ppb in the U.S. and 2000 ppb in Australia/New Zealand [110]. These data provide a basis for comparing the regulatory frameworks, emphasizing the need for harmonized global standards to ensure food safety.
5. Current Challenges and Limitations
The effective management of mycotoxin contamination in food and feed presents several challenges and limitations, particularly concerning detection methods and control strategies. Resolving these issues is crucial for improving food safety and mitigating health risks associated with mycotoxins.
5.1. Detection Challenges
Detection of mycotoxins in food involves sophisticated analytical techniques that face several challenges, including sensitivity, specificity, and cost (Table 10), while Table 11 illustrates the various regulatory and monitoring approaches. One significant challenge is achieving the required sensitivity to detect low levels of mycotoxins, especially when they are present in complex food matrices. Techniques such as high-performance liquid chromatography (HPLC) and gas chromatography–mass spectrometry (GC-MS) offer high sensitivity but can be expensive and require extensive sample preparation [111]. In addition, the specificity of these methods must be high to accurately distinguish between mycotoxins and similar compounds that may interfere with results.

Table 10.
Post-harvest control measures.

Table 11.
Regulatory and monitoring approaches.
Another challenge is the cost of advanced detection methods, which may be prohibitive for routine testing in low-resource settings. While immunoassays like enzyme-linked immunosorbent assays (ELISAs) are more cost-effective, they may lack the sensitivity and specificity required for detecting low levels of mycotoxins in complex samples [118]. Furthermore, developing and validating new detection methods can be time-consuming and resource-intensive.
5.2. Control Measures Limitations
While various control strategies are employed to manage mycotoxin contamination, each has its limitations in terms of effectiveness and feasibility.
Managing mycotoxin contamination involves addressing several challenges related to detection and control measures. Detection methods must be sensitive and specific, but high costs and technical limitations can restrict their use. Control measures, including pre-harvest practices, post-harvest treatments, and chemical decontamination, face limitations in effectiveness and feasibility. Regulatory and monitoring systems are crucial for ensuring compliance but may encounter consistency and resource availability challenges. To improve food safety and mitigate mycotoxin risks, ongoing research, innovation, and investment in more effective and accessible methods are needed [119].
6. Future Directions
The management and control of mycotoxins continue to evolve as new challenges and opportunities arise. Further research and the adoption of emerging technologies are essential to address these issues effectively. Identifying research gaps and staying abreast of emerging trends, such as advanced biosensors, machine learning models, and genome editing tools, can help enhance our ability to detect, control, and mitigate the risks associated with mycotoxins. This proactive approach ensures that food safety strategies remain dynamic and effective in the face of evolving threats [120].
6.1. Research Gaps
In recent years, significant progress has been made in understanding mycotoxins and their impact on food safety. However, several critical research gaps remain, hindering the development of more effective detection, control, and management strategies. Table 12 highlights these gaps, the needs they address, and the opportunities for future research to improve food safety and mitigate the risks posed by mycotoxins [121]

Table 12.
Detection challenges.
6.2. Emerging Trends
As mycotoxin management evolves, several emerging trends are reshaping how mycotoxins are detected, controlled, and regulated. These advancements are driven by technological innovations, research into sustainable solutions, and enhanced global cooperation. Table 13 outlines the key emerging trends, highlighting their potential impact on food safety, agricultural practices, and regulatory frameworks [7]. In addition, Table 14 presents the research gaps, while Table 15 discusses key emerging trends.

Table 13.
Control measure limitations.

Table 14.
Research gaps.

Table 15.
Key emerging trends.
Addressing the challenges associated with mycotoxins requires ongoing research and adopting new technologies. Identifying research gaps, such as the need for advanced analytical methods and innovative control strategies, is crucial for improving mycotoxin management [113]. Emerging trends, including advancements in analytical technology, the integration of AI and ML, and the development of biocontrol agents, offer promising opportunities to enhance detection and control efforts [136]. By staying informed about these developments and investing in research and innovation, we can better manage the risks associated with mycotoxins [137] and protect public health.
7. Conclusions
This review examined the complex issue of mycotoxins in food, emphasizing their origin, significance, and impact on public health. Mycotoxins, toxic secondary metabolites produced by fungi such as Aspergillus, Penicillium, and Fusarium, represent a severe threat to food safety and public health. Key mycotoxins of concern include aflatoxins, ochratoxins, fumonisins, zearalenone, and patulin, each with specific sources and affected foods. The cancer risk associated with mycotoxins is notably significant, with aflatoxins being particularly carcinogenic and linked to liver cancer. Epidemiological evidence highlights a heightened risk in areas with substantial mycotoxin exposure, often exacerbated by inadequate control measures and regulatory oversight. Detection of mycotoxins remains challenging due to analytical methods’ sensitivity, specificity, and cost issues. While techniques such as chromatography and immunoassays are effective, they have limitations that affect their reliability and accessibility. Control measures, including pre-harvest and post-harvest strategies, along with regulatory frameworks, are crucial but have limitations regarding feasibility and effectiveness. Emerging trends, such as advancements in analytical technology, the integration of AI and machine learning, and the development of biocontrol agents, offer promising opportunities for enhancing mycotoxin management. However, research gaps persist, particularly in developing new methods, understanding mycotoxin interactions, and adapting to climate change. To improve the management of mycotoxin contamination and ensure food safety, it is recommended to invest in advanced analytical technologies, develop and implement innovative control strategies, address research gaps and emerging risks, strengthen regulatory and monitoring frameworks, and promote global collaboration and data sharing. Addressing these challenges requires a coordinated effort that includes advancing technology, enhancing control measures, and fostering international cooperation to manage mycotoxin risks and protect public health effectively.
Author Contributions
Conceptualization, A.N.M. and D.B.; methodology, A.N.M.; software, A.N.M.; validation, D.B.; investigation, A.N.M.; writing—original draft preparation, A.N.M.; writing—review and editing, A.N.M. and D.B.; visualization, A.N.M. and D.B.; supervision, D.B.; project administration, D.B.; funding acquisition, D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Priorities Research Program grant NPRP 14S-0311–210033; awarded to Dietrich Büsselberg, January 2023–Current) from the Qatar National Research Fund (QNRF, a member of Qatar Foundation). Publication costs for this work were covered by the Biomedical Research Program at Weill Cornell Medicine-Qatar, a program funded by Qatar Foundation. The statements made herein are solely the responsibility of the authors.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Yu, J.; Pedroso, I.R. Mycotoxins in Cereal-Based Products and Their Impacts on the Health of Humans, Livestock Animals and Pets. Toxins 2023, 15, 480. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.; Carreiró, F.; Freitas, A.; Barros, S.; Brites, C.; Ramos, F.; Sanches Silva, A. Mycotoxins Contamination in Rice: Analytical Methods, Occurrence and Detoxification Strategies. Toxins 2022, 14, 647. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; D’Amore, T.; Tarapoulouzi, M.; Agriopoulou, S.; Varzakas, T. Aflatoxins Contamination in Feed Commodities: From Occurrence and Toxicity to Recent Advances in Analytical Methods and Detoxification. Microorganisms 2023, 11, 2614. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Samota, M.K.; Kumar, A.; Silva, A.S.; Dubey, N.K. Fungal mycotoxins in food commodities: Present status and future concerns. Front. Sustain. Food Syst. 2023, 7, 1162595. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Akinola, S.A.; Mwanza, M. Fusarium Mycotoxins, Their Metabolites (Free, Emerging, and Masked), Food Safety Concerns, and Health Impacts. Int. J. Environ. Res. Public Health 2021, 18, 11741. [Google Scholar] [CrossRef]
- Khan, R.; Anwar, F.; Ghazali, F.M. A comprehensive review of mycotoxins: Toxicology, detection, and effective mitigation approaches. Heliyon 2024, 10, e28361. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef]
- Kappes, A.; Tozooneyi, T.; Shakil, G.; Railey, A.F.; McIntyre, K.M.; Mayberry, D.E.; Rushton, J.; Pendell, D.L.; Marsh, T.L. Livestock health and disease economics: A scoping review of selected literature. Front. Vet. Sci. 2023, 10, 1168649. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, Z.; Hou, J.; Yao, Y. Contamination and Control of Mycotoxins in Grain and Oil Crops. Microorganisms 2024, 12, 567. [Google Scholar] [CrossRef]
- Mukhtar, K.; Nabi, B.G.; Ansar, S.; Bhat, Z.F.; Aadil, R.M.; Mousavi Khaneghah, A. Mycotoxins and consumers’ awareness: Recent progress and future challenges. Toxicon 2023, 232, 107227. [Google Scholar] [CrossRef] [PubMed]
- Faour-Klingbeil, D.; Todd, E. A Review on the Rising Prevalence of International Standards: Threats or Opportunities for the Agri-Food Produce Sector in Developing Countries, with a Focus on Examples from the MENA Region. Foods 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Magnoli, A.P.; Poloni, V.L.; Cavaglieri, L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019, 29, 99–108. [Google Scholar] [CrossRef]
- Sirma, A.J.; Lindahl, J.F.; Makita, K.; Senerwa, D.; Mtimet, N.; Kang’ethe, E.K.; Grace, D. The impacts of aflatoxin standards on health and nutrition in sub-Saharan Africa: The case of Kenya. Glob. Food Sec. 2018, 18, 57–61. [Google Scholar] [CrossRef]
- Lamm, K.W.; Randall, N.L.; Diez-Gonzalez, F. Critical Food Safety Issues Facing the Food Industry: A Delphi Analysis. J. Food Prot. 2021, 84, 680–687. [Google Scholar] [CrossRef]
- Latham, R.L.; Boyle, J.T.; Barbano, A.; Loveman, W.G.; Brown, N.A. Diverse mycotoxin threats to safe food and feed cereals. Essays Biochem. 2023, 67, 797–809. [Google Scholar] [CrossRef]
- Nji, Q.N.; Babalola, O.O.; Ekwomadu, T.I.; Nleya, N.; Mwanza, M. Six Main Contributing Factors to High Levels of Mycotoxin Contamination in African Foods. Toxins 2022, 14, 318. [Google Scholar] [CrossRef]
- Ekwomadu, T.; Mwanza, M.; Musekiwa, A. Mycotoxin-Linked Mutations and Cancer Risk: A Global Health Issue. Int. J. Environ. Res. Public Health 2022, 19, 7754. [Google Scholar] [CrossRef]
- Huong, B.T.M.; Tuyen, L.D.; Madsen, H.; Brimer, L.; Friis, H.; Dalsgaard, A. Total Dietary Intake and Health Risks Associated with Exposure to Aflatoxin B1, Ochratoxin A and Fuminisins of Children in Lao Cai Province, Vietnam. Toxins 2019, 11, 638. [Google Scholar] [CrossRef]
- Soni, P.; Gangurde, S.S.; Ortega-Beltran, A.; Kumar, R.; Parmar, S.; Sudini, H.K.; Lei, Y.; Ni, X.; Huai, D.; Fountain, J.C.; et al. Functional Biology and Molecular Mechanisms of Host-Pathogen Interactions for Aflatoxin Contamination in Groundnut (Arachis hypogaea L.) and Maize (Zea mays L.). Front. Microbiol. 2020, 11, 227. [Google Scholar] [CrossRef]
- Goessens, T.; Mouchtaris-Michailidis, T.; Tesfamariam, K.; Truong, N.N.; Vertriest, F.; Bader, Y.; De Saeger, S.; Lachat, C.; De Boevre, M. Dietary mycotoxin exposure and human health risks: A protocol for a systematic review. Environ. Int. 2024, 184, 108456. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, X.; Yuan, L.; Li, J. Complicated interactions between bio-adsorbents and mycotoxins during mycotoxin adsorption: Current research and future prospects. Trends Food Sci. Technol. 2020, 96, 127–134. [Google Scholar] [CrossRef]
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ruiz, M.J.; Vila-Donat, P. Multi-mycotoxin occurrence in feed, metabolism and carry-over to animal-derived food products: A review. Food Chem. Toxicol. 2021, 158, 112661. [Google Scholar] [CrossRef] [PubMed]
- Gallage, S.; García-Beccaria, M.; Szydlowska, M.; Rahbari, M.; Mohr, R.; Tacke, F.; Heikenwalder, M. The therapeutic landscape of hepatocellular carcinoma. Med 2021, 2, 505–552. [Google Scholar] [CrossRef]
- Malvandi, A.M.; Shahba, S.; Mehrzad, J.; Lombardi, G. Metabolic Disruption by Naturally Occurring Mycotoxins in Circulation: A Focus on Vascular and Bone Homeostasis Dysfunction. Front. Nutr. 2022, 9, 915681. [Google Scholar] [CrossRef]
- Vörösházi, J.; Neogrády, Z.; Mátis, G.; Mackei, M. Pathological consequences, metabolism and toxic effects of trichothecene T-2 toxin in poultry. Poult. Sci. 2024, 103, 103471. [Google Scholar] [CrossRef]
- Gómez-Osorio, L.-M.; Vasiljevic, M.; Raj, J.; Chaparro-Gutierréz, J.J.; López-Osorio, S. Mycotoxins and coccidiosis in poultry—co-occurrence, interaction, and effects. Front. Vet. Sci. 2024, 11, 1387856. [Google Scholar] [CrossRef]
- Abraham, N.; Chan, E.T.S.; Zhou, T.; Seah, S.Y.K. Microbial detoxification of mycotoxins in food. Front. Microbiol. 2022, 13, 957148. [Google Scholar] [CrossRef]
- Kortei, N.K.; Badzi, S.; Nanga, S.; Wiafe-Kwagyan, M.; Amon, D.N.K.; Odamtten, G.T. Survey of knowledge, and attitudes to storage practices preempting the occurrence of filamentous fungi and mycotoxins in some Ghanaian staple foods and processed products. Sci. Rep. 2023, 13, 8710. [Google Scholar] [CrossRef]
- Liguori, J.; Trübswasser, U.; Pradeilles, R.; Le Port, A.; Landais, E.; Talsma, E.F.; Lundy, M.; Béné, C.; Bricas, N.; Laar, A.; et al. How do food safety concerns affect consumer behaviors and diets in low- and middle-income countries? A systematic review. Glob. Food Sec. 2022, 32, 100606. [Google Scholar] [CrossRef]
- Nada, S.; Nikola, T.; Bozidar, U.; Ilija, D.; Andreja, R. Prevention and practical strategies to control mycotoxins in the wheat and maize chain. Food Control 2022, 136, 108855. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Kępińska-Pacelik, J.; Biel, W. Mycotoxins—Prevention, Detection, Impact on Animal Health. Processes 2021, 9, 2035. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef]
- Alameri, M.M.; Kong, A.S.-Y.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Al Sallagi, M.; Cheng, W.-H.; Abushelaibi, A.; Lim, S.-H.E.; Loh, J.-Y.; et al. Aflatoxin Contamination: An Overview on Health Issues, Detection and Management Strategies. Toxins 2023, 15, 246. [Google Scholar] [CrossRef]
- Ding, L.; Han, M.; Wang, X.; Guo, Y. Ochratoxin A: Overview of Prevention, Removal, and Detoxification Methods. Toxins 2023, 15, 565. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Wang, J.; Xue, K.S.; Su, X.; Qu, H.; Duan, X.; Jiang, Y. The occurrence and management of fumonisin contamination across the food production and supply chains. J. Adv. Res. 2024, 60, 13–26. [Google Scholar] [CrossRef]
- Balló, A.; Busznyákné Székvári, K.; Czétány, P.; Márk, L.; Török, A.; Szántó, Á.; Máté, G. Estrogenic and Non-Estrogenic Disruptor Effect of Zearalenone on Male Reproduction: A Review. Int. J. Mol. Sci. 2023, 24, 1578. [Google Scholar] [CrossRef]
- Bacha, S.A.S.; Li, Y.; Nie, J.; Xu, G.; Han, L.; Farooq, S. Comprehensive review on patulin and Alternaria toxins in fruit and derived products. Front. Plant Sci. 2023, 14, 1139757. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control 2022, 144, 109350. [Google Scholar] [CrossRef]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, W.; Ma, Z.; Zhang, Q.; Li, H. The Occurrence and Contamination Level of Ochratoxin A in Plant and Animal-Derived Food Commodities. Molecules 2021, 26, 6928. [Google Scholar] [CrossRef] [PubMed]
- Yli-Mattila, T.; Sundheim, L. Fumonisins in African Countries. Toxins 2022, 14, 419. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Umereweneza, D.; Kamizikunze, T.; Muhizi, T. Assessment of mycotoxins types in some foodstuff consumed in Rwanda. Food Control 2018, 85, 432–436. [Google Scholar] [CrossRef]
- Peivasteh-roudsari, L.; Barzegar-bafrouei, R.; Sharifi, K.A.; Azimisalim, S.; Karami, M.; Abedinzadeh, S.; Asadinezhad, S.; Tajdar-oranj, B.; Mahdavi, V.; Alizadeh, A.M.; et al. Origin, dietary exposure, and toxicity of endocrine-disrupting food chemical contaminants: A comprehensive review. Heliyon 2023, 9, e18140. [Google Scholar] [CrossRef]
- Godebo, T.R.; Stoner, H.; Kodsup, P.; Bases, B.; Marzoni, S.; Weil, J.; Frey, M.; Daley, P.; Earnhart, A.; Ellias, G.; et al. Occurrence of heavy metals coupled with elevated levels of essential elements in chocolates: Health risk assessment. Food Res. Int. 2024, 187, 114360. [Google Scholar] [CrossRef]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023, 21, e06857. [Google Scholar] [CrossRef]
- Wang, X.; Nag, R.; Brunton, N.P.; Siddique, M.A.B.; Harrison, S.M.; Monahan, F.J.; Cummins, E. Human health risk assessment of bisphenol A (BPA) through meat products. Environ. Res. 2022, 213, 113734. [Google Scholar] [CrossRef]
- Kovač, M.; Bulaić, M.; Nevistić, A.; Rot, T.; Babić, J.; Panjičko, M.; Kovač, T.; Šarkanj, B. Regulated Mycotoxin Occurrence and Co-Occurrence in Croatian Cereals. Toxins 2022, 14, 112. [Google Scholar] [CrossRef]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- Warnatzsch, E.A.; Reay, D.S.; Camardo Leggieri, M.; Battilani, P. Climate Change Impact on Aflatoxin Contamination Risk in Malawi’s Maize Crops. Front. Sustain. Food Syst. 2020, 4, 591792. [Google Scholar] [CrossRef]
- Schrögel, P.; Wätjen, W. Insects for Food and Feed-Safety Aspects Related to Mycotoxins and Metals. Foods 2019, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Açar, Y.; Akbulut, G. Evaluation of Aflatoxins Occurrence and Exposure in Cereal-Based Baby Foods: An Update Review. Curr. Nutr. Rep. 2024, 13, 59–68. [Google Scholar] [CrossRef]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 Toxicity and Protective Effects of Curcumin: Molecular Mechanisms and Clinical Implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef]
- Ben Miri, Y.; Benabdallah, A.; Chentir, I.; Djenane, D.; Luvisi, A.; De Bellis, L. Comprehensive Insights into Ochratoxin A: Occurrence, Analysis, and Control Strategies. Foods 2024, 13, 1184. [Google Scholar] [CrossRef]
- Abrehame, S.; Manoj, V.R.; Hailu, M.; Chen, Y.-Y.; Lin, Y.-C.; Chen, Y.-P. Aflatoxins: Source, Detection, Clinical Features and Prevention. Processes 2023, 11, 204. [Google Scholar] [CrossRef]
- Oke, O.E.; Akosile, O.A.; Oni, A.I.; Opowoye, I.O.; Ishola, C.A.; Adebiyi, J.O.; Odeyemi, A.J.; Adjei-Mensah, B.; Uyanga, V.A.; Abioja, M.O. Oxidative stress in poultry production. Poult. Sci. 2024, 103, 104003. [Google Scholar] [CrossRef]
- Lumsangkul, C.; Tso, K.-H.; Fan, Y.-K.; Chiang, H.-I.; Ju, J.-C. Mycotoxin Fumonisin B1 Interferes Sphingolipid Metabolisms and Neural Tube Closure during Early Embryogenesis in Brown Tsaiya Ducks. Toxins 2021, 13, 743. [Google Scholar] [CrossRef]
- Sun, Y.; Song, Y.; Long, M.; Yang, S. Immunotoxicity of Three Environmental Mycotoxins and Their Risks of Increasing Pathogen Infections. Toxins 2023, 15, 187. [Google Scholar] [CrossRef]
- Ghazi, T.; Arumugam, T.; Foolchand, A.; Chuturgoon, A.A. The Impact of Natural Dietary Compounds and Food-Borne Mycotoxins on DNA Methylation and Cancer. Cells 2020, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Ráduly, Z.; Szabó, L.; Madar, A.; Pócsi, I.; Csernoch, L. Toxicological and Medical Aspects of Aspergillus-Derived Mycotoxins Entering the Feed and Food Chain. Front. Microbiol. 2020, 10, 2908. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef]
- Yang, L.; Liao, W.; Dong, J.; Chen, X.; Huang, L.; Yang, W.; Jiang, S. Zearalenone Promotes Uterine Hypertrophy through AMPK/mTOR Mediated Autophagy. Toxins 2024, 16, 73. [Google Scholar] [CrossRef]
- Kościelecka, K.; Kuć, A.; Kubik-Machura, D.; Męcik-Kronenberg, T.; Włodarek, J.; Radko, L. Endocrine Effect of Some Mycotoxins on Humans: A Clinical Review of the Ways to Mitigate the Action of Mycotoxins. Toxins 2023, 15, 515. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, H.; Niu, J.; Chen, X.; Li, H.; Rao, Z.; Guo, Y.; Zhang, W.; Wang, Z. Curcumin alleviates zearalenone-induced liver injury in mice by scavenging reactive oxygen species and inhibiting mitochondrial apoptosis pathway. Ecotoxicol. Environ. Saf. 2024, 277, 116343. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef]
- Kobets, T.; Smith, B.P.C.; Williams, G.M. Food-Borne Chemical Carcinogens and the Evidence for Human Cancer Risk. Foods 2022, 11, 2828. [Google Scholar] [CrossRef]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef]
- Cui, J.; Yin, S.; Zhao, C.; Fan, L.; Hu, H. Combining Patulin with Cadmium Induces Enhanced Hepatotoxicity and Nephrotoxicity In Vitro and In Vivo. Toxins 2021, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- Mulisa, G.; Pero-Gascon, R.; McCormack, V.; Bisanz, J.E.; Talukdar, F.R.; Abebe, T.; De Boevre, M.; De Saeger, S. Multiple mycotoxin exposure assessment through human biomonitoring in an esophageal cancer case-control study in the Arsi-Bale districts of Oromia region of Ethiopia. Int. J. Hyg. Environ. Health 2025, 263, 114466. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Andrews-Trevino, J.Y.; Webb, P.; Shively, G.; Kablan, A.; Baral, K.; Davis, D.; Paudel, K.; Shrestha, R.; Pokharel, A.; Acharya, S.; et al. Aflatoxin exposure and child nutrition: Measuring anthropometric and long-bone growth over time in Nepal. Am. J. Clin. Nutr. 2021, 113, 874–883. [Google Scholar] [CrossRef]
- Mupunga, I.; Mngqawa, P.; Katerere, D. Peanuts, Aflatoxins and Undernutrition in Children in Sub-Saharan Africa. Nutrients 2017, 9, 1287. [Google Scholar] [CrossRef]
- Ivanovics, B.; Gazsi, G.; Reining, M.; Berta, I.; Poliska, S.; Toth, M.; Domokos, A.; Nagy, B.; Staszny, A.; Cserhati, M.; et al. Embryonic exposure to low concentrations of aflatoxin B1 triggers global transcriptomic changes, defective yolk lipid mobilization, abnormal gastrointestinal tract development and inflammation in zebrafish. J. Hazard. Mater. 2021, 416, 125788. [Google Scholar] [CrossRef]
- Morales, F.; Montserrat-de la Paz, S.; Leon, M.J.; Rivero-Pino, F. Effects of Malnutrition on the Immune System and Infection and the Role of Nutritional Strategies Regarding Improvements in Children’s Health Status: A Literature Review. Nutrients 2023, 16, 1. [Google Scholar] [CrossRef]
- Salas, R.; Acosta, N.; Garza, A.d.J.; Tijerina, A.; Dávila, R.; Jiménez-Salas, Z.; Otero, L.; Santos, M.; Trujillo, A.-J. Levels of Aflatoxin M1 in Breast Milk of Lactating Mothers in Monterrey, Mexico: Exposure and Health Risk Assessment of Newborns. Toxins 2022, 14, 194. [Google Scholar] [CrossRef]
- Lukwago, F.B.; Mukisa, I.M.; Atukwase, A.; Kaaya, A.N.; Tumwebaze, S. Mycotoxins contamination in foods consumed in Uganda: A 12-year review (2006–18). Sci. Afr. 2019, 3, e00054. [Google Scholar] [CrossRef]
- Anato, A.; Headey, D.; Hirvonen, K.; Pokharel, A.; Tessema, M.; Wu, F.; Baye, K. Feed handling practices, aflatoxin awareness and children’s milk consumption in the Sidama region of southern Ethiopia. One Health 2024, 18, 100672. [Google Scholar] [CrossRef]
- Visser, M.E.; Schoonees, A.; Ezekiel, C.N.; Randall, N.P.; Naude, C.E. Agricultural and nutritional education interventions for reducing aflatoxin exposure to improve infant and child growth in low- and middle-income countries. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Enespa; Chandra, P. Aflatoxins: Food Safety, Human Health Hazards and Their Prevention. In Aflatoxins—Occurrence, Detoxification, Determination and Health Risks; IntechOpen: London, UK, 2022; p. 2. [Google Scholar]
- Boshra, M.H.; El-Housseiny, G.S.; Farag, M.M.S.; Aboshanab, K.M. Innovative approaches for mycotoxin detection in various food categories. AMB Express 2024, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Keskin, E.; Eyupoglu, O.E. Determination of mycotoxins by HPLC, LC-MS/MS and health risk assessment of the mycotoxins in bee products of Turkey. Food Chem. 2023, 400, 134086. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Gorniak, L.; Stela, M.; Bijak, M. The Existing Methods and Novel Approaches in Mycotoxins’ Detection. Molecules 2021, 26, 3981. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Analysis and Detection of Major Mycotoxins in Foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef]
- Malachová, A.; Stránská, M.; Václavíková, M.; Elliott, C.T.; Black, C.; Meneely, J.; Hajšlová, J.; Ezekiel, C.N.; Schuhmacher, R.; Krska, R. Advanced LC–MS-based methods to study the co-occurrence and metabolization of multiple mycotoxins in cereals and cereal-based food. Anal. Bioanal. Chem. 2018, 410, 801–825. [Google Scholar] [CrossRef]
- Liew, W.-P.-P.; Sabran, M.-R. Recent advances in immunoassay-based mycotoxin analysis and toxicogenomic technologies. J. Food Drug Anal. 2022, 30, 549–561. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, Q.; Li, P. Advances in Visual Immunoassays for Sensitive Detection of Mycotoxins in Food—A Review. Chem. Proc. 2021, 5, 25. [Google Scholar] [CrossRef]
- Shahjahan, T.; Javed, B.; Sharma, V.; Tian, F. Overview of Various Components of Lateral-Flow Immunochromatography Assay for the Monitoring of Aflatoxin and Limit of Detection in Food Products: A Systematic Review. Chemosensors 2023, 11, 520. [Google Scholar] [CrossRef]
- Inglis, A.; Parnell, A.C.; Subramani, N.; Doohan, F.M. Machine Learning Applied to the Detection of Mycotoxin in Food: A Systematic Review. Toxins 2024, 16, 268. [Google Scholar] [CrossRef]
- Hafez, E.; Abd El-Aziz, N.M.; Darwish, A.M.G.; Shehata, M.G.; Ibrahim, A.A.; Elframawy, A.M.; Badr, A.N. Validation of New ELISA Technique for Detection of Aflatoxin B1 Contamination in Food Products versus HPLC and VICAM. Toxins 2021, 13, 747. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Wang, S.; Fotina, H.; Wang, Z. A Novel Lateral Flow Immunochromatographic Assay for Rapid and Simultaneous Detection of Aflatoxin B1 and Zearalenone in Food and Feed Samples Based on Highly Sensitive and Specific Monoclonal Antibodies. Toxins 2022, 14, 615. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, S.; Barda, O.; Zakin, V.; Reifen, R.; Sionov, E. Rapid Detection and Quantification of Patulin and Citrinin Contamination in Fruits. Molecules 2021, 26, 4545. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Ji, Y.; Chen, J.; Ye, J.; Ni, B.; Li, L.; Yang, Y. Multicolor Visual Detection of Deoxynivalenol in Grain Based on Magnetic Immunoassay and Enzymatic Etching of Plasmonic Gold Nanobipyramids. Toxins 2023, 15, 351. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Bassolli Borsatto, J.V.; Maciel, E.V.S.; Lanças, F.M. Current role of modern chromatography and mass spectrometry in the analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2021, 135, 116156. [Google Scholar] [CrossRef]
- Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors 2021, 21, 5185. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, Y.; Zhou, Y.; Wei, B.; Feng, X. Recent Insights into Sample Pretreatment Methods for Mycotoxins in Different Food Matrices: A Critical Review on Novel Materials. Toxins 2023, 15, 215. [Google Scholar] [CrossRef]
- Alahmad, W.; Kaya, S.I.; Cetinkaya, A.; Varanusupakul, P.; Ozkan, S.A. Green chemistry methods for food analysis: Overview of sample preparation and determination. Adv. Sample Prep. 2023, 5, 100053. [Google Scholar] [CrossRef]
- Donnelly, R.; Elliott, C.; Zhang, G.; Baker, B.; Meneely, J. Understanding Current Methods for Sampling of Aflatoxins in Corn and to Generate a Best Practice Framework. Toxins 2022, 14, 819. [Google Scholar] [CrossRef]
- Zhang, K.; Banerjee, K. A Review: Sample Preparation and Chromatographic Technologies for Detection of Aflatoxins in Foods. Toxins 2020, 12, 539. [Google Scholar] [CrossRef]
- Colombo, R.; Papetti, A. Pre-Concentration and Analysis of Mycotoxins in Food Samples by Capillary Electrophoresis. Molecules 2020, 25, 3441. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, N.; Combès, A.; Pichon, V. Immunoaffinity Extraction and Alternative Approaches for the Analysis of Toxins in Environmental, Food or Biological Matrices. Toxins 2020, 12, 795. [Google Scholar] [CrossRef]
- Fumagalli, F.; Ottoboni, M.; Pinotti, L.; Cheli, F. Integrated Mycotoxin Management System in the Feed Supply Chain: Innovative Approaches. Toxins 2021, 13, 572. [Google Scholar] [CrossRef] [PubMed]
- Habschied, K.; Krstanović, V.; Zdunić, Z.; Babić, J.; Mastanjević, K.; Šarić, G.K. Mycotoxins Biocontrol Methods for Healthier Crops and Stored Products. J. Fungi 2021, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Zadravec, M.; Markov, K.; Lešić, T.; Frece, J.; Petrović, D.; Pleadin, J. Biocontrol Methods in Avoidance and Downsizing of Mycotoxin Contamination of Food Crops. Processes 2022, 10, 655. [Google Scholar] [CrossRef]
- Martín, I.; Gálvez, L.; Guasch, L.; Palmero, D. Fungal Pathogens and Seed Storage in the Dry State. Plants 2022, 11, 3167. [Google Scholar] [CrossRef]
- Bartholomew, H.P.; Bradshaw, M.; Jurick, W.M.; Fonseca, J.M. The Good, the Bad, and the Ugly: Mycotoxin Production During Postharvest Decay and Their Influence on Tritrophic Host–Pathogen–Microbe Interactions. Front. Microbiol. 2021, 12, 611881. [Google Scholar] [CrossRef]
- Loi, M.; Logrieco, A.F.; Pusztahelyi, T.; Leiter, É.; Hornok, L.; Pócsi, I. Advanced mycotoxin control and decontamination techniques in view of an increased aflatoxin risk in Europe due to climate change. Front. Microbiol. 2023, 13, 1085891. [Google Scholar] [CrossRef]
- Abou Dib, A.; Assaf, J.C.; El Khoury, A.; El Khatib, S.; Koubaa, M.; Louka, N. Single, Subsequent, or Simultaneous Treatments to Mitigate Mycotoxins in Solid Foods and Feeds: A Critical Review. Foods 2022, 11, 3304. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dhole, A.; Krishnan, A.A.; Banerjee, K. Mycotoxin Monitoring, Regulation and Analysis in India: A Success Story. Foods 2023, 12, 705. [Google Scholar] [CrossRef]
- Imade, F.; Ankwasa, E.M.; Geng, H.; Ullah, S.; Ahmad, T.; Wang, G.; Zhang, C.; Dada, O.; Xing, F.; Zheng, Y.; et al. Updates on food and feed mycotoxin contamination and safety in Africa with special reference to Nigeria. Mycology 2021, 12, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Frank, K.; Wang, T.; Wu, F. Global wheat trade and Codex Alimentarius guidelines for deoxynivalenol: A mycotoxin common in wheat. Glob. Food Sec. 2021, 29, 100538. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; Lizarraga Pérez, E.; González-Peñas, E. Monitoring Mycotoxin Exposure in Food-Producing Animals (Cattle, Pig, Poultry, and Sheep). Toxins 2024, 16, 218. [Google Scholar] [CrossRef] [PubMed]
- Ngure, F.M.; Makule, E.; Mgongo, W.; Phillips, E.; Kassim, N.; Stoltzfus, R.; Nelson, R. Processing complementary foods to reduce mycotoxins in a medium scale Tanzanian mill: A hazard analysis critical control point (HACCP) approach. Food Control 2024, 162, 110463. [Google Scholar] [CrossRef]
- Hao, W.; Li, A.; Wang, J.; An, G.; Guan, S. Mycotoxin Contamination of Feeds and Raw Materials in China in Year 2021. Front. Vet. Sci. 2022, 9, 929904. [Google Scholar] [CrossRef]
- Hu, X.; Li, H.; Yang, J.; Wen, X.; Wang, S.; Pan, M. Nanoscale Materials Applying for the Detection of Mycotoxins in Foods. Foods 2023, 12, 3448. [Google Scholar] [CrossRef]
- Bhatia, D.; Paul, S.; Acharjee, T.; Ramachairy, S.S. Biosensors and their widespread impact on human health. Sens. Int. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Kizis, D.; Vichou, A.-E.; Natskoulis, P.I. Recent Advances in Mycotoxin Analysis and Detection of Mycotoxigenic Fungi in Grapes and Derived Products. Sustainability 2021, 13, 2537. [Google Scholar] [CrossRef]
- Papatheocharidou, C.; Samanidou, V. Two-Dimensional High-Performance Liquid Chromatography as a Powerful Tool for Bioanalysis: The Paradigm of Antibiotics. Molecules 2023, 28, 5056. [Google Scholar] [CrossRef]
- Janssen, E.M.; Mourits, M.C.M.; van der Fels-Klerx, H.J.; Lansink, A.G.J.M.O. Pre-harvest measures against Fusarium spp. infection and related mycotoxins implemented by Dutch wheat farmers. Crop Prot. 2019, 122, 9–18. [Google Scholar] [CrossRef]
- Odjo, S.; Alakonya, A.E.; Rosales-Nolasco, A.; Molina, A.L.; Muñoz, C.; Palacios-Rojas, N. Occurrence and postharvest strategies to help mitigate aflatoxins and fumonisins in maize and their co-exposure to consumers in Mexico and Central America. Food Control 2022, 138, 108968. [Google Scholar] [CrossRef]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; Obidiegwu, J.E.; Chilaka, A.C.; Atanda, O.O.; Mally, A. Mycotoxin Regulatory Status in Africa: A Decade of Weak Institutional Efforts. Toxins 2022, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Eshelli, M.; Qader, M.M.; Jambi, E.J.; Hursthouse, A.S.; Rateb, M.E. Current Status and Future Opportunities of Omics Tools in Mycotoxin Research. Toxins 2018, 10, 433. [Google Scholar] [CrossRef]
- Furlong, E.B.; Buffon, J.G.; Cerqueira, M.B.; Kupski, L. Mitigation of Mycotoxins in Food—Is It Possible? Foods 2024, 13, 1112. [Google Scholar] [CrossRef]
- Magoke, G.Z.; Alders, R.G.; Krockenberger, M.; Bryden, W.L. Aflatoxin and Mycotoxin Analysis: An Overview Including Options for Resource-limited Settings. In Aflatoxins—Occurrence, Detection and Novel Detoxification Strategies; IntechOpen: London, UK, 2022. [Google Scholar]
- Ayaz, M.; Li, C.-H.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Shafiq, M.; Ali, F.; Yu, X.-Y.; Yu, Q.; Zhao, J.-T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Lootens, O.; Vermeulen, A.; Croubels, S.; De Saeger, S.; Van Bocxlaer, J.; De Boevre, M. Possible Mechanisms of the Interplay between Drugs and Mycotoxins—Is There a Possible Impact? Toxins 2022, 14, 873. [Google Scholar] [CrossRef]
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.-J.; Caloni, F. Climate Change and Effects on Molds and Mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Zeng, X.; Liu, C.; Wu, Y.; Fu, C. Advances in Aptamer-Based Biosensors for the Detection of Foodborne Mycotoxins. Molecules 2024, 29, 3974. [Google Scholar] [CrossRef]
- Camardo Leggieri, M.; Mazzoni, M.; Battilani, P. Machine Learning for Predicting Mycotoxin Occurrence in Maize. Front. Microbiol. 2021, 12, 661132. [Google Scholar] [CrossRef]
- Makhuvele, R.; Naidu, K.; Gbashi, S.; Thipe, V.C.; Adebo, O.A.; Njobeh, P.B. The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon 2020, 6, e05291. [Google Scholar] [CrossRef]
- Stoev, S.D. Food Security and Foodborne Mycotoxicoses—What Should Be the Adequate Risk Assessment and Regulation? Microorganisms 2024, 12, 580. [Google Scholar] [CrossRef] [PubMed]
- Susha, I.; Rukanova, B.; Zuiderwijk, A.; Gil-Garcia, J.R.; Gasco Hernandez, M. Achieving voluntary data sharing in cross sector partnerships: Three partnership models. Inf. Organ. 2023, 33, 100448. [Google Scholar] [CrossRef]
- González-Rodríguez, V.E.; Izquierdo-Bueno, I.; Cantoral, J.M.; Carbú, M.; Garrido, C. Artificial Intelligence: A Promising Tool for Application in Phytopathology. Horticulturae 2024, 10, 197. [Google Scholar] [CrossRef]
- Logrieco, A.F.; Miller, J.D.; Eskola, M.; Krska, R.; Ayalew, A.; Bandyopadhyay, R.; Battilani, P.; Bhatnagar, D.; Chulze, S.; De Saeger, S.; et al. The Mycotox Charter: Increasing Awareness of, and Concerted Action for, Minimizing Mycotoxin Exposure Worldwide. Toxins 2018, 10, 149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).