Bioactivity-Guided Isolation of Secondary Metabolites from Camellia fascicularis: Antioxidative Antibacterial Activities and Anti-Inflammatory Hypoglycemic Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Chemicals and Reagents
2.3. Plant Material
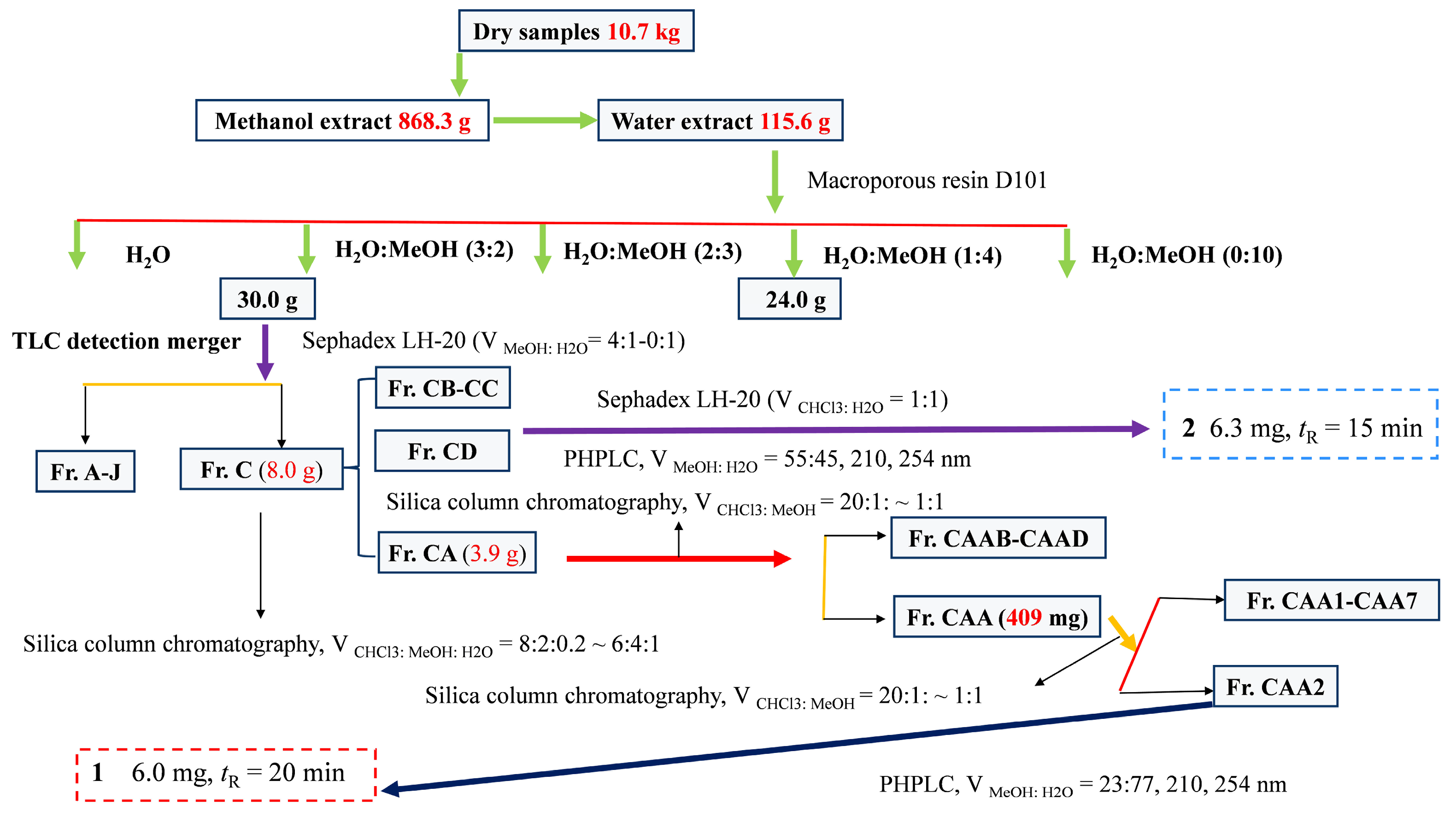
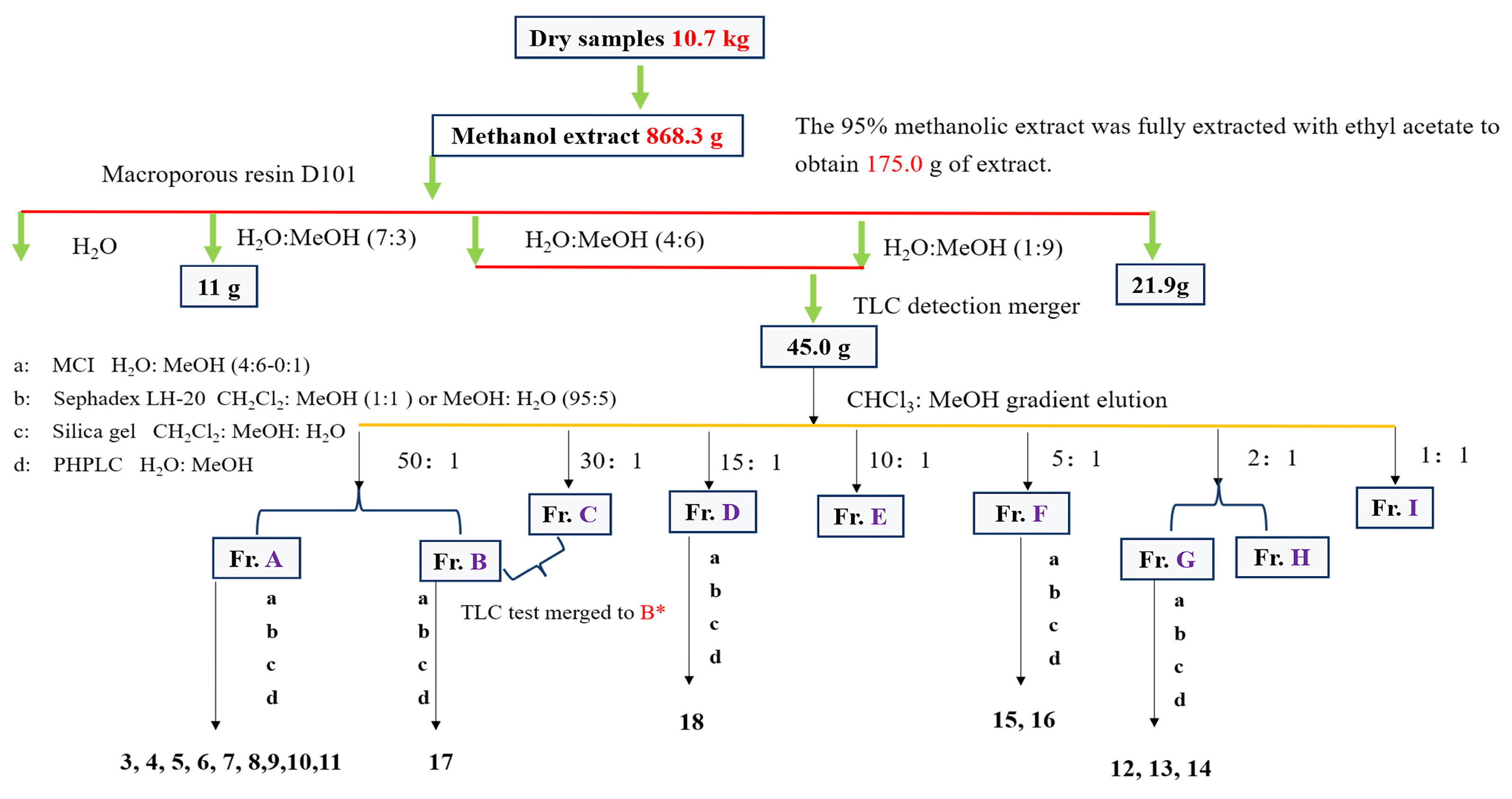
2.4. Extraction and Isolation
2.5. Bioactivity-Guided Analysis
2.5.1. Antioxidative and Antibacterial Activities
2.5.2. Anti-Inflammatory Activity
2.6. Activitive of Secondary Metabolites
2.6.1. Antioxidative Activity
2.6.2. Antimicrobial Activity
2.6.3. Molecular Docking
2.7. Statistical Analysis
3. Results and Discussion
3.1. Bioactivity-Guided Evaluations
3.1.1. Screening for Antioxidative and Antibacterial Activities
3.1.2. Screening for Anti-Inflammatory Activity
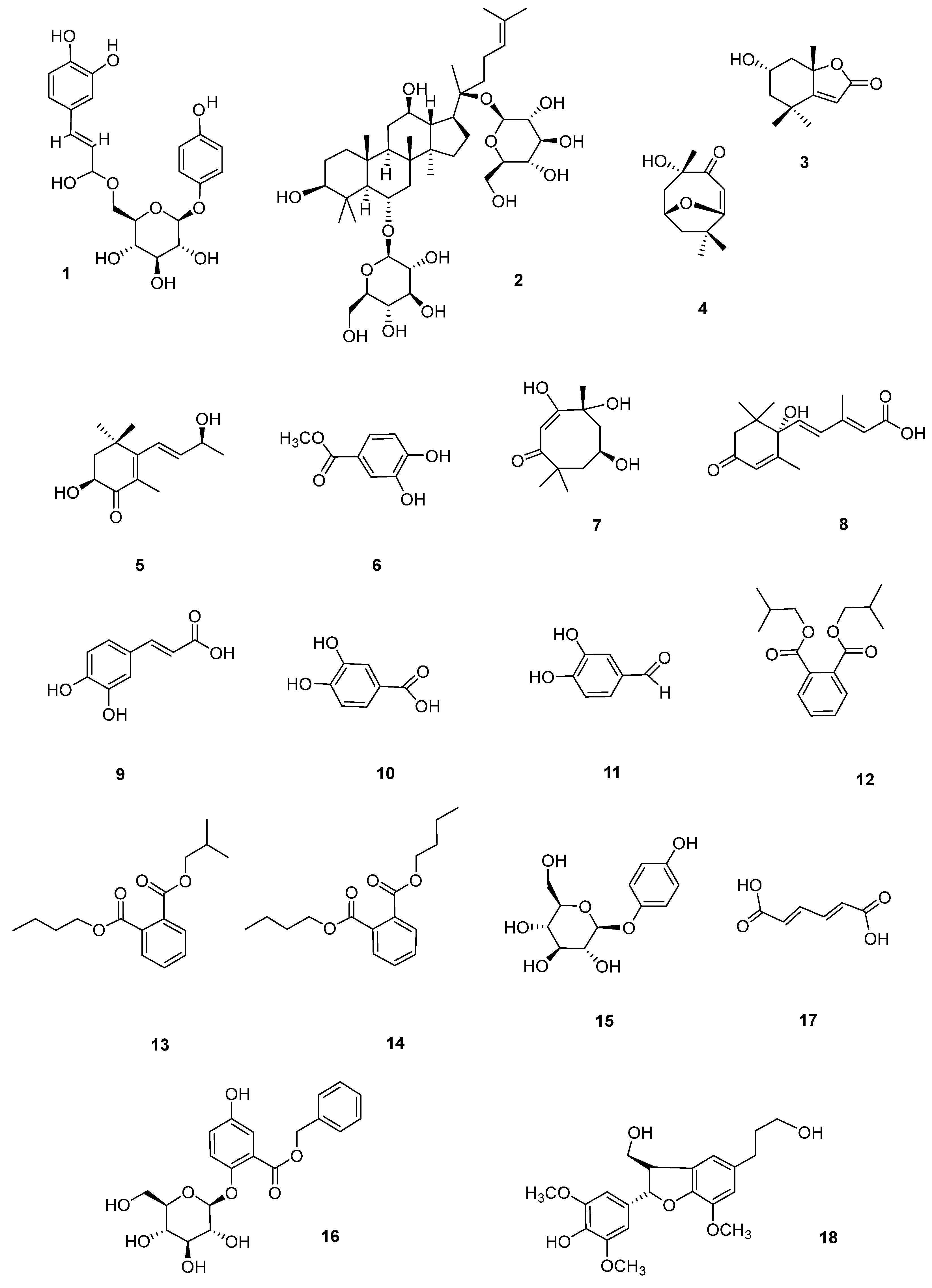
3.2. Analysis of Secondary Metabolites
3.3. Antioxidative Activity of Secondary Metabolites
3.4. Antibacterial Activity of Secondary Metabolites
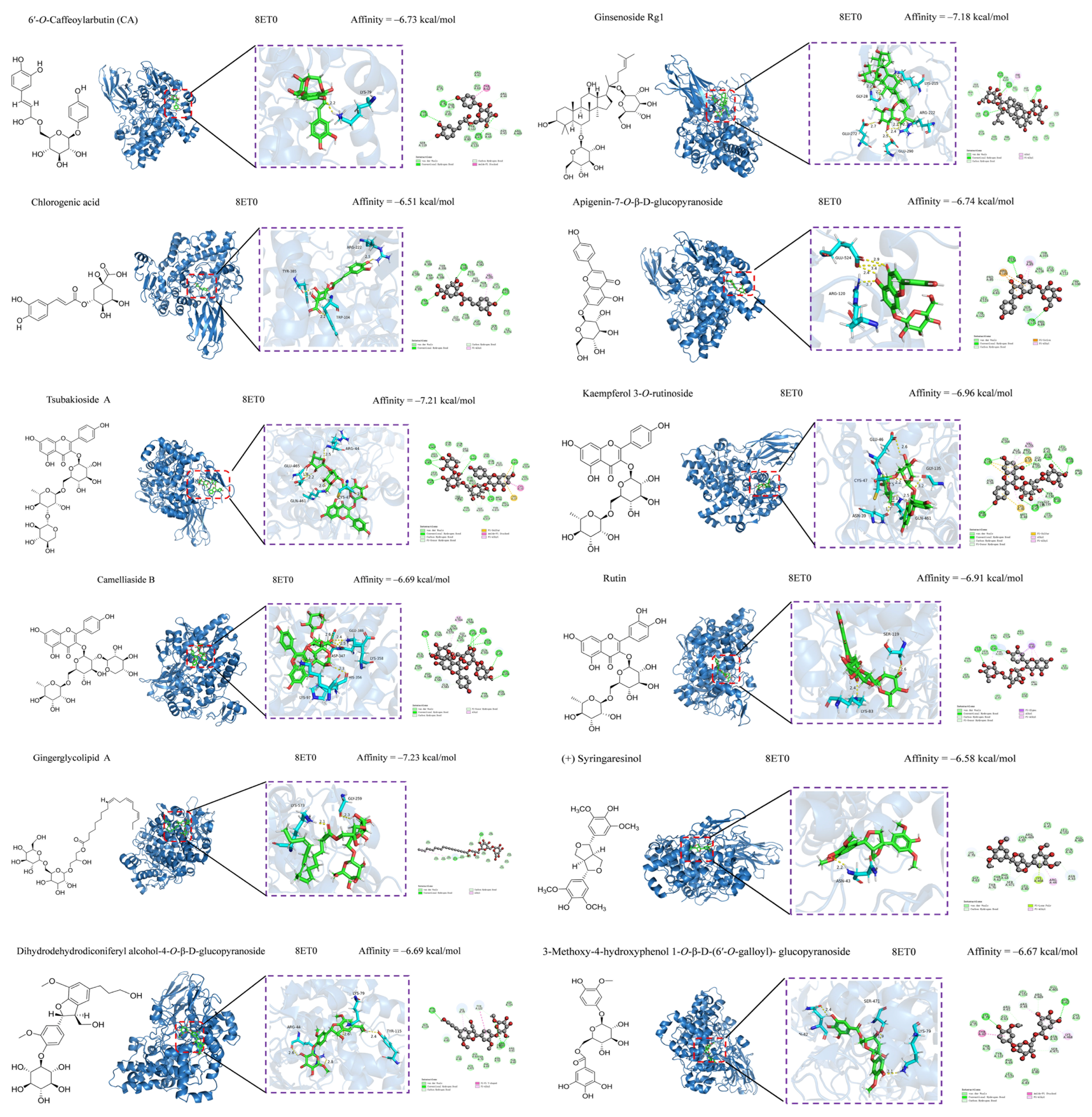
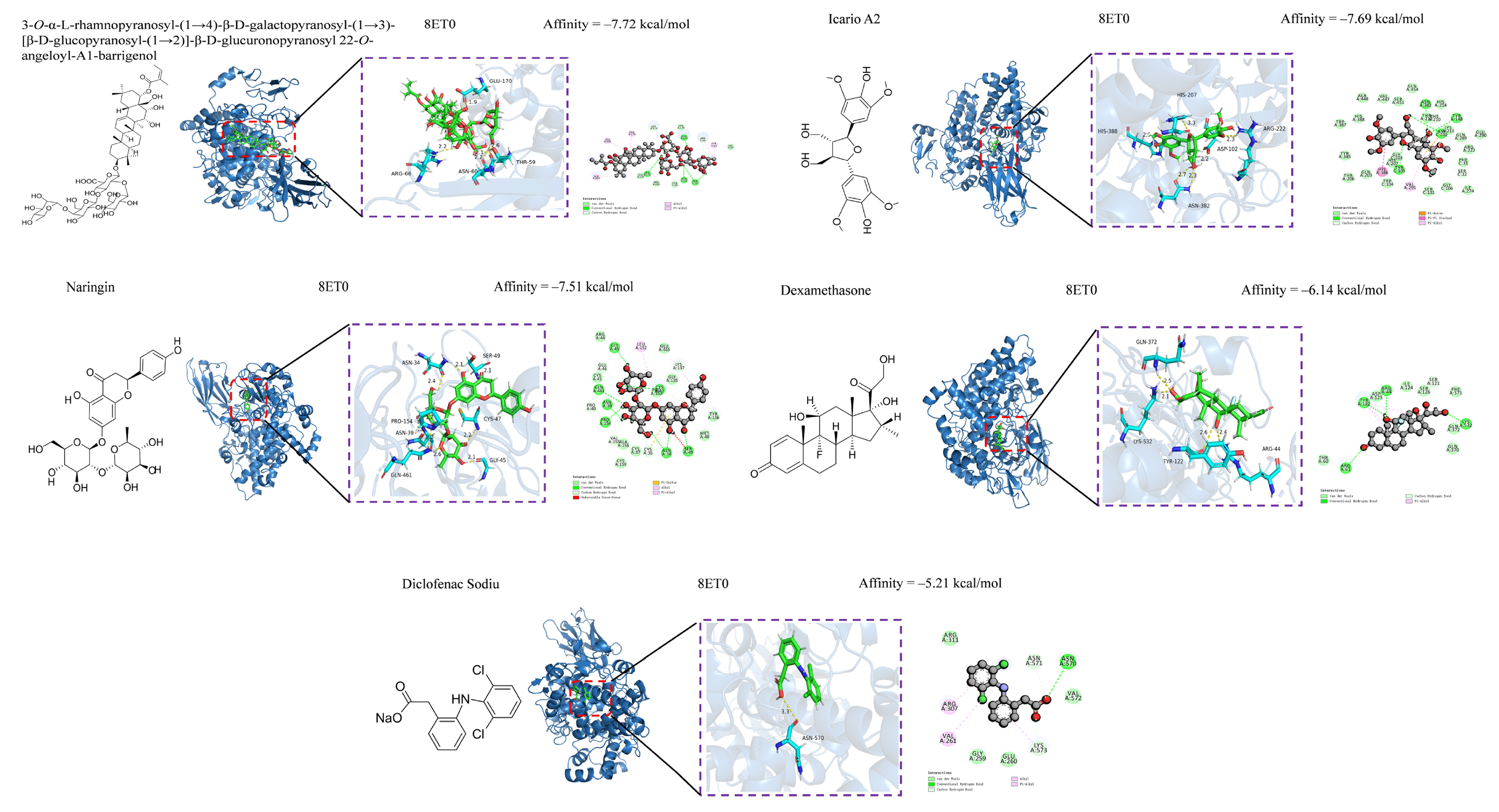
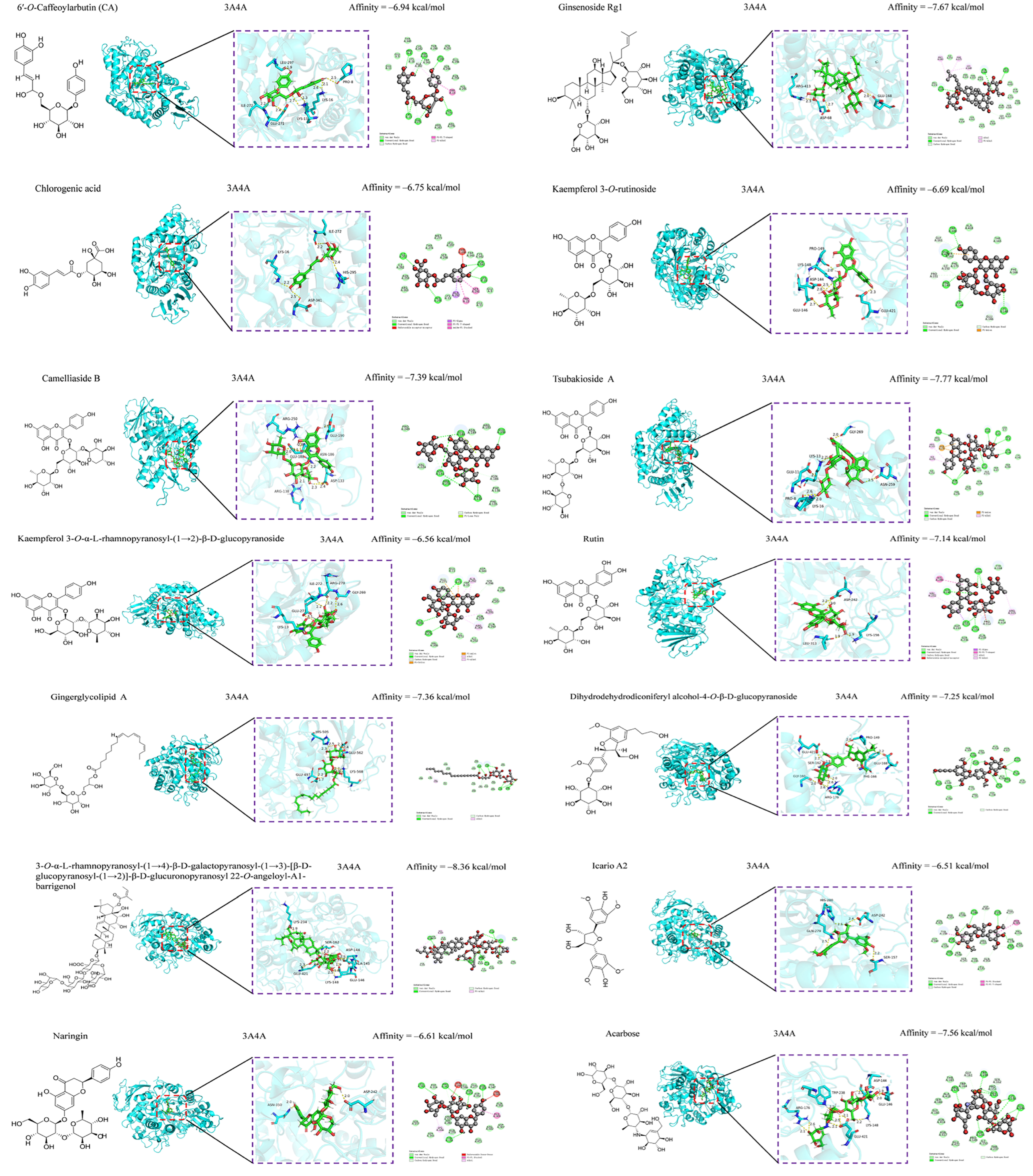
3.5. Anti-Inflammatory and Hypoglycemic Molecular Docking of Secondary Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Luo, X.L.; Lan, Z.Q.; Tang, J.R.; Zhao, P.; Kan, H. Ultrasonic-assisted extraction and antioxidant capacities of flavonoids from Camellia fascicularis leaves. CyTA-J. Food. 2018, 16, 105–112. [Google Scholar] [CrossRef]
- Hu, X.; Tang, J.R.; Zhang, G.L.; Deng, J.; Kan, H.; Zhang, Y.J.; Zhao, P.; Liu, Y. Optimization of extraction process and antioxidant activities of saponins from Camellia fascicularis leaves. J. Food Meas. Charact. 2021, 15, 1889–1898. [Google Scholar] [CrossRef]
- Liu, Y.; Kan, H.; Fan, F.Y.; Tang, J.R.; Zhang, Y.J.; Zhao, P. Microwave-assisted extraction and antioxidant activities of polyphenols from Camellia fascicularis Leaves. Curr. Top. Nutraceut. Res. 2019, 17, 164–171. [Google Scholar] [CrossRef]
- Tang, J.D.; Liu, Y.; Wang, W.H.; Wu, B.X.; Tang, J.R.; Zhang, Y.J.; Zhang, G.L.; Kan, H.; Zhao, P. Chemcical consitiuents from the leaves of Camellia fascicularis. J. Southwest For. Univ. (Nat. Sci.) 2025, 45, 1–6. [Google Scholar]
- Tang, J.D.; Li, R.L.; Wu, B.X.; Tang, J.R.; Kan, H.; Zhao, P.; Zhang, Y.J.; Wang, W.H.; Liu, Y. Secondary metabolites with antioxidant and antimicrobial activities from Camellia fascicularis. Curr. Issues Mol. Biol. 2024, 46, 404. [Google Scholar] [CrossRef]
- Li, R.L.; Tang, J.D.; Li, J.J.; Wu, B.X.; Tang, J.R.; Kan, H.; Zhao, P.; Zhang, Y.J.; Wang, W.H.; Liu, Y. Bioactivity-guided isolation of secondary metabolites with antioxidant and antimicrobial activities from Camellia fascicularis. Foods 2024, 13, 2266. [Google Scholar] [CrossRef]
- Peng, X.W.; Hu, X.; Zhang, Y.J.; Xu, H.; Tang, J.R.; Zhang, G.L.; Deng, J.; Kan, H.; Zhao, P.; Liu, Y. Extraction, characterization, antioxidant and anti-tumor activities of polysaccharides from Camellia fascicularis leaves. Int. J. Biol. Macromol. 2022, 222, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.W.; He, X.H.; Tang, J.R.; Xiang, J.Y.; Deng, J.; Kan, H.; Zhang, Y.J.; Zhang, G.L.; Zhao, P.; Liu, Y. Evaluation of the in vitro antioxidant and antitumor activity of extracts from Camellia fascicularis leaves. Front. Chem. 2022, 10, 1035949. [Google Scholar] [CrossRef]
- Shi, Z.J.; Tang, J.R.; Xiang, J.Y.; Deng, J.; Kan, H.; Shi, Z.J.; Zhao, P.; Zhang, Y.J.; Liu, Y. UHPLC Q-Exactive Orbitrap MS based metabolomics and biological activities of Camellia fascicularis from different geographical regions. Ind. Crops Prod. 2024, 213, 118432. [Google Scholar] [CrossRef]
- Gao, M.Z.; Peng, X.W.; Tang, J.R.; Deng, J.; Wang, F.; Zhang, Y.J.; Zhao, P.; Kan, H.; Liu, Y. Anti-inflammatory effects of Camellia fascicularis polyphenols via attenuation of NF-κB and MAPK pathways in LPS-induced THP-1 macrophages. J. Iinflamm. Res. 2022, 15, 851–864. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Gomes, D.C.; Lyra, F.H.; Santos, G.F.; Martins, L.R. The remarkable structural diversity achieved in ent-kaurane diterpenes by fungal biotransformation. Molecules 2014, 19, 1856–1886. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wang, S.S.; Li, Z.R.; Chen, C.; Hou, J.Q.; Xu, D.Q.; Wang, R.Z.; Lin, Y.D.; Lou, J.G.; Kong, L.Y. Bioactivity-guided cut countercurrent chromatography for isolation of lysine-specific demethylase 1 inhibitors from Scutellaria baicalensis Georgi. Anal. Chim. Acta 2018, 1016, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.W.; Li, L.G.; Huo, C.H.; Zhang, M.L.; Wan, Y.F. Study on natural medicinal chemistry and new drug development. Chin. Tradit. Herb. Drugs. 2010, 41, 1583–1589. [Google Scholar]
- He, X.H.; Shi, Z.J.; Peng, X.W.; Kan, H.; Zhao, P.; Liu, Y. Antioxidant activities analysis of different polar solvent extracts from Camellia fascicularis H. T. Chang. Chem. Ind. For. Prod. 2022, 42, 61–68. [Google Scholar]
- Ajayi, F.F.; Alnuaimi, A.; Hamdi, M.; Mostafa, H.; Wakayama, M.; Mudgil, P.; Maqsood, S. Metabolomics approach for the identification of bioactive compounds released from young and mature soybean upon in vitro gastrointestinal digestion and their effect on health-related bioactive properties. Food Chem. 2023, 420, 136050. [Google Scholar] [CrossRef]
- Weiss, D.R.; Karpiak, J.; Huang, X.P.; Sassano, M.F.; Lyu, J.; Roth, B.L.; Shoichet, B.K. Selectivity challenges in docking screens for GPCR targets and antitargets. J. Med. Chem. 2018, 61, 6830–6845. [Google Scholar] [CrossRef]
- Paggi, J.M.; Pandit, A.; Dror, R.O. The art and science of molecular docking. Annu. Rev. Biochem. 2024, 93, 389–410. [Google Scholar] [CrossRef]
- Tao, X.; Huang, Y.K.; Wang, C.; Chen, F.; Yang, L.L.; Ling, L.; Chen, Z.M.; Chen, X.G. Recent developments in molecular docking technology applied in food science: A review. Int. J. Food Sci. Technol. 2020, 55, 33–45. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Santiago-López, L.; Peres, C.M.; Peres, C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef]
- Wang, Z.B.; Pei, J.J.; Ma, H.L.; Cai, P.F.; Yan, J.K. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohyd. Polym. 2014, 109, 49–55. [Google Scholar] [CrossRef]
- Rivero-Cruz, J.F.; Granados-Pineda, J.; Pedraza-Chaverri, J.; Pérez-Rojas, J.M.; Kumar-Passari, A.; Diaz-Ruiz, G.; Rivero-Cruz, B.E. Phytochemical constituents, antioxidant, cytotoxic, and antimicrobial activities of the ethanolic extract of Mexican brown propolis. Antioxidants 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput-Aid. Drug 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Shukla, R.; Madhu, S.V.; Gambhir, J.K.; Prabhu, K.M. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin. Biochem. 2003, 36, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef]
- Zou, D.F.; Qiu, Y.T.; Xie, A.Z.; Ye, M.; Wang, F.F. GC-MS analysis of ether extraction of Camellia chrysantha. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 129–132. [Google Scholar] [CrossRef]
- Huang, Y.L.; Chen, Y.Y.; Wen, Y.X.; Li, D.P.; Liu, J.L.; Wei, X. Analysis of volatile components in Camellia nitidssima by GC-MS. Mod. Food Sci. Technol. 2009, 34, 257–260. [Google Scholar] [CrossRef]
- Zhao, P.; Tanaka, T.; Hirabayashi, K.; Zhang, Y.J.; Yang, C.R.; Kouno, I. Caffeoyl arbutin and related compounds from the buds of Vaccinium dunalianum. Phytochemistry 2008, 69, 3087–3094. [Google Scholar] [CrossRef]
- Zhang, B.B.; Hu, X.L.; Wang, Y.Y.; Li, J.Y.; Pham, T.A.; Wang, H. Neuroprotective effects of dammarane-type saponins from Panax notoginseng on glutamate-induced cell damage in PC12 cells. Planta Med. 2019, 85, 692–700. [Google Scholar] [CrossRef]
- Park, K.E.; Kim, Y.A.; Jung, H.A.; Lee, H.J.; Ahn, J.W.; Lee, B.J.; Seo, Y.W. Three norisoprenoids from the brown alga Sargassum thunbergii. J. Korean Chem. Soc. 2004, 48, 394–398. [Google Scholar] [CrossRef]
- Wan, C.P.; Chen, C.Y.; Li, M.X.; Yang, Y.X.; Chen, M.; Chen, J.Y. Chemical constituents and antifungal activity of Ficus hirta Vahl. fruits. Plants 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.G.; Xu, X.Y.; Xie, H.H.; Lin, Q.; Wei, X.Y. Chemical constituents from Isodon lophanthoides var. graciliflora. J. Trop. Subtrop. Bot. 2010, 18, 564–568. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Wei, F.; Liu, H.; Qin, R.; Yang, X.Z. Two aromatic acid derivatives and a xanthone from Hypericum hengshanense. Nat. Prod. Res. 2024, 38, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Clardy, J.; Senger, D.; Cao, S. Chakyunglupulins A and B, two novel 4, 8, 8-trimethylcyclooct-2-enone derivatives from Barleria lupulina. Tetrahedron Lett. 2015, 56, 2732–2734. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Yu, Y.; Wang, Z.Z.; Meng, Z.Q.; Fang, H.; Ding, G.; Huang, W.Z.; Xiao, W.; Yao, X.S. Research on chemical constituents from Re-Du-Ning Injection (III). Chin. Tradit. Herb. Drugs 2016, 47, 1643–1649. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, F.R.; Lin, Y.J.; Wang, L.; Chen, J.F.; Wu, Y.C.; Wu, M.J. Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.). Food Chem. 2007, 105, 48–56. [Google Scholar] [CrossRef]
- Ge, L.L.; Xie, Q.J.; Wei, X.F.; Li, Y.F.; Shen, W.Y.; Hu, Y.G.; Yao, J.; Wang, S.L.; Du, X.; Zeng, X.B. Five undescribed plant-derived bisphenols from Artemisia capillaris aerial parts: Structure elucidation, anti-hepatoma activities and plausible biogenetic pathway. Arab. J. Chem. 2023, 16, 104580. [Google Scholar] [CrossRef]
- Pouységu, L.; Sylla, T.; Garnier, T.; Rojas, L.B.; Charris, J.; Deffieux, D.; Quideau, S. Hypervalent iodine-mediated oxygenative phenol dearomatization reactions. Tetrahedron 2010, 66, 5908–5917. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Qiao, Y.B.; Zhang, Z.J.; Deng, Y.F.; Chen, T.Q.; Tao, L.; Xu, Q.X.; Liu, J.J.; Sun, W.L.; Ye, Y.; et al. New polyketides with anti-inflammatory activity from the fungus Aspergillus rugulosa. Front. Pharmacol. 2021, 12, 700573. [Google Scholar] [CrossRef]
- Pan, Z.H.; Wu, Y.F.; Ning, D.S.; Wei, Y.L. Chemical constituents from the root of Croton lachynocarpus. Guihaia 2014, 34, 148–150+25. [Google Scholar]
- Dawar, P.; Raju, M.B.; Ramakrishna, R.A. One-pot esterification and amide formation via acid-catalyzed dehydration and ritter reactions. Synth. Commun. 2014, 44, 836–846. [Google Scholar] [CrossRef]
- Xu, Z.M.; Liu, S.; Lai, H.N.; You, L.J.; Zhao, Z.G. Green-efficient enzymatic synthesis and characterization of liposoluble 6′/6″-O-lauryl phenolic glycosides with enhanced intestinal permeability. J. Agric. Food Chem. 2023, 71, 7689–7702. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xie, H.H.; Jiang, Y.M.; Wei, X.Y. Phenolics from strawberry cv. Falandi and their antioxidant and α-glucosidase inhibitory activities. Food Chem. 2016, 194, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Batanero, B.; Barba, F. Facile conversion of O-quinones into 1, 3-dioxoles. Org. Lett. 2005, 7, 2567–2569. [Google Scholar] [CrossRef]
- Wang, Y.H.; Sun, Q.Y.; Yang, F.M.; Long, C.L.; Zhao, F.W.; Tang, G.H.; Niu, H.M.; Wang, H.; Huang, Q.Q.; Xu, J.J.; et al. Neolignans and caffeoyl derivatives from Selaginella moellendorffii. Helv. Chim. Acta 2010, 93, 2467–2477. [Google Scholar] [CrossRef]
- Barbu, I.A.; Toma, V.A.; Moț, A.C.; Vlase, A.-M.; Butiuc-Keul, A.; Pârvu, M. Chemical composition and antioxidant activity of six Allium extracts using protein-based biomimetic methods. Antioxidants 2024, 13, 1182. [Google Scholar] [CrossRef]
- Xu, H.; Yu, S.L.; Lin, C.X.; Dong, D.J.; Xiao, J.B.; Ye, Y.B.; Wang, M.F. Roles of flavonoids in ischemic heart disease: Cardioprotective effects and mechanisms against myocardial ischemia and reperfusion injury. Phytomedicine 2024, 126, 155409. [Google Scholar] [CrossRef]
- Li, H.; Lin, J.Y.; Bai, B.Q.; Bo, T.; He, Y.F.; Fan, S.H.; Zhang, J.H. Study on purification, identification and antioxidant of flavonoids extracted from Perilla leaves. Molecules 2023, 28, 7273. [Google Scholar] [CrossRef]
- Xi, J.; Yan, L. Optimization of pressure-enhanced solid-liquid extraction of flavonoids from Flos Sophorae and evaluation of their antioxidant activity. Sep. Purif. Technol. 2017, 175, 170–176. [Google Scholar] [CrossRef]
- Zhang, M.; Shuai, X.X.; Wei, Z.; Dai, T.T.; Wei, C.B.; Li, Y.; He, J.J.; Du, L.Q. Characterization, antioxidant and antitumor activities of phenolic compounds from Amomum villosum Lour. Front. Nutr. 2024, 11, 1327164. [Google Scholar] [CrossRef]
- Li, K.; Fan, H.; Yin, P.P.; Yang, L.G.; Xue, Q.; Li, X.; Sun, L.W.; Liu, Y.J. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots. Arab. J. Chem. 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Li, N.; Zeng, W.L.; Luo, X.L.; Yang, C.R.; Zhang, Y.J.; Ding, Y.; Zhao, P. A new arbutin derivative from the leaves of Vaccinium dunalianum wight. Nat. Prod. Res. 2018, 32, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Lao, Q.C.; Zhao, P.; Zhu, X.Y.; Zhu, H.T.; Luo, X.L.; Yang, C.R.; He, J.H.; Li, C.Q.; Zhang, Y.J. 6′-O-Caffeoylarbutin inhibits melanogenesis in zebrafish. Nat. Prod. Res. 2014, 28, 932–934. [Google Scholar] [CrossRef]
- Tao, Y.P.; Zhang, H.W.; Wang, Y. Revealing and predicting the relationship between the molecular structure and antioxidant activity of flavonoids. LWT-Food Sci. Technol. 2023, 174, 114433. [Google Scholar] [CrossRef]
- Boo, Y.C. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef]
- Gao, M.Z.; Tang, J.R.; Deng, J.; Xiang, J.Y.; Kan, H.; Zhao, P.; Zhang, Y.J.; Zhang, G.L.; Liu, Y. Evaluation of anti-inflammatory effect of Camellia fascicularis polyphenols using zebrafish model and network pharmacology. Food Sci. 2023, 44, 134–142. [Google Scholar] [CrossRef]
- Chen, J.X.; Yang, J.; Ma, L.L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Soltan, O.I.A.; Gazwi, H.S.S.; Ragab, A.E.; Aljohani, A.S.M.; El-Ashmawy, I.M.; Batiha, G.E.S.; Hafiz, A.A.; Abdel-Hameed, S.M. Assessment of bioactive phytochemicals and utilization of Rosa canina fruit extract as a novel natural antioxidant for mayonnaise. Molecules 2023, 28, 3350. [Google Scholar] [CrossRef]
- Li, S.; Jiang, S.X.; Jia, W.T.; Guo, T.M.; Wang, F.; Li, J.; Yao, Z.L. Natural antimicrobials from plants: Recent advances and future prospects. Food Chem. 2023, 432, 137231. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Keikhaei, B.; Mottaghi, S. A review of molecular mechanisms involved in anticancer and antiangiogenic effects of natural polyphenolic compounds. Phytother. Res. 2019, 33, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure–activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Han, Q.H.; Wu, Z.L.; Huang, B.; Sun, L.Q.; Ding, C.B.; Yuan, S.; Zhang, Z.W.; Chen, Y.G.; Hu, C.; Zhou, L.J.; et al. Extraction, antioxidant and antibacterial activities of Broussonetia papyrifera fruits polysaccharides. Int. J. Biol. Macromol. 2016, 92, 116–124. [Google Scholar] [CrossRef]
- Avato, P.; Bucci, R.; Tava, A.; Vitali, C.; Rosato, A.; Bialy, Z.; Jurzysta, M. Antimicrobial activity of saponins from Medicago sp.: Structure-activity relationship. Phytother. Res. 2006, 20, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Zálešák, F.; Bon, D.J.Y.D.; Pospíšil, J. Lignans and neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef] [PubMed]
- Semchyshyn, H.M.; Lozinska, L.M. Fructose protects baker’s yeast against peroxide stress: Potential role of catalase and superoxide dismutase. FEMS Yeast Res. 2012, 12, 761–773. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, V.; Xiong, Y.; Ho, Y.S. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic. Bio. Med. 2006, 41, 1191–1196. [Google Scholar] [CrossRef]
- Le, D.; Han, S.; Ahn, J.; Yu, J.; Kim, C.K.; Lee, M. Analysis of antioxidant phytochemicals and anti-inflammatory effect from Vitex rotundifolia L.f. Antioxidants 2022, 11, 454. [Google Scholar] [CrossRef]
- Wu, T.; Zang, X.X.; He, M.Y.; Pan, S.Y.; Xu, X.Y. Structure–activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.J.; Chen, S.Y.; Lin, J.C.; Bian, J.S.; Huang, D.J. Anti-inflammation activity of flavones and their structure–activity relationship. J. Agric. Food Chem. 2021, 69, 7285–7302. [Google Scholar] [CrossRef]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef]
- Rosa, S.I.G.; Rios-Santos, F.; Balogun, S.O.; de Oliveira Martins, D.T. Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine 2016, 23, 9–17. [Google Scholar] [CrossRef]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Zoheir, K.M.; Iqbal, M.; Anwer, M.K.; Hoshani, A.R.A.; Attia, S.M.; Ahmad, S.F. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol. Res. 2015, 102, 1–11. [Google Scholar] [CrossRef]
- Saleem, A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamd, B.S.; Alharthy, S.A. Anticancer, cardio-protective and anti-inflammatory potential of natural-sources-derived phenolic acids. Molecules 2022, 27, 7286. [Google Scholar] [CrossRef]
- Xie, J.C.; Xiong, S.H.; Li, Y.M.; Xia, B.H.; Li, M.J.; Zhang, Z.M.; Shi, Z.; Peng, Q.X.; Li, C.; Lin, L.M.; et al. Phenolic acids from medicinal and edible homologous plants: A potential anti-inflammatory agent for inflammatory diseases. Front. Immunol. 2024, 15, 1345002. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Chen, J.H.; Shang, R.F.; Yang, F.; Xie, C.Q.; Liu, Y.F.; Wen, X.F.; Fu, J.P.; Xiong, W.; Wu, L. The mosquito larvicidal activity of lignans from branches of Cinnamomum camphora chvar. borneol. Molecules 2023, 28, 3769. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, K.; Chen, H.C.; He, R.J.; Cai, R.L.; Li, J.; Zhou, D.X.; Liu, W.; Huang, X.S.; Yang, R.Y.; et al. Anti-inflammatory lignans and phenylethanoid glycosides from the root of Isodon ternifolius (D. Don) Kudô. Phytochemistry 2018, 153, 36–47. [Google Scholar] [CrossRef]
- Santana Souza, M.T.; Almeida, J.R.G.D.S.; de Souza Araujo, A.A.; Duarte, M.C.; Gelain, D.P.; Moreira, J.C.F.; dos Santos, M.R.V.; Quintans-Júnior, L.J. Structure–activity relationship of terpenes with anti-inflammatory profile–A systematic review. Basic Clin. Pharmacol. 2014, 115, 244–256. [Google Scholar] [CrossRef]
- Wei, X.F.; Wang, Y.K.; Liu, R.T.; Wu, J.P.; Xu, K.P. Review of natural plant-derived seco-triterpenoids and derived saponins from 2020 to 2023: New compounds, distributions, diverse activities and structure–activity relationships. Phytochem. Rev. 2024, 1–50. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Y.N.; Huang, R.M.; Zhao, Z.G. Identification and structure–activity relationship of recovered phenolics with antioxidant and antihyperglycemic potential from sugarcane molasses vinasse. Foods 2022, 11, 3131. [Google Scholar] [CrossRef]
- Borges, P.H.; Pedreiro, S.; Baptista, S.J.; Geraldes, C.F.; Batista, M.T.; Silva, M.M.; Figueirinha, A. Inhibition of α-glucosidase by flavonoids of Cymbopogon citratus (DC) Stapf. J. Ethnopharmacol. 2021, 280, 114470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.F.; Nian, M.; Qiao, H.F.; Yang, X.H.; Wu, S.P.; Zheng, X.H. Review of bioactivity and structure-activity relationship on baicalein (5, 6, 7-trihydroxyflavone) and wogonin (5, 7-dihydroxy-8-methoxyflavone) derivatives: Structural modifications inspired from flavonoids in Scutellaria baicalensis. Eur. J. Med. Chem. 2022, 243, 114733. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, Y.Y.; Zhao, C.C.; Zhang, X.H.; Li, S.X.; Shao, J.H. Anti-diabetic potential of Viburnum betulifolium fruits: Sesquilignans with α-amylase, α-glucosidase, and PTP1B inhibitory activities. Ind. Crops Prod. 2022, 187, 115362. [Google Scholar] [CrossRef]
- Sciacca, C.; Cardullo, N.; Pulvirenti, L.; Travagliante, G.; D’Urso, A.; D’Agata, R.; Peri, E.; Cancemi, P.; Cornu, A.; Deffieux, D.; et al. Synthesis of obovatol and related neolignan analogues as α-glucosidase and α-amylase inhibitors. Bioorg. Chem. 2024, 147, 107392. [Google Scholar] [CrossRef]
- Ma, Q.G.; Wang, L.; Liu, R.H.; Yuan, J.B.; Xiao, H.; Shen, Z.Y.; Li, J.X.; Guo, J.Z.; Cao, L.; Huang, H.L.; et al. Phyllanthus emblica Linn: A comprehensive review of botany, traditional uses, phytonutrients, health benefits, quality markers, and applications. Food Chem. 2024, 446, 138891. [Google Scholar] [CrossRef]
| Anti-Inflammatory Activity (DSEC μg/mL) | ||||
|---|---|---|---|---|
| A | B* | D | E | F |
| 55.07 ± 9.75 a | 227.85 ± 31.48 e | 515.13 ± 38.24 h | 81.91 ± 10.64 b | 307.57± 38.62 f |
| G | H | I | Fr. II–III | EtOAc |
| 191.46 ± 26.54 d | 86.90 ± 13.76 bc | 341.93 ± 34.62 g | 489.26 ± 51.13 h | 529.63 ± 54.70 i |
| Compounds | ABTS B Assay (%) | |||
|---|---|---|---|---|
| 500 µg/mL | 100 µg/mL | 50 µg/mL | 10 µg/mL | |
| 1 | 98.68 ± 0.17 a | – | 90.85 ± 3.33 a | 24.22 ± 2.33 a |
| 2 | 9.11 ± 1.17 n | – | – | – |
| 3 | 61.78 ± 4.92 hi | – | – | – |
| 4 | 63.90 ± 2.37 h | – | – | – |
| 5 | 45.68 ± 2.34 k | – | – | – |
| 6 | 96.66 ± 0.56 b | 83.17 ± 4.27 d | 48.52 ± 1.27 f | 4.14 ± 0.82 e |
| 7 | 59.92 ± 2.82 hi | – | – | – |
| 8 | 52.34 ± 3.21 j | – | – | – |
| 9 | 100.28 ± 0.13 a | 99.87 ± 0.28 a | 71.04 ± 2.89 b | 18.78 ± 1.39 c |
| 10 | 99.30 ± 0.12 a | 93.67 ± 0.86 bc | 47.25 ± 2.54 f | 15.59 ± 2.23 d |
| 11 | 95.83 ± 0.32 b | 92.87 ± 1.57 bc | 57.46 ± 2.73 e | 16.15 ± 2.50 d |
| 12 | 24.20 ± 2.57 m | – | – | – |
| 13 | 46.38 ± 1.14 k | – | – | – |
| 14 | 42.63 ± 3.53 l | – | – | – |
| 15 | 89.01 ± 3.32 cd | 79.22 ± 1.97 de | 25.19 ± 2.02 g | – |
| 16 | 72.34 ± 1.69 f | 22.64 ± 3.69 j | – | – |
| 17 | 8.47 ± 1.11 n | – | – | – |
| 18 | – | – | – | – |
| 19 | 57.90 ± 1.37 i | – | – | – |
| 20 | 53.83 ± 2.69 j | 11.49 ± 1.66 m | – | – |
| 21 | 65.92 ± 3.92 g | – | – | – |
| 22 | 89.18 ± 1.97 cd | – | – | – |
| 23 | 92.12 ± 3.53 c | 15.29 ± 1.30 l | – | – |
| 24 | 86.55 ± 3.31 e | 36.25 ± 2.54 h | – | – |
| 25 | 8.81 ± 1.39 n | – | – | – |
| 26 | 99.16 ± 1.02 a | 70.73 ± 3.47 f | 21.52 ± 1.83 gh | – |
| 27 | 94.40 ± 1.18 b | 66.52 ± 2.62 g | 14.49 ± 2.16 i | – |
| 28 | 100.27 ± 0.63 a | 100.20 ± 0.11 a | 70.65 ± 1.94 b | 25.34 ± 1.57 a |
| 29 | 99.41 ± 1.69 a | – | – | 23.13 ± 1.61 ab |
| 30 | 99.52 ± 0.38 a | 93.67 ± 0.68 bc | 46.59 ± 0.72 f | 15.07 ± 2.90 d |
| 31 | 100.38 ± 0.47 a | 95.61 ± 2.13 b | 60.95 ± 2.00 d | 19.43 ± 1.84 c |
| 32 | 94.12 ± 0.99 b | 36.36 ± 1.64 h | – | – |
| 33 | 72.60 ± 2.58 f | 18.83 ± 2.45 k | – | – |
| 34 | 99.73 ± 0.59 a | 29.79 ± 2.54 i | – | – |
| 35 | 59.86 ± 2.91 hi | 18.92 ± 3.24 k | – | – |
| A AA | - | 98.35 ± 1.03 a | 65.12 ± 3.35 c | 23.00 ± 2.86 ab |
| Components | MIC b µg/mL | ||
|---|---|---|---|
| E. coli | S. aureus | P. aeruginosa | |
| 1 | 250.00 | 500.00 | 250.00 |
| 2 | 500.00 | 500.00 | 250.00 |
| 3 | >500.00 | >500.00 | 500.00 |
| 4 | 500.00 | 500.00 | 250.00 |
| 5 | 500.00 | 500.00 | 250.00 |
| 6 | 500.00 | 500.00 | 250.00 |
| 7 | 500.00 | 500.00 | 125.00 |
| 8 | 500.00 | 500.00 | 250.00 |
| 9 | 500.00 | 500.00 | 250.00 |
| 10 | 500.00 | 500.00 | 250.00 |
| 11 | 500.00 | 500.00 | 250.00 |
| 12 | 500.00 | 500.00 | – |
| 13 | 500.00 | 500.00 | – |
| 14 | 500.00 | 500.00 | – |
| 15 | 500.00 | 250.00 | 250.00 |
| 16 | 500.00 | 500.00 | – |
| 17 | >500.00 | >500.00 | 500.00 |
| 18 | 500.00 | 500.00 | 125.00 |
| 19 | >500.00 | >500.00 | 500.00 |
| 20 | 500.00 | 500.00 | 250.00 |
| 21 | 500.00 | 500.00 | 250.00 |
| 22 | 500.00 | 500.00 | 125.00 |
| 23 | 250.00 | 500.00 | 250.00 |
| 24 | 500.00 | 500.00 | 250.00 |
| 25 | >500.00 | >500.00 | 250.00 |
| 26 | 500.00 | 500.00 | 250.00 |
| 27 | 500.00 | 500.00 | 125.00 |
| 28 | 500.00 | 500.00 | 250.00 |
| 29 | 500.00 | 500.00 | 250.00 |
| 30 | 500.00 | 500.00 | 250.00 |
| 31 | 500.00 | 500.00 | 250.00 |
| 32 | 500.00 | 500.00 | 250.00 |
| 33 | 500.00 | 500.00 | 250.00 |
| 34 | 500.00 | 500.00 | 250.00 |
| 35 | 500.00 | 500.00 | 250.00 |
| a Penicillin | 62.50 | 62.50 | 250.00 |
| a Tetracycline | 15.62 | 31.25 | 125.00 |
| Compounds | 1 BE (kcal/mol) | 2 BE (kcal/mol) | Compounds | 1 BE (kcal/mol) | 2 BE (kcal/mol) |
|---|---|---|---|---|---|
| 1 | −6.73 | −6.94 | 35 | −6.28 | −6.22 |
| 2 | −7.18 | −7.67 | 36 | −7.21 | −7.77 |
| 3 | - | −4.24 | 37 | −6.96 | −6.69 |
| 4 | - | −4.37 | 38 | −6.69 | −7.39 |
| 5 | −5.13 | −4.81 | 39 | −6.33 | −6.56 |
| 6 | - | −4.24 | 40 | −6.91 | −7.14 |
| 7 | −4.03 | −4.62 | 41 | −7.23 | −7.36 |
| 8 | −4.91 | −3.07 | 42 | −5.82 | −6.05 |
| 9 | −4.35 | −4.82 | 43 | −6.58 | −6.09 |
| 10 | - | −4.82 | 44 | −5.97 | −6.18 |
| 11 | - | −5.73 | 45 | −5.83 | −5.64 |
| 12 | −5.53 | −5.89 | 46 | −6.15 | −6.39 |
| 13 | −5.51 | −5.92 | 47 | −4.59 | −4.94 |
| 14 | −5.21 | −5.75 | 48 | −4.25 | −4.33 |
| 15 | −5.17 | −5.52 | 49 | −2.85 | −3.53 |
| 16 | −5.72 | −5.43 | 50 | - | −3.89 |
| 17 | −3.13 | −3.61 | 51 | −3.15 | −4.65 |
| 18 | −6.14 | −6.39 | 52 | −4.61 | −4.47 |
| 19 | −4.11 | −4.94 | 53 | −4.85 | −4.69 |
| 20 | −4.63 | −4.88 | 54 | −5.70 | −5.78 |
| 21 | −4.05 | −4.65 | 55 | −4.84 | −4.74 |
| 22 | −4.00 | −4.70 | 56 | −5.09 | −4.99 |
| 23 | - | - | 57 | −5.35 | −5.71 |
| 24 | - | −3.97 | 58 | −5.36 | −5.99 |
| 25 | - | - | 59 | −5.86 | −5.73 |
| 26 | −6.51 | −6.75 | 60 | −5.67 | −5.67 |
| 27 | −5.19 | −4.10 | 61 | - | −5.35 |
| 28 | −4.40 | −4.47 | 62 | −3.88 | −5.12 |
| 29 | - | −4.40 | 63 | −6.66 | −7.25 |
| 30 | −5.17 | −5.21 | 64 | −6.67 | −6.53 |
| 31 | −5.54 | −6.09 | 65 | −7.72 | −8.36 |
| 32 | −5.71 | −5.46 | 66 | −7.69 | −6.51 |
| 33 | −6.74 | −6.42 | 67 | −6.51 | −6.61 |
| 34 | −6.38 | −6.26 | |||
| A (DXMS and DSEC) | −6.14 and −5.12 | ||||
| A (Acarbose) | −7.56 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Li, J.; Wu, B.; Li, R.; Tang, J.; Kan, H.; Zhao, P.; Zhang, Y.; Wang, W.; Liu, Y. Bioactivity-Guided Isolation of Secondary Metabolites from Camellia fascicularis: Antioxidative Antibacterial Activities and Anti-Inflammatory Hypoglycemic Molecular Docking. Foods 2024, 13, 3435. https://doi.org/10.3390/foods13213435
Tang J, Li J, Wu B, Li R, Tang J, Kan H, Zhao P, Zhang Y, Wang W, Liu Y. Bioactivity-Guided Isolation of Secondary Metabolites from Camellia fascicularis: Antioxidative Antibacterial Activities and Anti-Inflammatory Hypoglycemic Molecular Docking. Foods. 2024; 13(21):3435. https://doi.org/10.3390/foods13213435
Chicago/Turabian StyleTang, Jiandong, Jingjing Li, Boxiao Wu, Ruonan Li, Junrong Tang, Huan Kan, Ping Zhao, Yingjun Zhang, Weihua Wang, and Yun Liu. 2024. "Bioactivity-Guided Isolation of Secondary Metabolites from Camellia fascicularis: Antioxidative Antibacterial Activities and Anti-Inflammatory Hypoglycemic Molecular Docking" Foods 13, no. 21: 3435. https://doi.org/10.3390/foods13213435
APA StyleTang, J., Li, J., Wu, B., Li, R., Tang, J., Kan, H., Zhao, P., Zhang, Y., Wang, W., & Liu, Y. (2024). Bioactivity-Guided Isolation of Secondary Metabolites from Camellia fascicularis: Antioxidative Antibacterial Activities and Anti-Inflammatory Hypoglycemic Molecular Docking. Foods, 13(21), 3435. https://doi.org/10.3390/foods13213435









