Abstract
In recent years, the market has seen a growing demand for healthy and convenient food options, such as fruit and cereal bars, driven by shifts in eating habits. These changes are primarily attributed to time constraints in meal preparation and the need for ready-to-eat foods. Consequently, this has promoted interest in creating a nutritious, high-quality snack combining oats and mango. This study employed a response surface analysis of extreme vertex mixtures, incorporating constraints and three components: oats, mango peel, and dehydrated mango pulp. This resulted in ten different mixtures, each with unique combinations and proportions of the three components. It evaluated the microbiological quality, proximal composition, total phenolic content, tannins, Aw, color, texture, and chemical properties during storage at room temperature. The optimal blend, which demonstrated the best quality characteristics, consisted of 44.38% oats, 5.36% mango peel, and 29.24% mango pulp. This formulation yielded a protein content of 7.1 g, dietary fiber of 20.3 g per 100 g, total phenols of 3.4 mg gallic acid per g, and no pathogenic microorganisms. According to the obtained data, Aw > 0.3, the estimated shelf life could be 12 months at room temperature. Developing a stable oat-mango snack with excellent nutritional, nutraceutical, chemical quality, and microbiological properties is technologically feasible.
1. Introduction
Mango (Mangifera indica L.) is a delicious tropical fruit and a nutritional powerhouse, making it one of the most sought-after fruits in the global market. Its sensory, nutritional, and functional properties have economically secured its position as the third most important fruit after bananas and oranges [1,2]. In 2022, global production of mango, guava, and mangosteen fruits reached 59.15 million tons, with Mexico ranking fifth with 1.8 mt, following India, China, Indonesia, and Thailand [3].
The “Ataulfo” variety is native to Mexico, and its widespread commercialization worldwide is attributed to its distinctive flavor, firm texture, sweet flesh, low acidity, and intense aroma [1,4]. Due to these appealing characteristics, a portion of the production is allocated for processed foods, including juices, nectars, jams, and canned and dehydrated products [5]. However, this industrial processing generates up to 60% of waste, primarily peel, seed, and fiber, leading to economic losses, environmental challenges, and possible uses [6].
Over the past two decades, research has focused on characterizing and processing mango peels for their potential reuse in food development. This not only offers a new source of income for the mango processing industry [5,6] but also emphasizes the nutritional value of this by-product. Mango peels are rich in dietary fiber, polyphenols, proteins, and carotenoids, making them a promising source of functional ingredients [6,7,8,9].
Mango by-products have been utilized in producing various processed foods, including fermented beverages, jellies, chips, pasta, cookies, bread, and snacks [10,11,12,13]. The results demonstrate that these products are rich in phenolic compounds, exhibit antioxidant activity, possess probiotic potential, and are high in dietary fiber. Consuming products containing these compounds is associated with several health benefits. For instance, a high dietary fiber intake is crucial in addressing obesity, coronary heart disease, cancer, and diabetes problems [14,15,16]. Additionally, the bioactive compounds in these products play a significant role by neutralizing free radicals, thus preventing cellular damage [17]. This information is essential for understanding the health benefits of these products.
The competitive landscape of the food industry continually drives the search for innovative product designs [18]. In recent years, there has been a growing demand for healthy foods that are convenient and sensorially appealing. As a result, fruit and protein bars, along with a variety of other healthy snacks, are gaining popularity. This research focuses on developing and optimizing a snack made from oats, mango pulp, and mango peel flour, offering substantial benefits in terms of nutritional value, nutraceutical properties, and quality stability at room temperature. This presents a promising opportunity for the food industry.
2. Materials and Methods
2.1. Materials
In this study, ripe mangos of the Ataulfo variety, cultivated in the South Pacific region of Chiapas, México, were used. Mango fruits were washed with water and sanitized with 20 ppm sodium hypochlorite for 15 min. Once sanitized, the fruits were peeled, cut into pieces, and crushed in a T12L stainless steel industrial blender (Rbanda, Mexico) to a homogeneous paste and stored at −17 °C. The fruit peels were placed in a stainless-steel strainer and blanched in water at 90 °C for 120 s. To inactivate the enzymes responsible for deterioration reactions such as browning, they were cut and dried for 20 h at 50 °C ± 2 °C in a hot air dehydrator (Excalibur Food Dehydrator, Burleigh Heads, QLD, Australia) until reaching a constant weight. The peel was pulverized in a mill (Pulvex 200) and stored at 2 °C. Commercial oat flakes Granvita (Grupo Industrial Vida S.A. de C.V.) were used, which contained protein = 10%, fat = 10%, total carbohydrates = 70%, and dietary fiber = 13.3% on dry matter.
2.2. Snack Design, Preparation, and Characterization
All mango-oat combinations described in Table 1 were added to 21% of the complement (15% sucrose, 5.3% butter, 0.5% salt, and 0.2% baking powder) to achieve 100%. All components were mixed to make a homogeneous mass, which was molded into an 8 cm long and 7 mm thick indented cylindrical shape. Each sample was left to rest for 15 min at room temperature and baked at 180 °C for 6 min, then allowed to rest for 8 min at room temperature (to allow the water in the product to migrate from the inside to the outside to reach equilibrium), followed by a second cooking stage of 10 min at 180 °C. The samples were removed from the oven, cooled to room temperature, and meticulously packaged in Ziploc® plastic bags for further analysis, ensuring the product’s quality.

Table 1.
Experimental runs, primary components (proportions), and experimental region of the extreme vertices design.
2.3. Proximal Analysis
Moisture, ash, protein (N × 5.83), fat, and carbohydrates (by difference) contents were determined according to AOAC methods [19]. Total dietary fiber was evaluated by the Megazyme total dietary fiber assay procedure, which is based on the AOAC 991.43 method (AOAC, 1998).
2.4. Hardness
The hardness of the samples was determined using a TA-XT2i texturometer (AMETEX, Cassatt Road Berwyn, FL, USA) measured at 2 points on the surface of the product with a double blade attachment at a speed of 20 mm/s and a contact force of 1 Newton until total breakage of the sample structure [20]. The results were reported in Newton (N).
2.5. Color Analysis
The color attributes of the samples were obtained in the CIELCh color space using a spectrophotometer (CM-700d; Konica Minolta, Ramsey, NJ, USA). The luminosity (L), hue angle (Hue = arc tan b/a), and chromaticity (Chroma = √ [a2 + b2]) were obtained with OnColor QC version 5 (CyberChrome, Stone Ridge, NY, USA) [21,22].
2.6. Chemical Analysis
The samples’ pH, acidity, and total soluble solids (TSS) were carried out according to [19]. To 10 g of sample, 50 mL of distilled H2O pH 7.0 was added, liquefied, and filtered. A total of 50 mL of filtered sample was taken and placed in a plastic cup. The pH and percentage of acidity were determined with a Mettler Toledo T50 automatic titrator using the LabX program, where the dilution factor and the milliequivalent of citric acid were indicated. One milliliter of the sample was placed in the Metter Toledo S470 refractometer cavity for total soluble solids. The result was multiplied by the dilution factor and reported in degrees Brix (°Brix).
2.7. Antioxidant Capacity
Extract preparation for antioxidant capacity involved 2 g of dry sample being added to 10 mL methanol, shaken at 200 rpm for 2 h, and centrifuged at 27,670× g for 20 min at 4 °C [23], using a centrifuge mod Z36 HK (Hermle Labortechnik, Wehigen, Germany).
The Folin Ciocalteu method was used to quantify the total phenolic compounds. A total of 10 µL of the extract, 230 µL of distilled water, and 10 µL of the 2 N Folin Ciocalteu reagent were placed in a clear flat-bottom 96-well transparent plate, incubated for 3 min, 25 µL of Na2CO3 4 N was added, and incubated for 2 h in the dark. The absorbance was measured at 725 nm using a Synergy HT Microplate Reader (BioTek, Instruments, Inc., Winooski, VT, USA). The results were expressed as mg of gallic acid equivalents (GAE) per gram [23].
The antioxidant capacity was determined by the DPPH (2,2-diphenyl-1-picrylhydrazyl) method. A 20 µL sample extract was prepared and added to the microplate. A 20 µL sample extract, using Trolox (0, 0.05, 0.1, 0.2, 0.25, 0.3, 0.35, 0.4, 0.5, 0.6, 0.8, and 1.0 mg∙mL−1); and 200 µL of DPPH (2,2-definil-1-picrylhydrazyl radical) reagent were added and incubated at 20 °C for 30 min, under dark conditions to determine the calibration curve. Absorbance was measured at 540 nm using a Synergy microplate reader HT (BioTek, Winooski, VT, USA). The results were expressed as mg Trolox equivalents/100 g sample dry weight [24].
2.8. Total Tannins
Total tannins were obtained as the difference between the content of phenolic compounds and the non-tannin phenolics compounds. First, it was necessary to determine the content of phenolic compounds using the Folin Ciocalteu assay, and then the content of non-tannin phenolics was subtracted from that value. For this, tannin precipitation was performed with PVPP. A total of 100 mg of polyvinyl polypyrrolidone (PVPP) and 1 mL of extract were added to Eppendorf tubes, shaken in a Vortex Maximix LP (Thermo Scientific, Waltham, MA, USA), and incubated for 15 min at 4 °C. Then, they were shaken rapidly and centrifuged for 10 min at 4427× g. The supernatant was recovered, and the content of phenolic compounds (non-tannin compounds) was determined using the Folin Ciocalteu assay. The results were expressed as mg catechin equivalents (CE)/g [25,26]. Total tannins = (the content of phenolic compounds minus the non-tannin phenolics compounds).
2.9. Water Activity
A 2 g mango bar was placed in a cell in the equipment cavity of Aqua LabCX-2 (Cromer, Australia) dew point hygrometer at 25 °C, previously calibrated with a NaCl standard of 0.75 water activity (Aw). Finally, Aw was measured and recorded in the sample.
2.10. Microbiological Analysis
Escherichia coli (E. coli), fecal coliforms, and total coliforms were determined following the Mexican Official Standard NOM-210-SSA1-2014 [27], appendix H, to Salmonella spp. appendix A and appendix B, the procedure for food. A sample of 25 g was placed in a sterile bag, 225 mL of phosphate butter was added, and the sample was homogenized manually for 2 min (stock sample). Coliforms from this homogenate were analyzed with decimal dilutions made using 9 mL of phosphate buffer. Biochemical tests were performed to evaluate E. coli, and the results were reported as the most probable number/mL (MPN/g) for Salmonella expressed as presence-absence in 25 g of sample. The fungi and yeasts were analyzed according to the Mexican Official Standard NOM-111-SSA1-1994 [28] for products intended for human consumption by plate count.
2.11. Data Analysis
A mixture design of degree 2, with extreme vertices, was implemented with three components (oats, mango peel flour, and mango pulp) with the following constraints: oats 30–55%, mango peel flour 0–30%, and mango pulp 25–65%. Oats and mango (peeled flour and pulp) constituted a balanced 79% of the total mixture, and 21% was the complement in the formulation (15% sucrose, 5.3% butter, 0.5% salt, and 0.2% baking powder). The mixture design consisted of 10 mixtures [29] and each mixture was replicated thrice (Table 1). Statistical significance of coefficients for the terms in the models were taken as p < 0.05.
3. Results and Discussion
3.1. Nutritional Characterization of Snack Ingredients
Table 2 shows the nutritional and nutraceutical content of the components used to prepare the oat-mango snack. The moisture content of the mango pulp, at 79%, served as the primary water source in the mixture, facilitating the kneading process with the other ingredients. The nutritional and nutraceutical characteristics of the snack were meticulously estimated, considering the protein contribution from oats (10%), dietary fiber (21.5%), and primarily tannins from mango peel. These estimations provide a solid basis for confidence in the snack’s health benefits. The raw materials’ analyzed content was consistent with values reported by various authors for oat and mango fruits and peels [5,7,8].

Table 2.
Characteristics of primary components for oat-mango snacks.
3.2. Optimization Process
Table 3 presents the protein, dietary fiber, and bioactive compound contents across the ten treatments derived from the experimental design, which were influenced by the proportions of oats, mango peel, and pulp. Higher ratios of oats and mango peel flour in the combinations enhanced the protein and dietary fiber content. Conversely, including mango pulp and mango peel flour improved the total phenol values, along with the incorporation of tannins. It was reported that mango peel is rich in total phenols, which help eliminate free radicals and function as natural antioxidants [8]. These findings offer a significative promise for future food development, opening opportunities for the creation of healthier and nutritious snack.

Table 3.
Means ± Standard deviation of the response variables of 10 mixtures of oats-mango snacks.
The dietary fiber functionality of oat-mango snacks, determined by the content and ratio of soluble and insoluble fractions [30], significantly influences glucose levels. The soluble fiber in these snacks helps stabilize glucose levels, providing health benefits. Furthermore, soluble fiber serves as a prebiotic, nourishing beneficial gut bacteria, which promotes a balanced microbiota and can enhance overall consumer health [16,31]. Furthermore, consuming foods that contain tannins could be therapeutic; they prevent cancer and the formation of carcinogens. Similarly, tannins enhance body’s natural detoxification defenses [25].
3.3. Regression Models
The ANOVA for the response variable is shown in Table 4. Protein and dietary fiber were adjusted by second-order models, while total phenols and total tannins were adjusted by linear models. The significant regression coefficients (p < 0.05) for each response are shown in Table 5. The corresponding polynomial models and their goodness of fit (R2) are given in Table 6.

Table 4.
Analysis of variance for protein, dietary fiber, total phenols, and total tannins.

Table 5.
Estimated regression coefficients.

Table 6.
Regression models and coefficient of determination.
3.4. Optimization
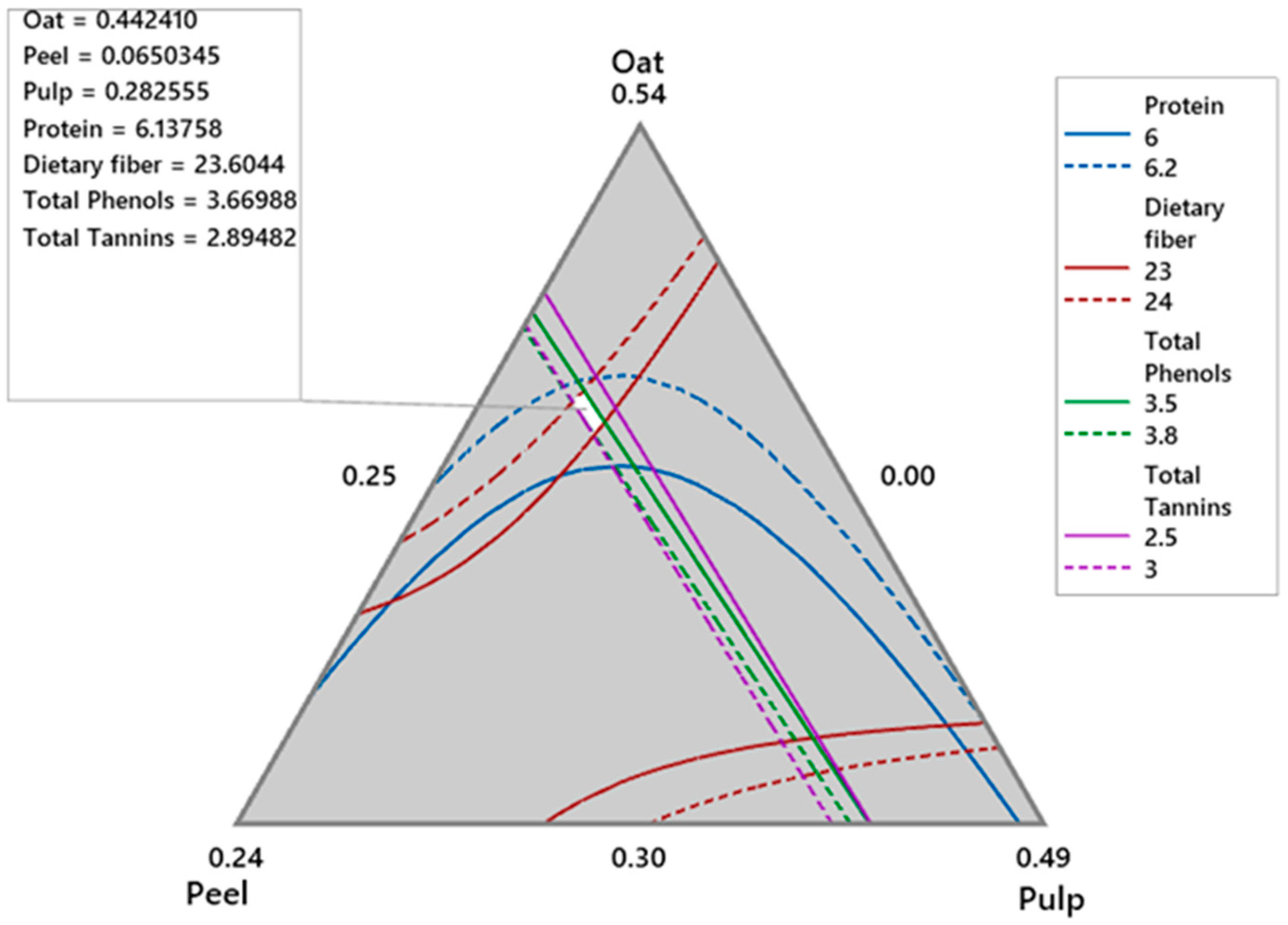
Using Minitab 19, an overlap of the contour plots of all response models over the restricted experimental region showed a small area where all responses intersect (Figure 1). A feasible solution in this region was oat = 0.441 (44.1%), mango peel flour = 0.0648 (6.5%), and mango pulp = 0.284 (28.4%). The response values at this point were protein (%) = 6.12, dietary fiber = 23.45 (%), total phenols (mg GAE∙g−1 snack) = 3.67, and total tannins mg Catechin∙g−1 snack = 2.89. This result was corroborated by the optimization procedure based on the optimal desirability functions for the four desired variables of 0.805.

Figure 1.
Overlaid contours of response variables of the oat-mango snack.
3.5. Physicochemical Properties of the Optimized Mango-Oat Snack
The snack made with the feasible oat-mango blend demonstrated important protein, dietary fiber, total phenols, and tannin contributions (Table 7), which were consistent with the results predicted by the model (Figure 1), indicating excellent reproducibility of the experiment. Overall, the optimized snack contained 3.8% moisture (Table 8), which falls within the acceptable range for baked goods according to NOM-247-SSA1-2008 [32] (moisture < 8%). The protein content is primarily derived from oats, followed by mango peel. With a high dietary fiber content of 20.3% (with a ratio of 3:1 insoluble to soluble fiber), this snack has three times the fiber found in other snacks enhanced with mango peel [4,33,34], potentially offering significant health benefits.

Table 7.
Results for predicted and experimental values in the feasible mixture of dehydrated mango and walnuts for conditions of 60 °C and 3 min.

Table 8.
Quality characteristics of the optimized oat-mango snack expressed on dry matter.
A serving of baked cereal products with fruit is 40 g by regulations (Grain-based bars with or without filling or coating, e.g., breakfast bars, granola bars, rice cereal bars, piece or g [35] from USA) and NOM-086-SSA1-1994 [36] from México. In this context, a serving of the oat-mango snack (40 g) provides 8.1 g of dietary fiber, which accounts for 29% of the recommended daily intake (DI), where 20% DI or more of a nutrient per serving is considered high from USA [35], qualifying it as “high in dietary fiber”. The insoluble dietary fiber in the snack primarily comes from the mango peel, while the soluble dietary fiber is derived mainly from the oat content [37]. The nutritional properties and quality of fiber are influenced by the composition and ratio of soluble and insoluble fractions; oats contain over 50% soluble fiber [38], whereas mango peel and pulp are richer in insoluble fiber [39]. However, a 40 g serving of the oat-mango snack contains 6.5 g of sugars, representing 26% of the daily value recommended by the World Health Organization (maximum 25 g/days). This suggests that sensory studies should be conducted to reduce the 15% added sugar content and assess its impact on acceptability.
Fiber-rich by-products such as oatmeal and mango can be incorporated into food products as substitutes for flour, fat, or sugar, enhancing water and oil retention and improving emulsion and oxidative stability [38]. Dietary fiber plays a crucial role in the body, it helps reduce glucose absorption in the bloodstream, leading to better insulin control and maintaining a healthy intestinal ecosystem, among other benefits [16]. The consumption of foods with soluble dietary fiber (SDF) to a total dietary fiber (TDF) ratio of 0.5 is associated with these positive characteristics [40]. In the optimized oat-mango snack, this ratio was 0.22, indicating a higher contribution of insoluble dietary fiber (Table 8).
In recent years, the food industry has increasingly focused on developing products that contain bioactive ingredients, utilizing by-products from fruit processing and marketing them as functional foods. These foods offer health benefits beyond basic nutrition, often due to the inclusion of bioactive compounds. Our study found that the oat-mango snack has a phenolic content of 3.4 mg of GAE per gram (Table 7), comparable to the 5.3 mg of GAE per gram reported by [4] for bread made with mango peel flour.
Mango peel, a significant contributor to our oat and mango snacks, is rich in antioxidant phenolics and tannin compounds. Tannin incorporation was meticulously considered in our mixture combinations, resulting in a snack with beneficial properties that boasted 2.2 mg of catechin per gram (Table 7). Although specific values for tannin consumption are unavailable, it is generally recommended not to exceed 2.5 g per day [41].
The antioxidant capacity of the optimal oat and mango snack was 41.4 µmol ET∙g−1 (Table 8), this value is in a range of results found in various foods [33,34,42]. It is important to note that the antioxidant capacity of a product is influenced not only by the content of phenolic compounds but also by the presence and levels of other bioactive compounds [40].
The optimized oat-mango snack offers benefits with its excellent physical, chemical, and physicochemical qualities (Table 8). Products with water activity (Aw) values less than 0.3, such as our snack, exhibit superior stability due to the low availability of water, which inhibits microbial growth and reduces biochemical changes during storage and distribution [43]. The low Aw value in the oat-mango snack was achieved using mango peel, oats, and dehydrated ingredients, with only the moisture from the mango pulp being added. Additionally, the snack achieved 22°Brix and 60.6 N, values comparable to those reported for cookies made with 20% mango peel flour [44].
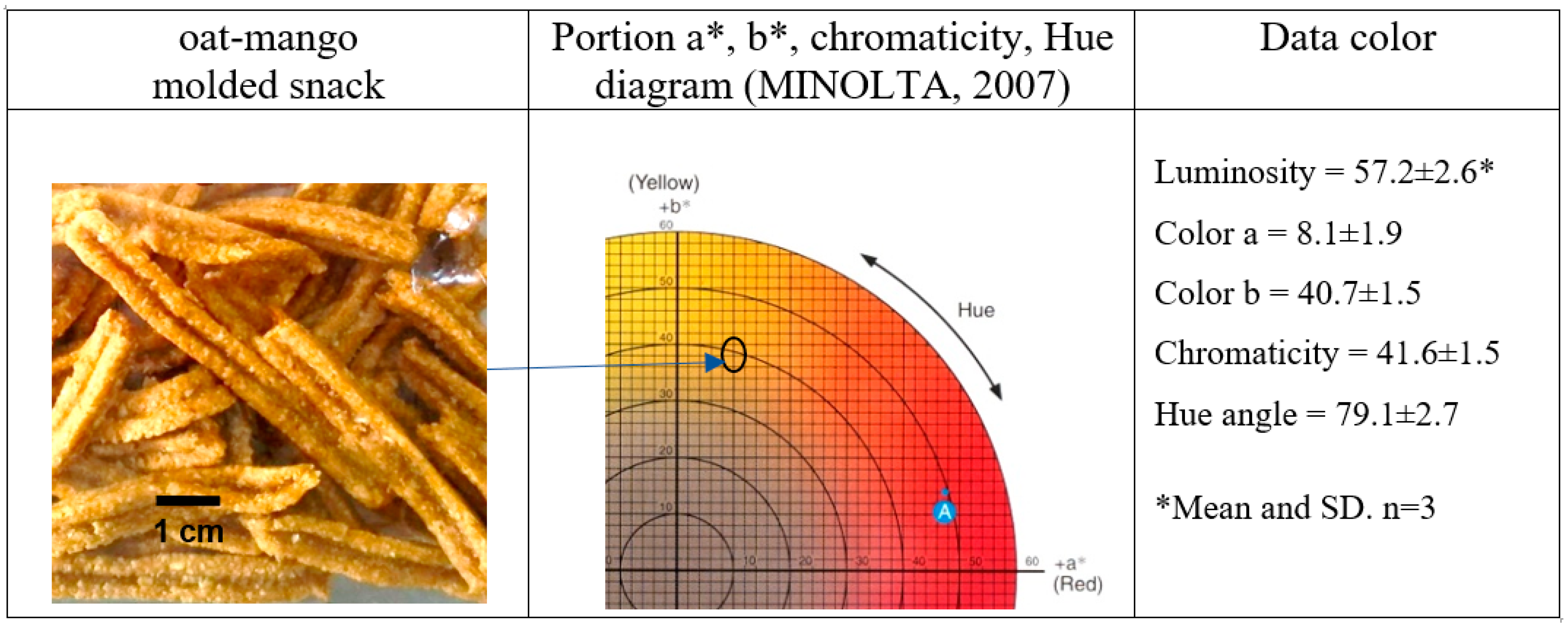
The color of food plays a crucial role in its marketing and consumption. An appealing color can enhance a product’s acceptability, often independent of its flavor and nutritional content [45]. The oat-mango snacks exhibit orange hues (Figure 2), characteristic of both the pulp and peel of the mango [46,47]. These snacks display luminosity values of 57.2, chromaticity of 41.6, and a hue angle of 79.1, indicating that the optimized snack appears dark orange due to the heat treatment during baking at 180 °C. In contrast, Ataulfo mango pulp, fresh ripe, has luminosity values of 60.6, chromaticity of 63.4, and a hue angle of 62.9 [44]. Ref. [47] reported a luminosity of 51.5 and a hue angle of 55.7 for Ataulfo mango pulp powder. The color values for the oat-mango snack align closely with those of the Ataulfo mango, falling within similar orange ranges. The combination of the ingredients and the baking process results in lower color saturation and a slight product darkening, as reflected in the reduced luminosity values.

Figure 2.
Picture and the color variables in the oat-mango snack.
3.6. Microbiological
The feasible oat-mango snack demonstrated acceptable microbiological quality, attributed to the high quality of the ingredients and the baking process conducted at 180 °C. No presence of Escherichia coli, total coliforms, fecal coliforms, fungi, or yeasts was detected. Additionally, Salmonella spp. was absent in 25 g of the sample, and only 50 colony-forming units of aerobic mesophiles were found per gram, significantly below the limit of 30,000 CFU·g−1 established for cookies according to NMX-F-006-1983 [48]. Furthermore, the aerobic mesophile counts are acceptable for cereal-based foods, edible seeds, flours, semolina, mixtures, and bakery products NOM-247-SSA1-2008 [32].
4. Conclusions
From a technological standpoint, the oat and mango snacks offer acceptable nutritional and nutraceutical benefits and low water activity (Aw) values that enhance stability. The optimal formulation identified through the optimization process for the specified study region consists of 44.1% oats, 6.5% mango peel, and 28.5% mango pulp. The mixture yields a snack rich in protein, high in dietary fiber, and contains significant phenolic compounds; however, adjustment of the added sugars is necessary. Ultimately, it successfully elaborates a snack that is microbiologically safe, nutritious, easy to handle, visually appealing, and capable of maintaining its shape during distribution. The stability of this product ensures a longer shelf life, making it an attractive option for food product developers, including food design and nutrition innovation. Moreover, this approach opens new avenues for utilizing mango peel as a valuable ingredient in fortified foods for human consumption. However, it is crucial to continue research on suitable packaging and shelf life for the mango oat snack to ensure it retains its nutritional and nutraceutical quality as much as possible.
Author Contributions
Conceptualization, M.D.M.-R. and J.B.H.; methodology, Y.A.A.-R., J.B.V.-T., J.P.C.-S. and J.H.-G.; software, Y.A.A.-R. and J.B.V.-T.; validation, Y.A.A.-R., J.B.V.-T., J.P.C.-S. and M.D.M.-R.; formal analysis, Y.A.A.-R., J.P.C.-S., J.B.H. and J.H.-G.; investigation, Y.A.A.-R.; resources, Y.A.A.-R. and M.D.M.-R.; data curation, Y.A.A.-R., J.B.V.-T. and J.P.C.-S.; writing—original draft preparation, Y.A.A.-R., J.P.C.-S., J.B.H. and M.D.M.-R.; writing—review and editing, J.B.V.-T., J.B.H. and M.D.M.-R.; visualization, M.D.M.-R. and J.B.V.-T.; supervision, M.D.M.-R.; project administration, M.D.M.-R.; funding acquisition, M.D.M.-R. and J.B.V.-T. All authors have read and agreed to the published version of the manuscript.
Funding
Consejo Nacional de Humanidades, Ciencia y Tecnologías (CONAHCYT) for the financial support (FORDECYT # 292474).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors thank the Centro de Investigación en Alimentación y Desarrollo, A. C. (CIAD, A. C.) Culiacán, Sinaloa, México for its facilities. and to the for conducting this research, as well as the support of the Laura Aracely Contreras Angulo, and Verónica Pérez Rubio, researchers at the CIAD-Culiacán, Sinaloa, Mexico.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yahia, E.; Ornelas-Paz, J.; Brecht, J.; García-Solís, P.; Maldonado, M. The contribution of mango fruit (Mangifera indica L.) to human nutrition and health. Arab. J. Chem. 2023, 16, 104860. [Google Scholar] [CrossRef]
- Afifa, A.; Farooq, U.; Akram, K.; Hayat, Z.; Shafi, A.; Sarfraz, F.; Sidhu, M.; Rehman, H.; Aftab, S. Therapeutic potentials of bioactive compounds from mango fruit wastes. Trends Food Sci. Technol. 2016, 53, 102–112. [Google Scholar] [CrossRef]
- FAO Statistics Division. Major Tropical Fruits Market Review-Preliminary Results 2022. Food and Agriculture Organization of the United Nations Rome. 2023. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/852265a4-9006-4d54-a792-51f1d9c44673/content (accessed on 18 April 2024).
- Ramírez-Maganda, J.; Blancas-Benítez, F.J.; Zamora-Gasga, V.M.; García-Magaña, M.; Bello-Pérez, L.A.; Tovar, J.; Sáyago-Ayerdi, S.G. Nutritional properties and phenolic content of a bakery product substituted with a mango (Mangifera indica) “Ataulfo” processing by-product. Food Res. Int. 2015, 73, 117–123. [Google Scholar] [CrossRef]
- Marçal, S.; Pintado, M. Mango peels as food ingredient/additive: Nutritional value, processing, safety and applications. Trends Food Sci. Technol. 2021, 114, 472–489. [Google Scholar] [CrossRef]
- García-Mahecha, M.; Soto-Valdez, H.; Carvajal-Millan, E.; Madera-Santana, T.; Lomelí-Ramírez, M.; Colín-Chávez, C. Bioactive compounds in extracts from the agro-industrial waste of mango. Molecules 2023, 28, 458. [Google Scholar] [CrossRef]
- Pacheco-Jiménez, A.A.; Basilio Heredia, J.; Gutiérrez-Grijalva, E.P.; Quintana-Obregón, E.A.; Muy-Rangel, M.D. Potencial industrial de la cáscara de mango (Mangifera indica L.) para la obtención de pectina en México. TIP Rev. Espec. Cienc. Químico-Biológicas 2022, 25, 1–13. [Google Scholar] [CrossRef]
- Quintana-Obregón, E.; San-Martín-Hernández, C.; Muy-Rangel, M.; Vargas-Ortiz, M. Valoración de polvos de cáscara de mango (Mangifera indica L.) como una alternativa para la generación de alimentos funcionales. TIP Rev. Espec. Cienc. Químico-Biológicas 2019, 22, 1–5. [Google Scholar] [CrossRef]
- Brecht, J.K.; Sidhu, J.S. Handbook of Mango Fruit: Production, Postharvest Science, Processing Technology and Nutrition; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Kumar, A.G.; Singh, P.G.; Beer, K.; Pongener, P.; Ravi, C.S.; Singh, S.; Verma, A.; Singh, A.; Thakur, M.; Tripathy, S.; et al. A review on valorization of different byproducts of mango (Mangifera indica L.) for functional food and human health. Food Biosci. 2022, 48, 101783. [Google Scholar] [CrossRef]
- Mayo-Mayo, G.; Navarrete-García, A.; Maldonado-Astudillo, Y.I.; Jiménez-Hernández, J.; Santiago-Ramos, D.; Arámbula-Villa, G.; Álvarez-Fitz, P.; Ramirez, M.; Salazar, R. Addition of roselle and mango peel powder in tortilla chips: A strategy for increasing their functionality. J. Food Meas. Charact. 2020, 14, 1511–1519. [Google Scholar] [CrossRef]
- Azura, N.; Radhiah, S.; Zunairah, W.; Shazini, N.; Hanani, N.; Ismail-Fitry, M.R. Physicochemical, cooking quality and sensory characterization of yellow alkaline noodle: Impact of mango peel powder level. Food Res. 2020, 4, 70–76. [Google Scholar] [CrossRef]
- Vicenssuto, G.M.; de Castro, R.J. Development of a novel probiotic milk product with enhanced antioxidant properties using mango peel as a fermentation substrate. Biocatal. Agric. Biotechnol. 2020, 24, 101564. [Google Scholar] [CrossRef]
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 63, 8752–8767. [Google Scholar] [CrossRef] [PubMed]
- Niño-Medina, G.; Muy-Rangel, D.; De La Garza, A.L.; Rubio-Carrasco, W.; Pérez-Meza, B.; Araujo-Chapa, A.P.; Gutiérrez-Álvarez, K.A.; Urías-Orona, V. Dietary fiber from chickpea (Cicer arietinum) and soybean (Glycine max) husk byproducts as baking additives: Functional and nutritional properties. Molecules 2019, 24, 991. [Google Scholar] [CrossRef] [PubMed]
- Sáyago-Ayerdi, S.G.; Velázquez-López, C.; Montalvo-González, E.; Goñi, I. By-product from decoction process of Hibiscus sabdariffa L. calyces as a source of polyphenols and dietary fiber. J. Sci. Food Agric. 2014, 94, 898–904. [Google Scholar] [CrossRef]
- Geiner, S.T.; Ari, A.; Dohi, H.; Dikdik, K. A Comprehensive review of free radicals, oxidative stress, and antioxidants: Overview, clinical applications, global perspectives, future directions, and mechanisms of antioxidant activity of flavonoid compounds. J. Chem. 2024, 1–21. [Google Scholar] [CrossRef]
- Parasecoli, F. Food design, nutrition, and innovation. Front. Public Health 2022, 10, 1039795. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Análisis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995; Volume 1. [Google Scholar]
- Márquez-Villacorta, L.F.; Pretell Vásquez, C.C. Evaluación de características de calidad en barras de cereales con alto contenido de fibra y proteína. Biotecnol. Sect. Agropecu. Agroind. 2018, 16, 69–78. [Google Scholar] [CrossRef]
- Konica Minolta. Precise Color Communication: Color Control from Perception to Instrumentation; Konica Minolta Sensing Inc.: Osaka, Japan, 2007. [Google Scholar]
- Báez-Sañudo, M.A.; Muy-Rangel, M.D.; Heredia, J.B.; Adriana, J. Reducing postharvest softening of papaya (Carica papaya cv. Maradol) by using an aqueous 1-methylcyclopropene application. Int. J. Environ. Agric. Res. 2017, 3, 144–151. [Google Scholar]
- Swain, T.; Hillis, W. The phenolics constituents of Prunus domestica I.—The quantitative analysis of phenolics constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.P. In vitro methods of assay of antioxidants: An overview. Food Rev. Int. 2008, 24, 392–415. [Google Scholar] [CrossRef]
- Barman, K. Biodegradation of Tanniniferous Feeds and Their Influence on Nutrient Utilization and Productivity of the Dairy Animals. Ph.D. Thesis, NDRI, Karnal, India, 2004. [Google Scholar]
- Heil, M.; Baumann, B.; Andary, C.; Linsenmair, E.K.; McKey, D. Extraction and quantification of “condensed tannins” as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften 2002, 89, 519–524. [Google Scholar] [CrossRef] [PubMed]
- NOM-210-SSA1-2014-DOF: 26-06-2015; Productos y Servicios, Métodos de Prueba Microbiológicos, Determinación de Microorganismos Indicadores, Determinación de Microorganismos Patógenos. Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 2015. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5398468&fecha=26/06/2015#gsc.tab=0 (accessed on 13 May 2024).
- NOM-111-SSA1-1994-DOF: 13-09-1995; Bienes y Servicios. Método para la Cuenta de Mohos y Levaduras en Alimentos. Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 1995. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4881226&fecha=13/09/1995#gsc.tab=0 (accessed on 15 May 2024).
- Minitab. Minitab Statistical Software. Simple Correspondence Analysis, Version 20.4. 2021. Available online: www.minitab.com (accessed on 23 August 2024).
- García-Gabarra, A. Ingesta de nutrientes: Conceptos y recomendaciones internacionales (1st part). Nutr. Hosp. 2006, 21, 291–299. [Google Scholar] [PubMed]
- Castañeda-Guillo, C. Actualización en prebióticos. Rev. Cuba. Pediatr. 2018, 90, e648. [Google Scholar]
- NOM-247-SSA1-2008-DOF: 27-07-2009; Productos y Servicios, Cereales y Sus Productos, Cereales, Harinas de Cereales, Sémolas o Semolinas, Alimentos a Base de: Cereales, Semillas Comestibles, de Harinas, Sémolas o Semolinas o Sus Mezclas, Productos de Panificación—Disposiciones y Especificaciones Sanitarias y Nutrimentales. Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 2009. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5100356&fecha=27/07/2009#gsc.tab=0 (accessed on 7 June 2024).
- Chen, Y.; Zhao, L.; He, T.; Ou, Z.; Hu, Z.; Wang, K. Effects of mango peel powder on starch digestion and quality characteristics of bread. Int. J. Biol. Macromol. 2019, 140, 647–652. [Google Scholar] [CrossRef]
- Sairam, S.; Krishna, G.; Urooj, A. Physico-chemical characteristics of defatted rice bran and its utilization in a bakery product. J. Food Sci. Technol. 2011, 48, 478–483. [Google Scholar] [CrossRef]
- FDA. U.S. Food and Drug Administration. Health Daily Value and Percent Daily Value on the Nutrition and Supplement Facts Labels. 2023. And Food Labeling: Serving Sizes of Foods that Can Reasonably Be Consumed at One Eating Occasion. 2018. Available online: https://www.fda.gov/Foodguidances (accessed on 11 October 2024).
- NOM-086-SSA1-1994-DOF: 26-06-1996; Bienes y Servicios. Alimentos y Bebidas no Alcohólicas con Modificaciones en Su Composición—Especificaciones Nutrimentales. Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 1996. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4890075&fecha=26/06/1996#gsc.tab=0 (accessed on 10 June 2024).
- Pathak, D.; Majumdar, J.; Raychaudhuri, U.; Chakraborty, R. Characterization of physicochemical properties in whole wheat bread after incorporation of ripe mango peel. J. Food Meas. Charact. 2016, 10, 554–561. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Pacheco-Jiménez, A.; Lizardi-Mendoza, J.; Heredia, J.B.; Gutiérrez-Grijalva, E.; Quintana-Obregón, E.; Muy-Rangel, M.D. Physicochemical characterization of pectin and mango peel (Mangifera indica L.) from Mexican cultivars. Heliyon 2024, 10, e35184. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; Avena-Bustillos, R.J.; Montalvo-González, E.; Sáyago-Ayerdi, S.G.; McHugh, H.T. Addition of dried ‘Ataulfo’ mango (Mangifera indica L.) by-products as a source of dietary fiber and polyphenols in starch molded mango snacks. J. Food Sci. Technol. 2015, 52, 7393–7400. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2019, 40, 432–444. [Google Scholar] [CrossRef]
- Hernández-Sánchez, C.; Moreno, I.; Cameán, A.; González, A.G.; González-Weller, D.; Castilla, A.; Gutiérrez, A.; Rubio, C.; Hardisson, A. Differentiation of mangoes (Magnifera indica L.) conventional and organically cultivated according to their mineral content by using support vector machines. Talanta 2012, 97, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Rockland, L.B.; Nishi, S.K. Influence of water activity on food product quality and stability. Food Technol. 1980, 34, 42–46. [Google Scholar]
- Ajila, C.M.; Naidu, K.A.; Bhat, S.G.; Rao, U.J. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Krokida, M.K.; Maroulis, Z.B. Kinetics on color changes during drying of some fruits and vegetables. Dry. Technol. 1998, 16, 667–685. [Google Scholar] [CrossRef]
- Jiménez-Maldonado, M.I.; Tovar-Pedraza, J.M.; León Félix, J.; Muy Rangel, M.D.; Islas-Osuna, M.A. Respuesta fisiológica y calidad de mango cv. Ataulfo infectado por Colletotrichum spp. Rev. Mex. Cienc. Agríc. 2022, 13, 1103–1115. [Google Scholar] [CrossRef]
- San-Martín-Hernández, C.; Pérez-Rubio, V.; Muy-Rangel, M.D.; Vargas-Ortiz, M.A.; Quintana-Obregón, E.A. Caracterización del polvo y pectina del pericarpio del mango “Ataulfo” maduro y análisis FODA para su procesamiento. TIP Rev. Espec. Cienc. Químico-Biológicas 2020, 23, 1–10. [Google Scholar] [CrossRef]
- NMX-F-006-1983; Alimentos-Galletas-Food-Cookie-Normas Mexicanas. Dirección General de Normas, Secretaría de Comercio y Fomento Industrial. Available online: https://sitios1.dif.gob.mx/alimentacion/docs/NMX-F-006-1983_GALLETAS.pdf (accessed on 3 June 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).