Comparative Study on the Fermentation Characteristics of Selective Lactic Acid Bacteria in Shanxi Aged Vinegar: Pure Culture Versus Co-Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Samples
2.1.2. Chemical Reagents
2.2. Methods
2.2.1. Enumeration and Cultivation Techniques of Lactic Acid Bacteria
2.2.2. Biomass Measurement Methods
2.2.3. Physical and Chemical Index Determination Method
- (1)
- Determination of total acid, reducing sugar, total esters, and acetoin
- (2)
- Organic acid
- (3)
- Determination of volatile aroma compounds
2.2.4. Sample Preparation, DNA Fragment Amplification, and Miseq Library Establishment
2.2.5. Interaction of Different Combinations of Lactic Acid Bacteria
2.3. Data Analysis
3. Results and Discussion
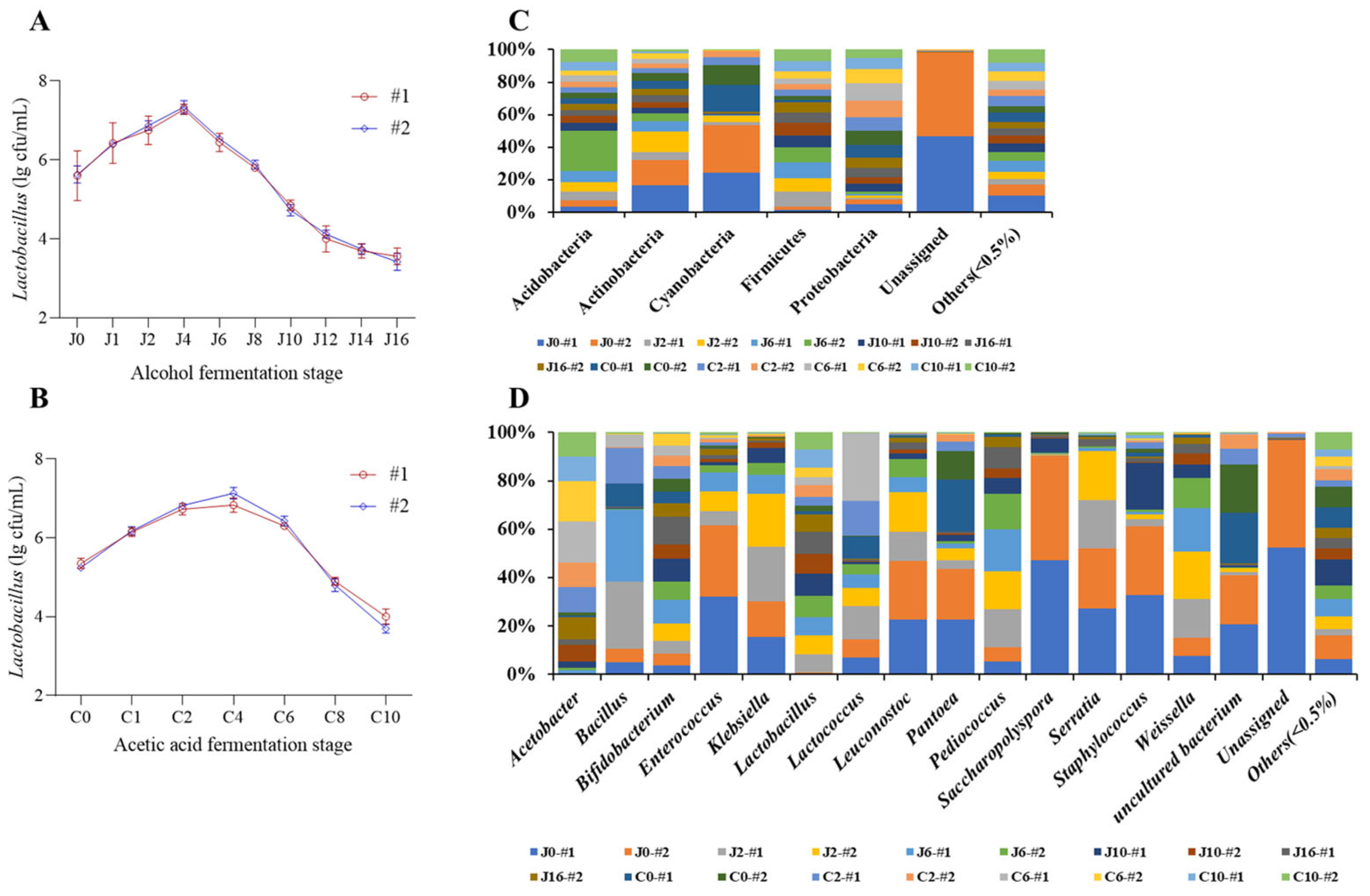
3.1. Dynamic Changes in Lactic Acid Bacteria Biomass During Alcohol and Acetic Acid Fermentation Stages
3.2. Dynamic Changes in Bacterial Flora Structure During the Traditional Fermentation Process of Shanxi Aged Vinegar
3.3. Fermentation Characteristics of Selected Lactic Acid Bacteria L7, L19, L729, and L2422 from Shanxi Aged Vinegar in Different Culture Media
3.3.1. Morphological Characteristics of Lactic Acid Bacteria L7, L19, L729, and L2422 in Different Media
3.3.2. Study of the Growth Characteristics of Lactobacillus Strains L7, L19, L729, and L2422 in Different Media and the Characteristics of Total Acid Production, Reducing Sugar, and Acetoin Production
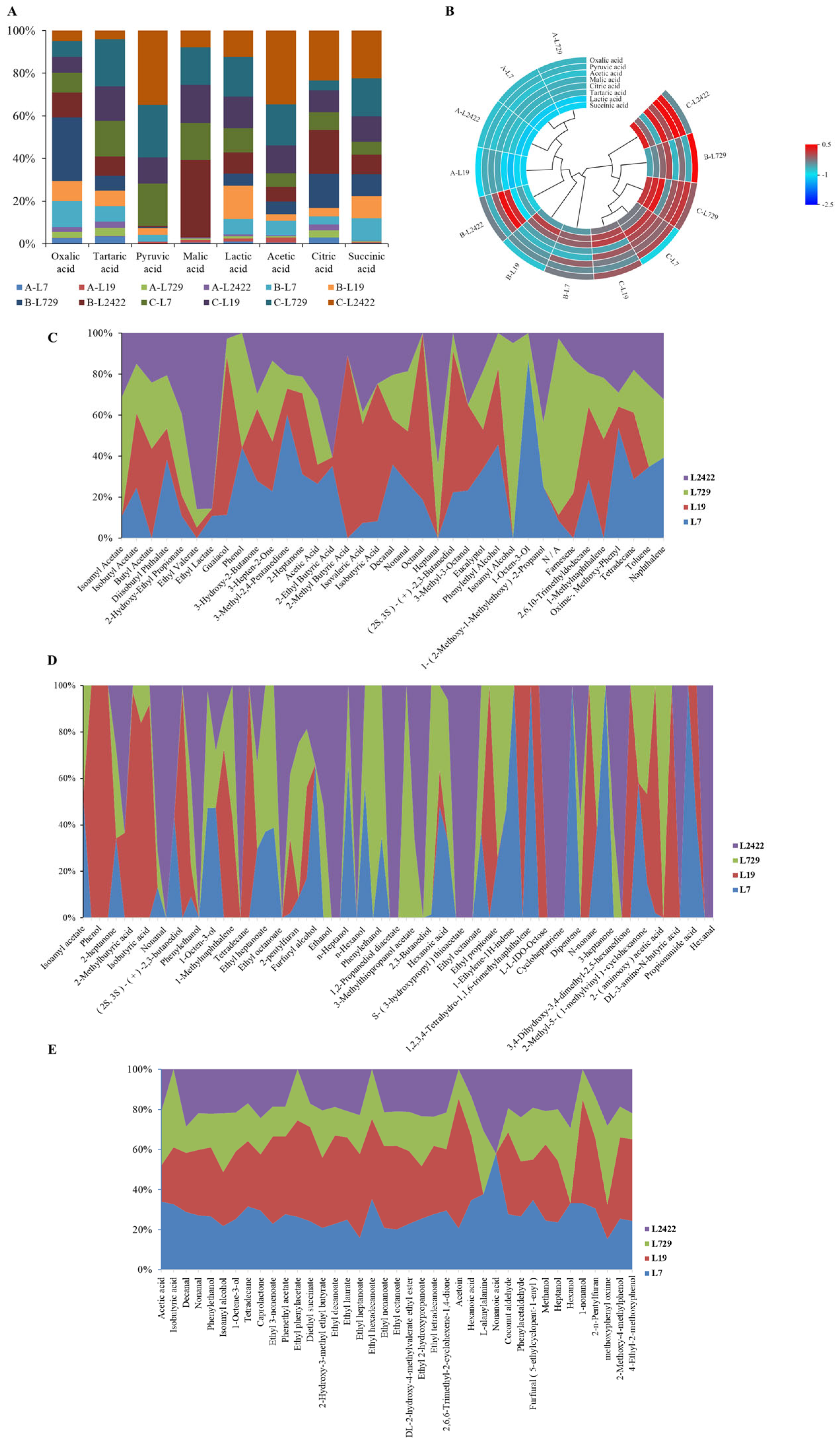
3.3.3. Study of the Characteristics of Organic Acids and Volatile Aroma Substances Produced by Lactic Acid Bacteria Strains L7, L19, L729, and L2422 in Different Media
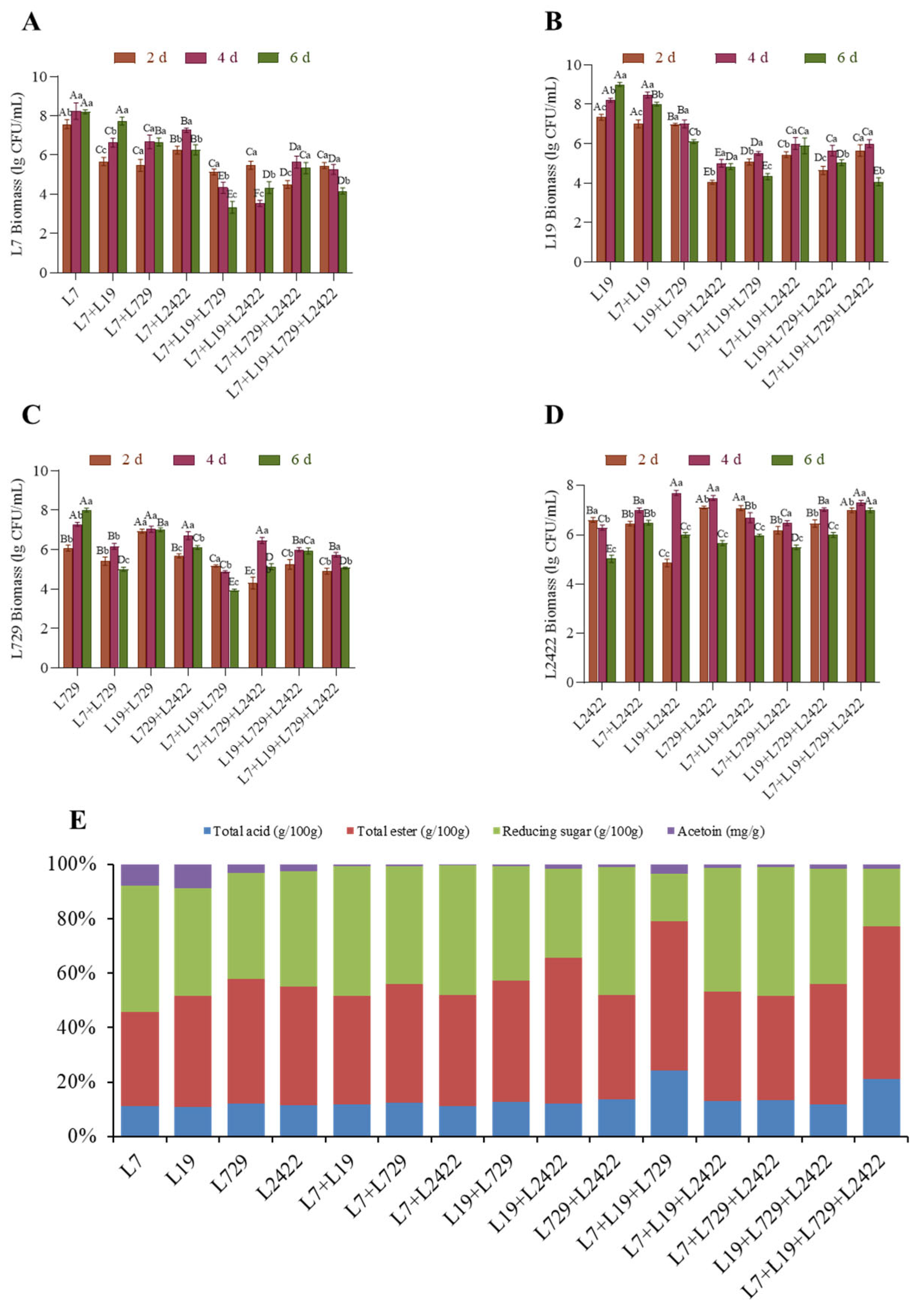
3.3.4. Determination of Optimal Lactic Acid Bacteria Combination and Determination and Analysis of Biomass and Physicochemical Indicate in Pure Culture and Co-Culture Systems
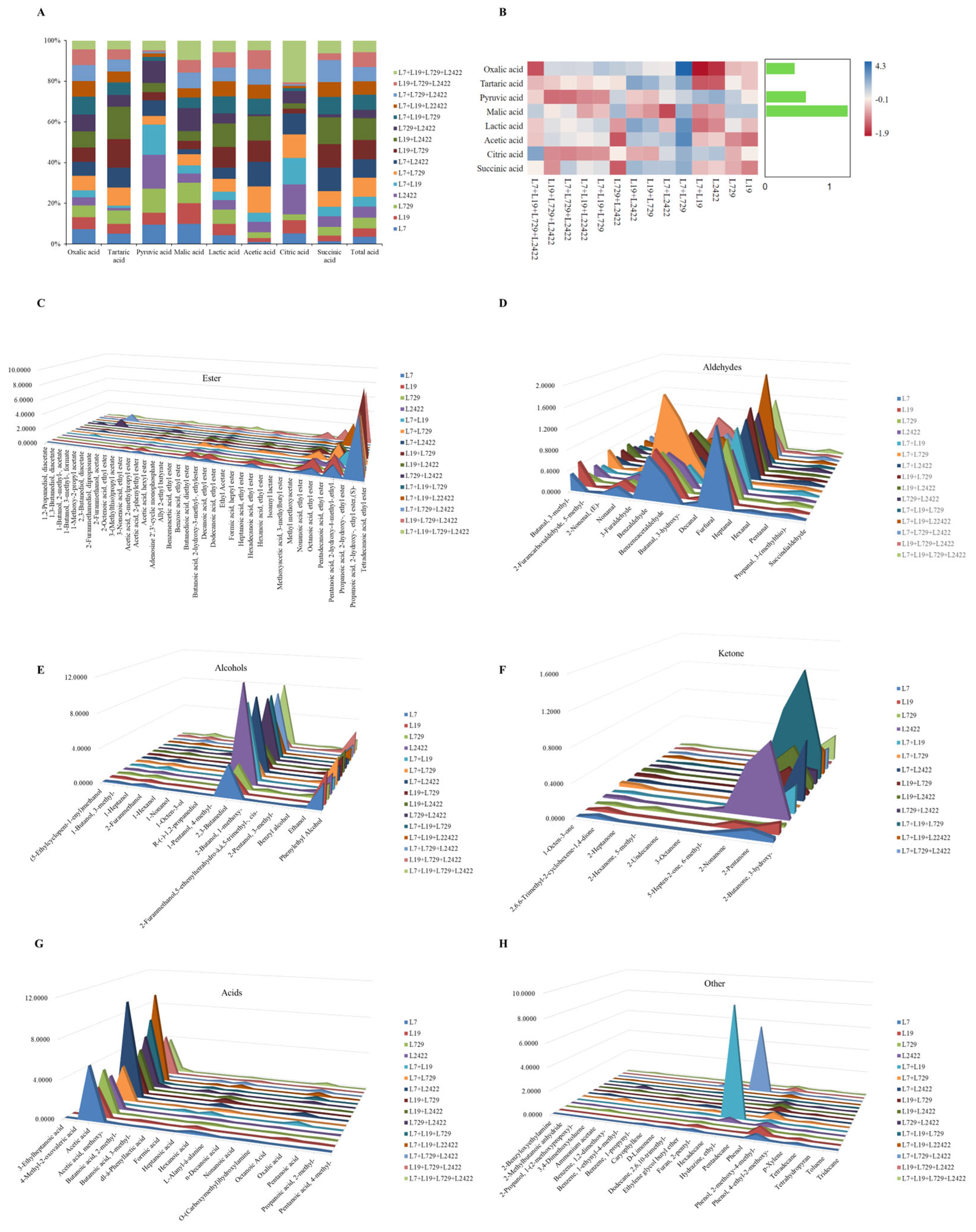
3.3.5. Determination and Analysis of Organic Acid Content and Volatile Aroma Components in Pure Culture and Co-Culture Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Li, P.; Liu, X.; Luo, L.; Lin, W. Bacterial Dynamics and Metabolite Changes in Solid-State Acetic Acid Fermentation of Shanxi Aged Vinegar. Appl. Microbiol. Biotechnol. 2016, 100, 4395–4411. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Ma, Y.K.; Zhang, F.F.; Chen, F.S. Biodiversity of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria in the Fermentation of “Shanxi Aged Vinegar”, a Traditional Chinese Vinegar. Food Microbiol. 2012, 30, 289–297. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Zhang, C.; Yang, L.; Fan, G.; Xu, Y.; Sun, B.; Li, X. Dynamic Microbial Succession of Shanxi Aged Vinegar and Its Correlation with Flavor Metabolites during Different Stages of Acetic Acid Fermentation. Sci. Rep. 2018, 8, 8612. [Google Scholar] [CrossRef]
- Sankuan, X.; Cuimei, Z.; Bingqian, F.; Yu, Z.; Menglei, X.; Linna, T.; Jia, S.; Xinyi, Z.; Min, W. Metabolic Network of Ammonium in Cereal Vinegar Solid-State Fermentation and Its Response to Acid Stress. Food Microbiol. 2021, 95, 103684. [Google Scholar] [CrossRef]
- Li, P.; Lin, W.; Liu, X.; Wang, X.; Gan, X.; Luo, L.; Lin, W.-T. Effect of Bioaugmented Inoculation on Microbiota Dynamics during Solid-State Fermentation of Daqu Starter Using Autochthonous of Bacillus, Pediococcus, Wickerhamomyces and Saccharomycopsis. Food Microbiol. 2017, 61, 83–92. [Google Scholar] [CrossRef]
- Li, P.; Aflakpui, F.W.K.; Yu, H.; Luo, L.; Lin, W.T. Characterization of Activity and Microbial Diversity of Typical Types of Daqu for Traditional Chinese Vinegar. Ann. Microbiol. 2015, 65, 2019–2027. [Google Scholar] [CrossRef]
- Chen, D.; Ye, Y.; Chen, J.; Yan, X. Evolution of Metabolomics Profile of Crab Paste during Fermentation. Food Chem. 2016, 192, 886–892. [Google Scholar] [CrossRef]
- Wang, Z.M.; Lu, Z.M.; Shi, J.S.; Xu, Z.-H. Exploring Flavour-Producing Core Microbiota in Multispecies Solid-State Fermentation of Traditional Chinese Vinegar. Sci. Rep. 2016, 6, 26818. [Google Scholar] [CrossRef]
- Leal Maske, B.; Murawski de Mello, A.F.; da Silva Vale, A.; Prado Martin, J.G.; de Oliveira Soares, D.L.; De Dea Lindner, J.; Soccol, C.R.; de Melo Pereira, G.V. Exploring Diversity and Functional Traits of Lactic Acid Bacteria in Traditional Vinegar Fermentation: A Review. Int. J. Food Microbiol. 2024, 412, 110550. [Google Scholar] [CrossRef]
- Sui, Y.; Liu, J.; Liu, Y.; Wang, Y.; Xiao, Y.; Gao, B.; Zhu, D. In Vitro Probiotic Characterization of Lactobacillus Strains from Fermented Tangerine Vinegar and Their Cholesterol Degradation Activity. Food Biosci. 2021, 39, 100843. [Google Scholar] [CrossRef]
- Haruta, S.; Ueno, S.; Egawa, I.; Hashiguchi, K.; Fujii, A.; Nagano, M.; Ishii, M.; Igarashi, Y. Succession of Bacterial and Fungal Communities during a Traditional Pot Fermentation of Rice Vinegar Assessed by PCR-Mediated Denaturing Gradient Gel Electrophoresis. Int. J. Food Microbiol. 2006, 109, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Zheng, Y.; Xie, S.; Zhang, X.; Song, J.; Xia, M.; Wang, M. Unraveling the Correlation between Microbiota Succession and Metabolite Changes in Traditional Shanxi Aged Vinegar. Sci. Rep. 2017, 7, 9240. [Google Scholar] [CrossRef]
- Li, Y.N.; Peng, M.Y.; Lu, Z.M.; Dong, Y.L.; Chai, L.J.; Shi, J.S.; Zhang, X.J.; Xu, Z.H. Lactiplantibacillus Plantarum and Komagataeibacter Europaeus Enhance Energy Metabolism, Acetic Acid and Aromatic Amino Acids Catabolism Flux in Cider Vinegar Fermentation. LWT 2024, 198, 115968. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, X.; Zhang, C.; Zheng, Y.; Zheng, B.; Duan, X.; Tian, Y. Microbial Dynamics and Flavor Formation during the Traditional Brewing of Monascus Vinegar. Food Res. Int. 2019, 125, 108531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, P.; Xu, D.; Wang, W.; Zhao, Y. Aroma Patterns of Beijing Rice Vinegar and Their Potential Biomarker for Traditional Chinese Cereal Vinegars. Food Res. Int. 2019, 119, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Nojima, N.; Yoshida, K.; Hirayama, S.; Ogihara, H.; Morinaga, Y. The Importance of Inter-Species Cell-Cell Co-Aggregation between Lactobacillus plantarum ML11-11 and Saccharomyces cerevisiae BY4741 in Mixed-Species Biofilm Formation. Biosci. Biotechnol. Biochem. 2011, 75, 1430–1434. [Google Scholar] [CrossRef]
- Cui, M.; Wang, M.; Sun, H.; Yu, L.; Su, Z.; Zhang, X.; Zheng, Y.; Xia, M.; Shen, Y.; Wang, M. Identifying and Characterization of Novel Broad-Spectrum Bacteriocins from the Shanxi Aged Vinegar Microbiome: Machine Learning, Molecular Simulation, and Activity Validation. Int. J. Biol. Macromol. 2024, 270, 132272. [Google Scholar] [CrossRef]
- Gong, X.; Lv, L.; Wu, Z. Study on Acid Producing Functional Bacteria of Shanxi Aged Vinegar. Food Ind. Technol. 2010, 31, 170–173. [Google Scholar]
- Yu, D.; Qiao, Y.; Fan, Z. Identification of High-Yielding Lactic Acid Bacteria During the Fermentation Process of Shanxi Aged Vinegar. Chin. Seas. 2018, 43, 4–8. [Google Scholar]
- Peng, M.Y.; Zhang, X.J.; Huang, T.; Zhong, X.Z.; Chai, L.J.; Lu, Z.M.; Shi, J.S.; Xu, Z.H. Komagataeibacter Europaeus Improves Community Stability and Function in Solid-State Cereal Vinegar Fermentation Ecosystem: Non-Abundant Species Plays Important Role. Food Res. Int. 2021, 150, 110815. [Google Scholar] [CrossRef]
- GB 12456-2021; National Health Commission of the People, Republic of China State Administration for Market Regulation. National Food Safety Standards Determination of Total Acid in Food. National Standards of the People’s Republic of China: Beijing, China, 2021.
- GB 19777-2005; General Administration of Quality Supervision, Inspection and Quarantine of the Peoples Republic of China, China National Standardization Administration Committee. Product of Designations of Origin or Geographical Indication-Shanxi Extra Aged Vinegar. National Standards of the People’s Republic of China: Beijing, China, 2005.
- Chen, J.C.; Chen, Q.H.; Guo, Q.; Ruan, S.; Ruan, H.; He, G.Q.; Gu, Q. Simultaneous Determination of Acetoin and Tetramethylpyrazine in Traditional Vinegars by HPLC Method. Food Chem. 2010, 122, 1247–1252. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, M.; Zhao, N.; Tu, L.; Xue, D.; Zhang, X.; Zhao, C.; Cheng, Y.; Zheng, Y.; Wang, M. Metabolic Profile of Main Organic Acids and Its Regulatory Mechanism in Solid-State Fermentation of Chinese Cereal Vinegar. Food Res. Int. 2021, 145, 110400. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Fu, J.; Zhang, G.; Liu, J.; Li, Z.; Luo, R.; Li, L. Investigating the Mechanism of the Flavor Formation in Sichuan Sun Vinegar Based on Flavor-Orientation and Metagenomics. Curr. Res. Food Sci. 2023, 6, 100460. [Google Scholar] [CrossRef]
- Nie, Z.; Zheng, Y.; Du, H.; Xie, S.; Wang, M. Dynamics and Diversity of Microbial Community Succession in Traditional Fermentation of Shanxi Aged Vinegar. Food Microbiol. 2015, 47, 62–68. [Google Scholar] [CrossRef]
- Xia, T.; Kang, C.; Qiang, X.; Zhang, X.; Li, S.; Liang, K.; Wang, Y.; Wang, J.; Cao, H.; Wang, M. Beneficial Effect of Vinegar Consumption Associated with Regulating Gut Microbiome and Metabolome. Curr. Res. Food Sci. 2024, 8, 100566. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, X.; Li, C.; Fu, Q.; Xu, X.; Sun, J.; Wang, C.; Du, J.; Wang, B.; Shi, X. Investigation of Microbial Succession and Volatile Compounds Dynamics during the Fermentation of Traditional Cereal Vinegar in Xinjiang. LWT 2023, 186, 115258. [Google Scholar] [CrossRef]
- Li, L.; Huang, C.; Li, Z.; Zhao, Y.; Liu, J.; Zheng, Y.; Cao, R.; Liao, Y. Investigating the Temporal Evolution of Physicochemical Attributes and Flavorome Profiles in Sichuan Shai Vinegar Utilizing Diverse Aging Techniques. Food Res. Int. 2024, 196, 115080. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Q.; Zhang, F.; Tang, Y.; Wang, J.; Sun, J. Changes in Bioactive and Volatile Aroma Compounds in Vinegar Fermented in a Rotary Drum Bioreactor. J. Food Compos. Anal. 2023, 121, 105345. [Google Scholar] [CrossRef]
- Li, Y.; Wang, A.; Dang, B.; Yang, X.; Nie, M.; Chen, Z.; Lin, R.; Wang, L.; Wang, F.; Tong, L.T. Deeply Analyzing Dynamic Fermentation of Highland Barley Vinegar: Main Physicochemical Factors, Key Flavors, and Dominate Microorganisms. Food Res. Int. 2024, 177, 113919. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Z.; Zhang, L.; Jiang, Y.; Zhu, J. Dynamic Changes of Quality and Flavor Characterization of Zhejiang Rosy Vinegar during Fermentation and Aging Based on Untargeted Metabolomics. Food Chem. 2023, 404, 134702. [Google Scholar] [CrossRef]
- Wang, J.; Huang, M.; Zhang, J.; Wu, J. Flavor Chemistry in Baijiu with Sesame Flavor: A Review. In ACS Symposium Series; Guthrie, B., Beauchamp, J.D., Buettner, A., Toth, S., Qian, M.C., Eds.; American Chemical Society: Washington, DC, USA, 2019; Volume 1321, pp. 177–224. ISBN 978-0-8412-3467-3. [Google Scholar]
- Fang, G.Y.; Chai, L.J.; Zhong, X.Z.; Jiang, Y.J. Deciphering the Succession Patterns of Bacterial Community and Their Correlations with Environmental Factors and Flavor Compounds during the Fermentation of Zhejiang Rosy Vinegar. Int. J. Food Microbiol. 2021, 341, 109070. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Feng, J.; Zhang, G.; Liu, J.; Li, N.; Xu, H.; Zhang, Y.; Cao, R.; Li, L. Role of Bacterial Community Succession in Flavor Formation during Sichuan Sun Vinegar Grain (Cupei) Fermentation. J. Biosci. Bioeng. 2023, 135, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.J.; Qiu, T.; Lu, Z.M.; Deng, Y.J.; Zhang, X.J.; Shi, J.S.; Xu, Z.H. Modulating Microbiota Metabolism via Bioaugmentation with Lactobacillus Casei and Acetobacter Pasteurianus to Enhance Acetoin Accumulation during Cereal Vinegar Fermentation. Food Res. Int. 2020, 138, 109737. [Google Scholar] [CrossRef] [PubMed]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef]
- Safari, R.; Hoseinifar, S.H.; Nejadmoghadam, S.; Khalili, M. Apple Cider Vinegar Boosted Immunomodulatory and Health Promoting Effects of Lactobacillus Casei in Common Carp (Cyprinus carpio). Fish. Shellfish Immunol. 2017, 67, 441–448. [Google Scholar] [CrossRef]
- Bakir, S.; Devecioglu, D.; Kayacan, S.; Toydemir, G.; Karbancioglu-Guler, F.; Capanoglu, E. Investigating the Antioxidant and Antimicrobial Activities of Different Vinegars. Eur. Food Res. Technol. 2017, 243, 2083–2094. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Aheto, J.H.; Xu, F.; Dai, C.; Ren, Y.; Wang, L.; Yu, S. Rapid Non-Destructive Monitoring and Quality Assessment of the Fumigation Process of Shanxi Aged Vinegar Based on Vis-NIR Hyperspectral Imaging Combined with Multiple Chemometric Algorithms. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 320, 124539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.; He, R.; Zhao, G.; Yu, Y.; Zhang, R.; Gao, X. Research Advances in Technologies and Mechanisms to Regulate Vinegar Flavor. Food Chem. 2024, 460, 140783. [Google Scholar] [CrossRef]
- Durán-Guerrero, E.; Castro, R.; García-Moreno, M.D.V.; Rodríguez-Dodero, M.D.C.; Schwarz, M.; Guillén-Sánchez, D. Aroma of Sherry Products: A Review. Foods 2021, 10, 753. [Google Scholar] [CrossRef]
- Lu, Z.M.; Liu, N.; Wang, L.J.; Wu, L.H.; Gong, J.S.; Yu, Y.J.; Li, G.Q.; Shi, J.S.; Xu, Z.H. Elucidating and Regulating the Acetoin Production Role of Microbial Functional Groups in Multispecies Acetic Acid Fermentation. Appl. Environ. Microbiol. 2016, 82, 5860–5868. [Google Scholar] [CrossRef]
- Zhou, Z.; Jian, D.; Gong, M.; Zhu, S.; Li, G.; Zhang, S.; Zhong, F.; Mao, J. Characterization of the Key Aroma Compounds in Aged Zhenjiang Aromatic Vinegar by Gas Chromatography-Olfactometry-Mass Spectrometry, Quantitative Measurements, Aroma Recombination and Omission Experiments. Food Res. Int. 2020, 136, 109434. [Google Scholar] [CrossRef] [PubMed]


| Bacterial Strain | Fermentation Characteristics | Tolerance Property | Cell Morphology | Colonial Morphology |
|---|---|---|---|---|
| Lactobacillus plantarum SAVndL 7 | High acid production (24.30 g/L), high acetoin production (1.21 mg/mL). | Alcohol (10%), high-temperature-resistant (50 °C), the initial sugar (250 g/L) | Slender, short rod | Colony diameter 1 mm, round, flat, smooth surface, shiny, neat edges, milky white, opaque. |
| Lactobacillusplantarum SAVndL 19 | Acid production (21.95 g/L), acetoin content (1.09 mg/mL) | Alcohol (10%), high-temperature-resistant (50 °C), the initial sugar (250 g/L) | Slender, short rod | Colony diameter 0.8 mm, round, flat, smooth surface, shiny, neat edges, milky white, opaque. |
| Pediococcus acidilactici SAVndL 729 | Acid production (18.55 g/L), acetoin content (0.92 mg/mL) | Alcohol (10%), high-temperature-resistant (50 °C), the initial sugar (250 g/L) | Rod shape | Colony diameter 1.2 mm, round, slightly convex, smooth surface, glossy, edge. |
| Pediococcus pentosaceus SAVndL 2422 | Acid production (10.08 g/L), acetoin content (0.90 mg/mL) | Alcohol (8%), high-temperature-resistant (45 °C), the initial sugar (200 g/L) | Rod shape | Colony diameter 1 mm, round, raised, smooth surface, shiny, neat edges, milky white, opaque. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhang, Y.; Wang, C.; Zhang, X.; Wei, R.; Li, Y.; Li, Q.; Xu, N. Comparative Study on the Fermentation Characteristics of Selective Lactic Acid Bacteria in Shanxi Aged Vinegar: Pure Culture Versus Co-Culture. Foods 2024, 13, 3374. https://doi.org/10.3390/foods13213374
Li Q, Zhang Y, Wang C, Zhang X, Wei R, Li Y, Li Q, Xu N. Comparative Study on the Fermentation Characteristics of Selective Lactic Acid Bacteria in Shanxi Aged Vinegar: Pure Culture Versus Co-Culture. Foods. 2024; 13(21):3374. https://doi.org/10.3390/foods13213374
Chicago/Turabian StyleLi, Qi, Yujing Zhang, Chaomin Wang, Xiaoyu Zhang, Ruteng Wei, Yunlong Li, Qiqiong Li, and Nv Xu. 2024. "Comparative Study on the Fermentation Characteristics of Selective Lactic Acid Bacteria in Shanxi Aged Vinegar: Pure Culture Versus Co-Culture" Foods 13, no. 21: 3374. https://doi.org/10.3390/foods13213374
APA StyleLi, Q., Zhang, Y., Wang, C., Zhang, X., Wei, R., Li, Y., Li, Q., & Xu, N. (2024). Comparative Study on the Fermentation Characteristics of Selective Lactic Acid Bacteria in Shanxi Aged Vinegar: Pure Culture Versus Co-Culture. Foods, 13(21), 3374. https://doi.org/10.3390/foods13213374






