The Protective Effects of Polygala tenuifolia and Tenuifolin on Corticosterone-Evoked Ferroptosis, Oxidative Stress, and Neuroinflammation: Insights from Molecular Dynamics Simulations and In Vitro Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Treatment
2.3. Cell Viability Assay
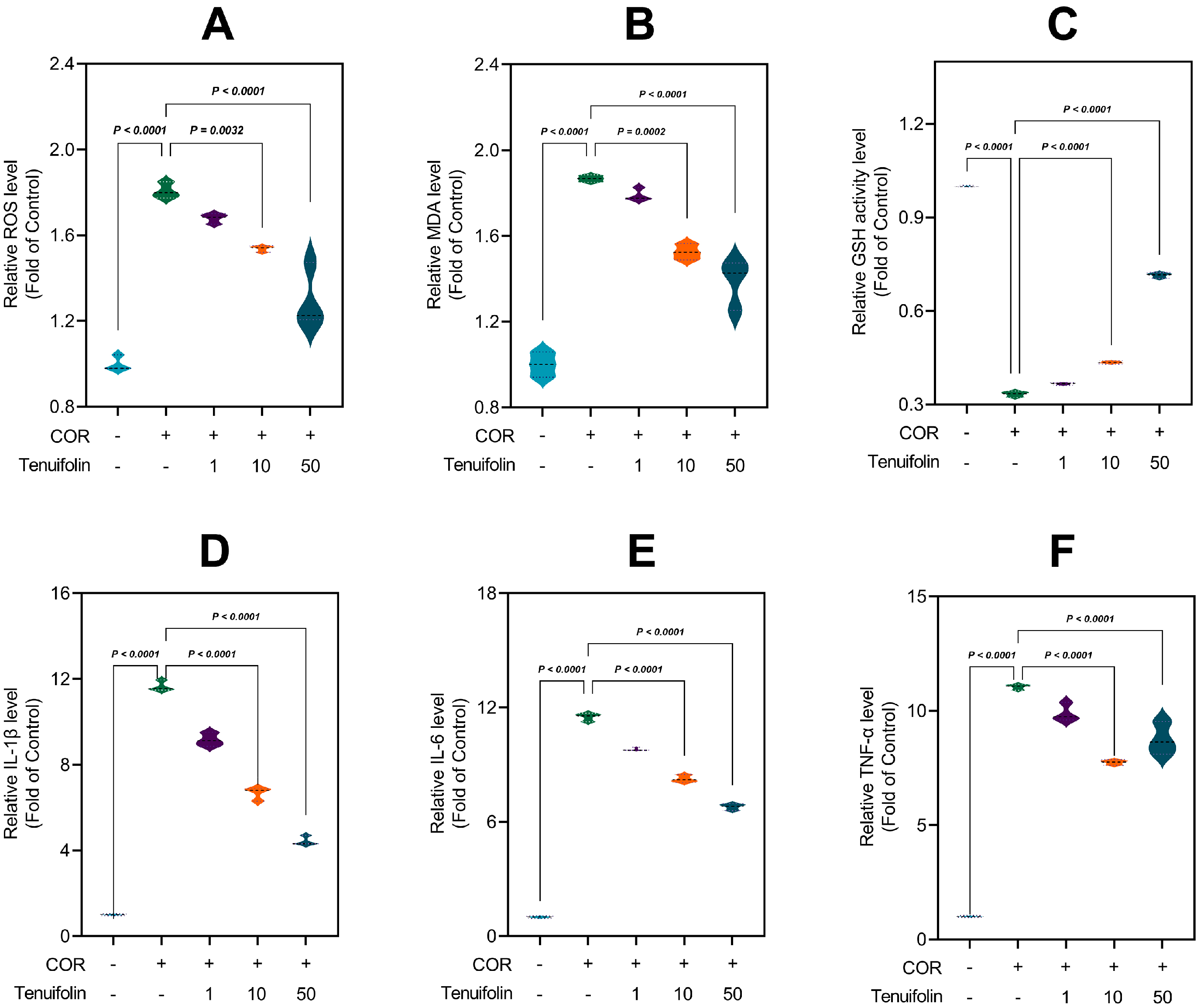
2.4. Determination of Iron Ions, MDA, Inflammatory Factors, ROS, GPX, SOD, and GSH Activity
2.5. Western Blotting
2.6. Immunofluorescence Analysis
2.7. Molecular Docking and Molecular Dynamics Simulations
2.8. Statistical Analysis
3. Results and Discussion
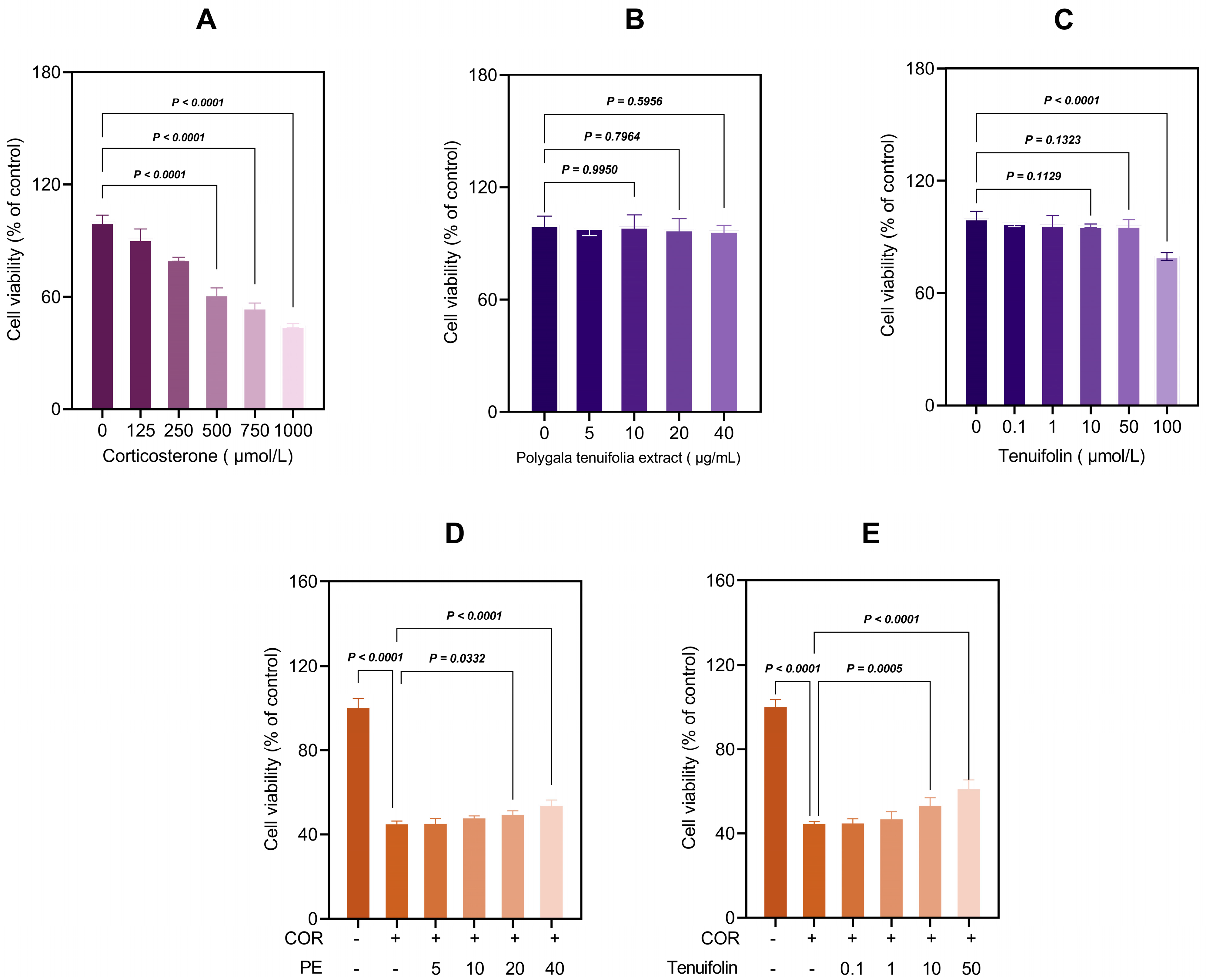
3.1. PTE and Tenuifolin Protected PC12 Cells from Corticosterone-Induced Cytotoxicity
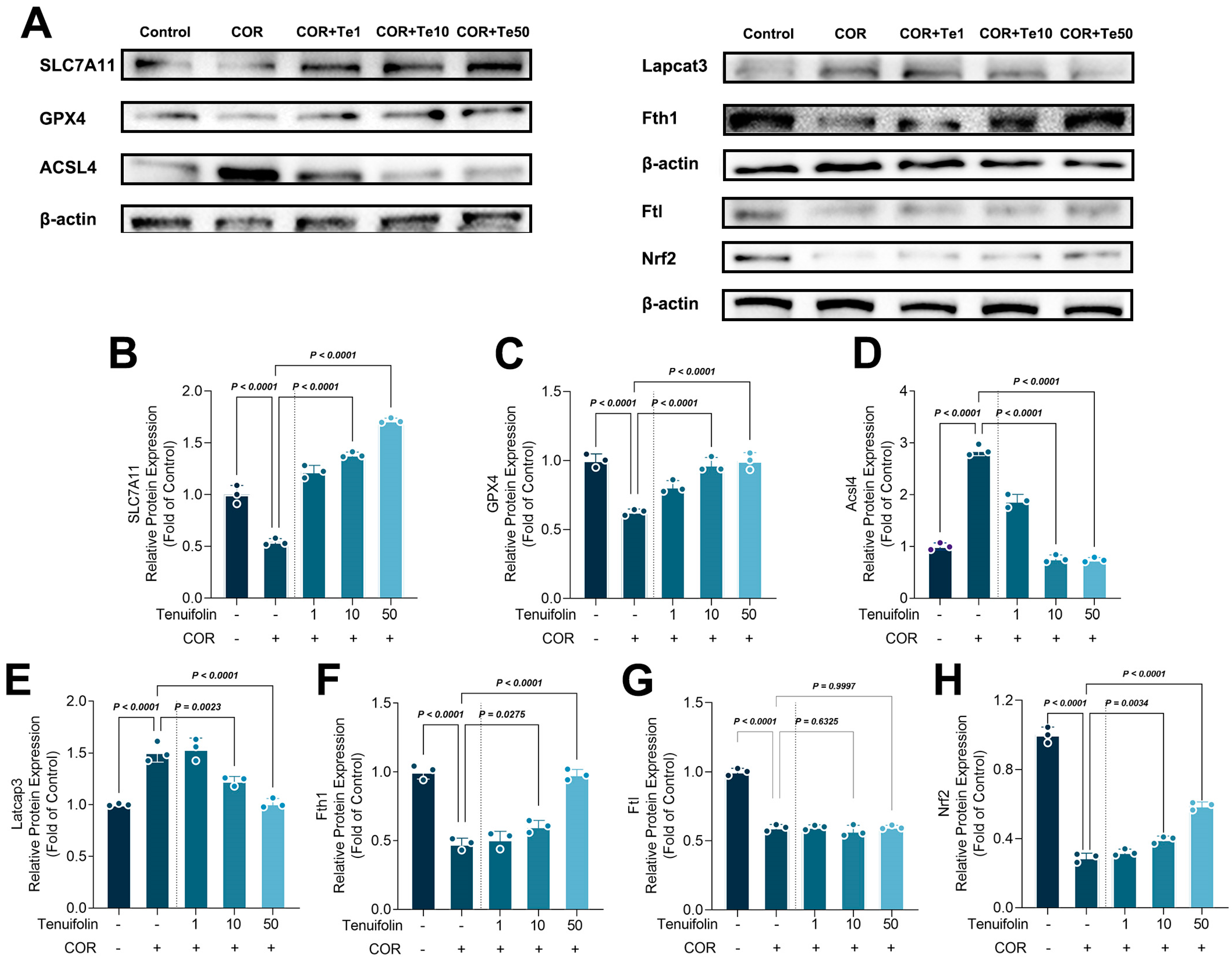
3.2. Tenuifolin Inhibited Ferroptosis-Related Protein Expression in PC12 Cells Exposed to Corticosterone
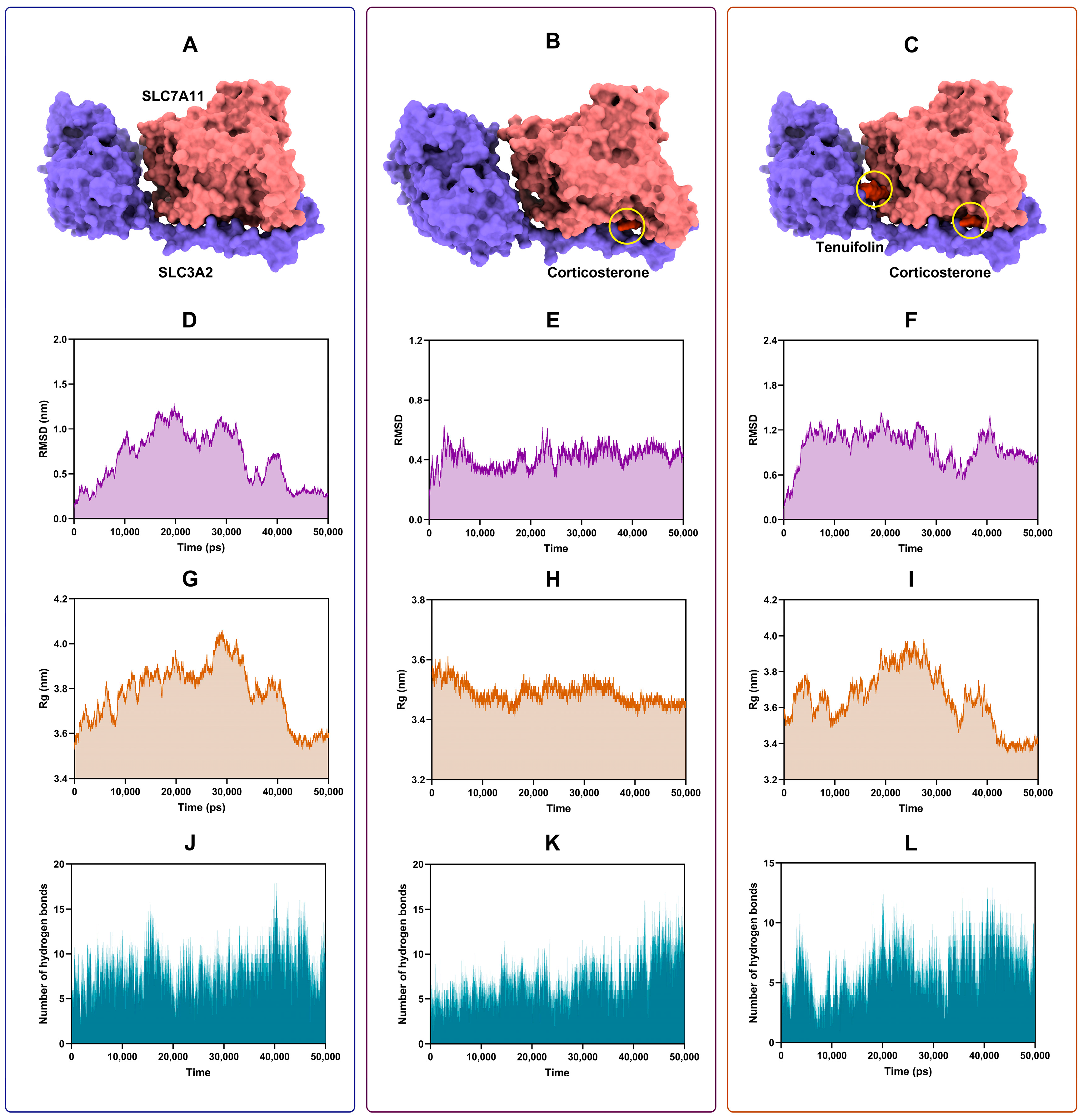
3.3. Tenuifolin Attenuated Structural Change in the SCL7A11/SCL3A2 Complex
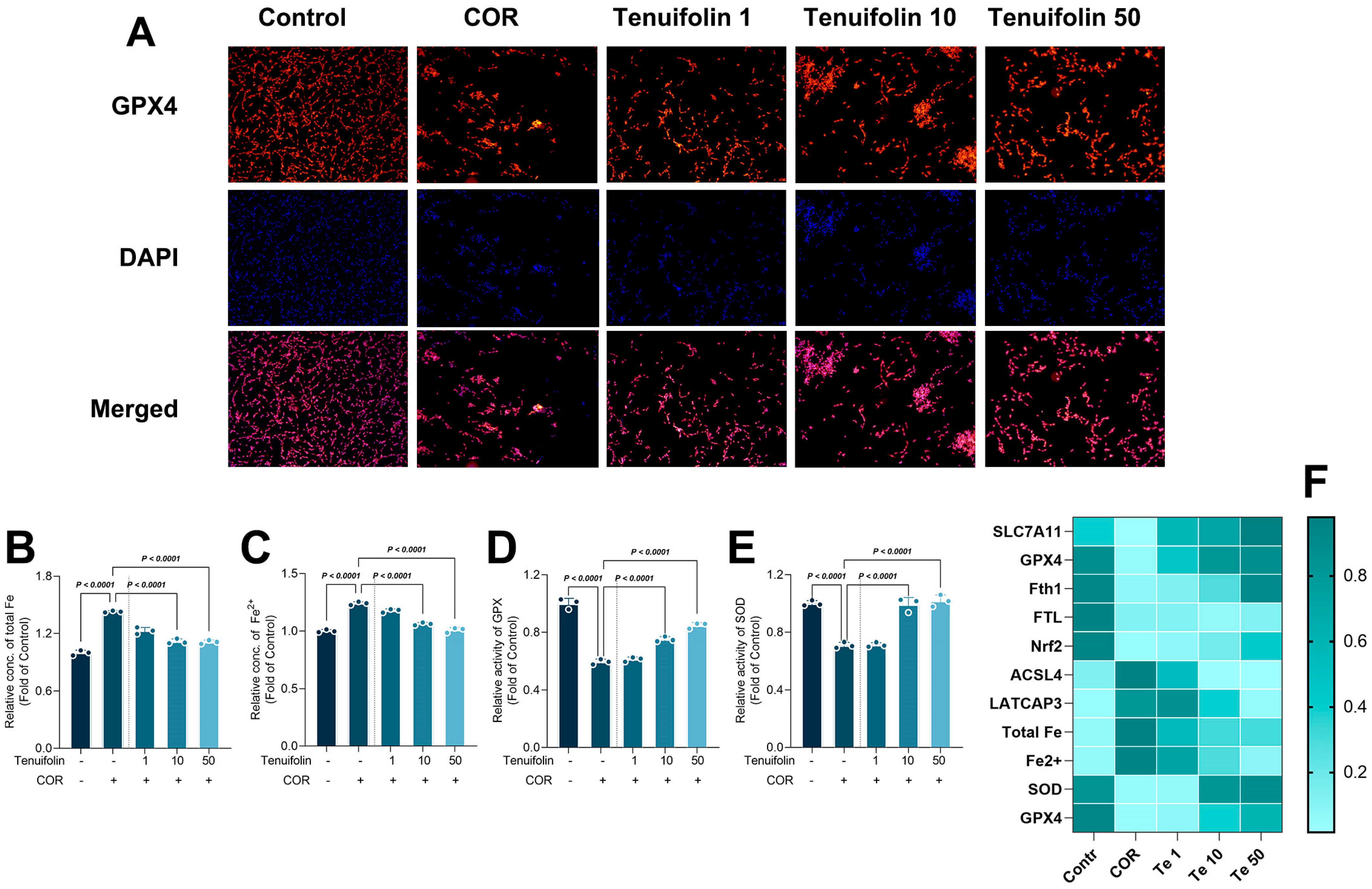
3.4. Tenuifolin Attenuated Corticosterone-Induced Oxidative Stress and Neuroinflammation in PC12 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monroe, S.M.; Harkness, K.L. Major depression and its recurrences: Life course matters. Annu. Rev. Clin. Psychol. 2022, 18, 329–357. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The bidirectional relationship of depression and inflammation: Double trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.J.; Carpenter, J.S.; Song, Y.J.C.; Hockey, S.J.; Naismith, S.L.; Grunstein, R.R.; Scott, E.M.; Merikangas, K.R.; Scott, J.; Hickie, I.B. Circadian rhythm sleep–wake disturbances and depression in young people: Implications for prevention and early intervention. Lancet Psychiatry 2021, 8, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Jiao, H.; Yang, H.; Yan, Z.; Chen, J.; Xu, M.; Jiang, Y.; Liu, Y.; Xue, Z.; Ma, Q.; Li, X.; et al. Traditional Chinese formula Xiaoyaosan alleviates depressive-like behavior in CUMS mice by regulating PEBP1-GPX4-mediated ferroptosis in the hippocampus. Neuropsychiatr. Dis. Treat. 2021, 17, 1001–1019. [Google Scholar] [CrossRef]
- Ashraf, A.; Jeandriens, J.; Parkes, H.G.; So, P.W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer’s disease: Evidence of ferroptosis. Redox Biol. 2020, 32, 101494. [Google Scholar] [CrossRef]
- Chen, L.; Dar, N.J.; Na, R.; McLane, K.D.; Yoo, K.; Han, X.; Ran, Q. Enhanced defense against ferroptosis ameliorates cognitive impairment and reduces neurodegeneration in 5xFAD mice. Free. Radic. Biol. Med. 2022, 180, 1–12. [Google Scholar] [CrossRef]
- Dang, Y.; He, Q.; Yang, S.; Sun, H.; Liu, Y.; Li, W.; Tang, Y.; Zheng, Y.; Wu, T. FTH1-and SAT1-induced astrocytic ferroptosis is involved in Alzheimer’s disease: Evidence from single-cell transcriptomic analysis. Pharmaceuticals 2022, 15, 1177. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H.; Liu, H.; Chen, S.; Guo, H.; Ma, S.; Han, W.; Li, Y.; Wang, D. Isoforsythiaside confers neuroprotection against Alzheimer’s disease by attenuating ferroptosis and neuroinflammation in vivo and in vitro. Food Sci. Hum. Wellness 2023, 12, 1730–1742. [Google Scholar] [CrossRef]
- Fiore, A.; Zeitler, L.; Russier, M.; Groß, A.; Hiller, M.-K.; Parker, J.L.; Stier, L.; Köcher, T.; Newstead, S.; Murray, P.J. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol. Cell 2022, 82, 920–932.e927. [Google Scholar] [CrossRef]
- Parker, J.L.; Deme, J.C.; Kolokouris, D.; Kuteyi, G.; Biggin, P.C.; Lea, S.M.; Newstead, S. Molecular basis for redox control by the human cystine/glutamate antiporter system xc−. Nat. Commun. 2021, 12, 7147. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: New approaches to hippocampal neurogenesis. J. Neuroinflamm. 2023, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Koo, B.-B.; Michalovicz, L.T.; Calderazzo, S.; Kelly, K.A.; Sullivan, K.; Killiany, R.J.; O’Callaghan, J.P. Corticosterone potentiates DFP-induced neuroinflammation and affects high-order diffusion imaging in a rat model of Gulf War Illness. Brain Behav. Immun. 2018, 67, 42–46. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, S.; Liu, X.; Han, L.; Wang, R.; Hao, H.; Jiao, Y.; Han, S.; Bai, C. Polygala tenuifolia: A source for anti-Alzheimer’s disease drugs. Pharm. Biol. 2020, 58, 410–416. [Google Scholar] [CrossRef]
- Borges, F.R.; Silva, M.D.; Córdova, M.M.; Schambach, T.R.; Pizzolatti, M.G.; Santos, A.R. Anti-inflammatory action of hydroalcoholic extract, dichloromethane fraction and steroid α-spinasterol from Polygala sabulosa in LPS-induced peritonitis in mice. J. Ethnopharmacol. 2014, 151, 144–150. [Google Scholar] [CrossRef]
- Yan, W.F.; Shao, Q.H.; Zhang, D.M.; Yuan, Y.H.; Chen, N.H. The molecular mechanism of polygalasaponin F-mediated decreases in TNFα: Emphasizing the role of the TLR4-PI3K/AKT-NF-κB pathway. J. Asian Nat. Prod. Res. 2015, 17, 662–670. [Google Scholar] [CrossRef]
- Fan, Z.; Liang, Z.; Yang, H.; Pan, Y.; Zheng, Y.; Wang, X. Tenuigenin protects dopaminergic neurons from inflammation via suppressing NLRP3 inflammasome activation in microglia. J. Neuroinflamm. 2017, 14, 256. [Google Scholar] [CrossRef]
- Huang, X.B.; Chen, Y.J.; Chen, W.Q.; Wang, N.Q.; Wu, X.L.; Liu, Y. Neuroprotective effects of tenuigenin on neurobehavior, oxidative stress, and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. Brain Circ. 2018, 4, 24–32. [Google Scholar]
- Garg, A.; Agrawal, R.; Deshmukh, R. Pharmacology of polygala tenuifolia and its significance in traditional Chinese medicine. Pharmacol. Res.-Mod. Chin. Med. 2023, 10, 100341. [Google Scholar] [CrossRef]
- Wang, L.; Jin, G.F.; Yu, H.H.; Lu, X.H.; Zou, Z.H.; Liang, J.Q.; Yang, H. Protective effects of tenuifolin isolated from Polygala tenuifolia Willd roots on neuronal apoptosis and learning and memory deficits in mice with Alzheimer’s disease. Food Funct. 2019, 10, 7453–7460. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, H.; Jiang, N.; Zhang, Y.; Lv, J.; Liu, X. Tenuifolin ameliorates chronic restraint stress-induced cognitive impairment in C57BL/6J mice. Phytother. Res. 2022, 36, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, F.; Qu, Y.; Zhao, P.; Wang, X.; Zhu, G. Tenuifolin in the prevention of Alzheimer’s disease-like phenotypes: Investigation of the mechanisms from the perspectives of calpain system, ferroptosis, and apoptosis. Phytother. Res. 2023, 37, 4621–4638. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, J.; Zhang, Q.; Xu, S.; Liu, Y.; Ma, S.; Hu, X.; Liu, Y.; Zhang, X.; Jiang, R.; et al. Polygala tenuifolia willd. Extract alleviates LPS-induced acute lung injury in rats via TLR4/NF-κB pathway and NLRP3 inflammasome suppression. Phytomedicine 2024, 132, 155859. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, G.-J.; Tu, B.-X.; Hu, Z.-L.; Luo, C.; Cui, Y.-H.; Xu, Y.; Li, F.; Dai, R.-P.; Bi, F.-F.; et al. Corticosterone induced the increase of proBDNF in primary hippocampal neurons via endoplasmic reticulum stress. Neurotox. Res. 2020, 38, 370–384. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Forli, S. Using AutoDock 4 and AutoDock vina with AutoDockTools: A tutorial. Scripps Res. Inst. Mol. Graph. Lab. 2012, 10550, 1000. [Google Scholar]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Carballo-Pacheco, M.; Ismail, A.E.; Strodel, B. On the applicability of force fields to study the aggregation of amyloidogenic peptides using molecular dynamics simulations. J. Chem. Theory Comput. 2018, 14, 6063–6075. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A new tool to perform end-state free energy calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, M.N.; Zhao, W.J. Research on ferroptosis as a therapeutic target for the treatment of neurodegenerative diseases. Ageing Res. Rev. 2023, 91, 102035. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Guo, H.; Jiang, H.; Liu, H.; Fu, H.; Wang, D. Forsythoside a mitigates alzheimer’s-like pathology by inhibiting ferroptosis-mediated neuroinflammation via Nrf2/GPX4 axis activation. Int. J. Biol. Sci. 2022, 18, 2075. [Google Scholar] [CrossRef]
- Ye, L.; Wen, X.; Qin, J.; Zhang, X.; Wang, Y.; Wang, Z.; Zhou, T.; Di, Y.; He, W. Metabolism-regulated ferroptosis in cancer progression and therapy. Cell Death Dis. 2024, 15, 196. [Google Scholar] [CrossRef]
- Pope, L.E.; Dixon, S.J. Regulation of ferroptosis by lipid metabolism. Trends Cell Biol. 2023, 33, 1077–1087. [Google Scholar] [CrossRef]
- Dai, Y.; Guo, J.; Zhang, B.; Chen, J.; Ou, H.; He, R.-R.; So, K.-F.; Zhang, L. Lycium barbarum (Wolfberry) glycopeptide prevents stress-induced anxiety disorders by regulating oxidative stress and ferroptosis in the medial prefrontal cortex. Phytomedicine 2023, 116, 154864. [Google Scholar] [CrossRef]
- Vidal-Limon, A.; Aguilar-Toalá, J.E.; Liceaga, A.M. Integration of molecular docking analysis and molecular dynamics simulations for studying food proteins and bioactive peptides. J. Agric. Food Chem. 2022, 70, 934–943. [Google Scholar] [CrossRef]
- Liang, F.; Shi, Y.; Shi, J.; Cao, W. Exploring the binding mechanism of pumpkin seed protein and apigenin: Spectroscopic analysis, molecular docking and molecular dynamics simulation. Food Hydrocoll. 2023, 137, 108318. [Google Scholar] [CrossRef]
- Dong, M.; Tian, H.; Xu, Y.; Han, M.; Xu, X. Effects of pulsed electric fields on the conformation and gelation properties of myofibrillar proteins isolated from pale, soft, exudative (PSE)-like chicken breast meat: A molecular dynamics study. Food Chem. 2021, 342, 128306. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, R.; Wang, C.; Zhang, X.; Wang, C. Deciphering the non-covalent binding patterns of three whey proteins with rosmarinic acid by multi-spectroscopic, molecular docking and molecular dynamics simulation approaches. Food Hydrocoll. 2022, 132, 107895. [Google Scholar] [CrossRef]
- Wu, N.; Li, P.; Shuang, Q. Screening and molecular dynamics simulation of ACE inhibitory tripeptides derived from milk fermented with Lactobacillus delbrueckii QS306. Food Funct. 2024, 15, 2655–2667. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Nian, B.; Shi, J.; Sun, X.; Du, R.; Tan, C.P.; Xu, Y.-J.; Liu, Y. Influence of extraction technology on rapeseed oil functional quality: A study on rapeseed polyphenols. Food Funct. 2022, 13, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Bartho, L.A.; Holland, O.J.; Moritz, K.M.; Perkins, A.V.; Cuffe, J.S. Maternal corticosterone in the mouse alters oxidative stress markers, antioxidant function and mitochondrial content in placentas of female fetuses. J. Physiol. 2019, 597, 3053–3067. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-Z.; Li, X.; Gong, W.-X.; Tian, J.-S.; Gao, X.-X.; Gao, L.; Zhang, X.; Du, G.-H.; Qin, X.-M. Protective effect of isoliquiritin against corticosterone-induced neurotoxicity in PC12 cells. Food Funct. 2017, 8, 1235–1244. [Google Scholar]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective effects of Hericium erinaceus (Bull.: Fr.) Pers. against high-dose corticosterone-induced oxidative stress in PC-12 cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, C.; Wang, G.; Luo, J.; Ma, H.; Xu, L.; Mu, Y.; Li, Y.; Seeram, N.P.; Huang, X.; et al. Urolithins attenuate LPS-induced neuroinflammation in BV2Microglia via MAPK, Akt, and NF-κB signaling pathways. J. Agric. Food Chem. 2018, 66, 571–580. [Google Scholar] [CrossRef]
- Li, X.; Qin, X.; Tian, J.; Gao, X.; Wu, X.; Du, G.; Zhou, Y. Liquiritin protects PC12 cells from corticosterone-induced neurotoxicity via regulation of metabolic disorders, attenuation ERK1/2-NF-κB pathway, activation Nrf2-Keap1 pathway, and inhibition mitochondrial apoptosis pathway. Food Chem. Toxicol. 2020, 146, 111801. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, C.; He, Z.; Zeng, M.; Wang, Z.; Chen, Q.; Qin, F.; Chen, M.; Ye, H.; Chen, J. The Protective Effects of Polygala tenuifolia and Tenuifolin on Corticosterone-Evoked Ferroptosis, Oxidative Stress, and Neuroinflammation: Insights from Molecular Dynamics Simulations and In Vitro Experiments. Foods 2024, 13, 3358. https://doi.org/10.3390/foods13213358
Xue C, He Z, Zeng M, Wang Z, Chen Q, Qin F, Chen M, Ye H, Chen J. The Protective Effects of Polygala tenuifolia and Tenuifolin on Corticosterone-Evoked Ferroptosis, Oxidative Stress, and Neuroinflammation: Insights from Molecular Dynamics Simulations and In Vitro Experiments. Foods. 2024; 13(21):3358. https://doi.org/10.3390/foods13213358
Chicago/Turabian StyleXue, Chaoyi, Zhiyong He, Maomao Zeng, Zhaojun Wang, Qiuming Chen, Fang Qin, Mingmin Chen, Hui Ye, and Jie Chen. 2024. "The Protective Effects of Polygala tenuifolia and Tenuifolin on Corticosterone-Evoked Ferroptosis, Oxidative Stress, and Neuroinflammation: Insights from Molecular Dynamics Simulations and In Vitro Experiments" Foods 13, no. 21: 3358. https://doi.org/10.3390/foods13213358
APA StyleXue, C., He, Z., Zeng, M., Wang, Z., Chen, Q., Qin, F., Chen, M., Ye, H., & Chen, J. (2024). The Protective Effects of Polygala tenuifolia and Tenuifolin on Corticosterone-Evoked Ferroptosis, Oxidative Stress, and Neuroinflammation: Insights from Molecular Dynamics Simulations and In Vitro Experiments. Foods, 13(21), 3358. https://doi.org/10.3390/foods13213358







