Boosting Health Benefits in Vegetables: A Novel Ultraviolet B (UVB) Device for Rapid At-Home Enhancement of Phytochemicals and Bioactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Plant Material
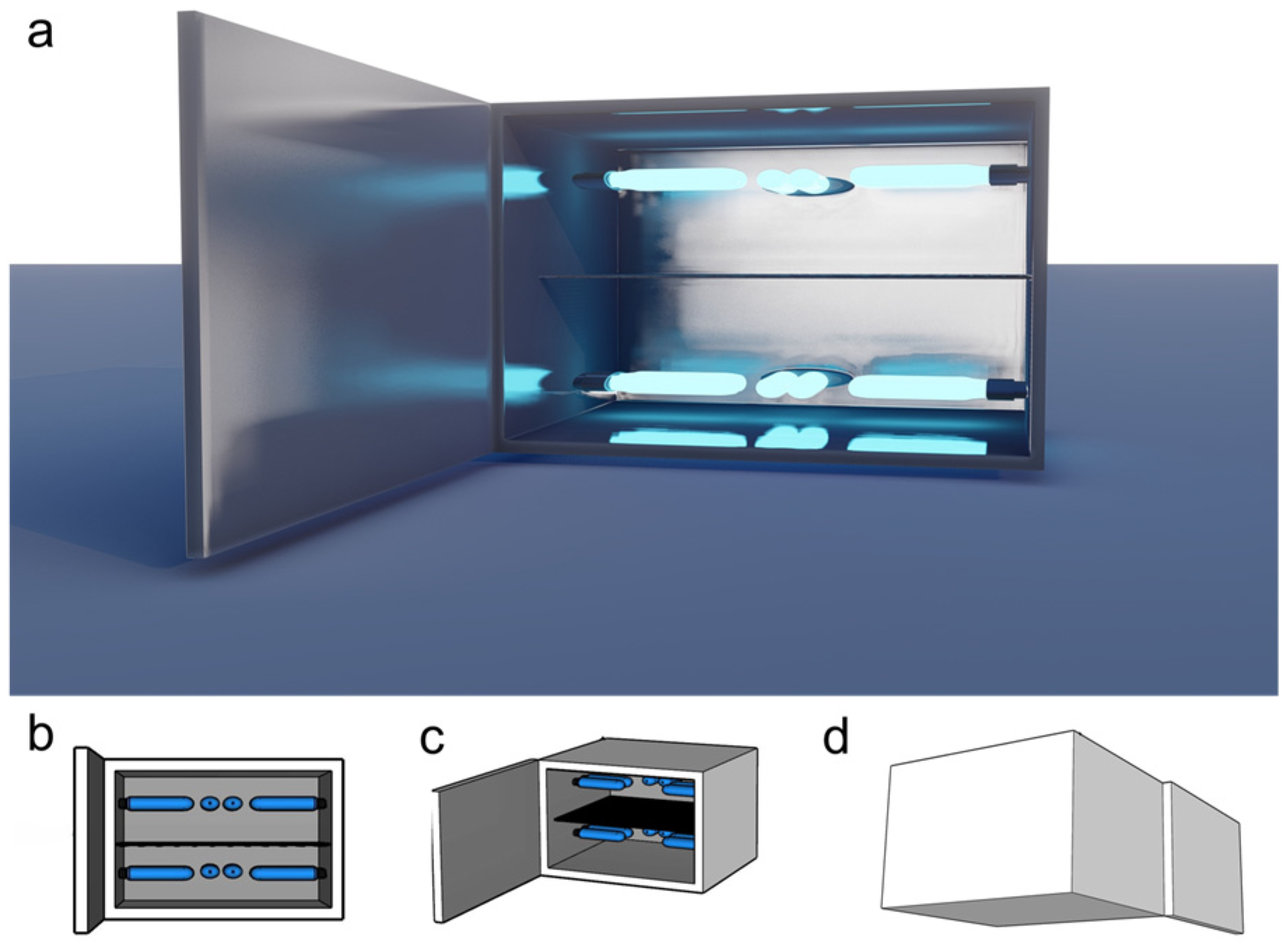
2.2. UVB Treatment Device Design and Construction
2.3. Fresh-Cut Processing and Storage Conditions
2.4. Optimization of UVB Radiation Conditions
2.5. Extraction, Identification, and Quantification of Phytochemicals
2.5.1. Phenolics
2.5.2. Carotenoids
2.5.3. Glucosinolates
2.6. Cell Culture
2.6.1. Extract Preparation
2.6.2. MTS Assay
2.6.3. Evaluation of Anti-Inflammatory Potential
2.6.4. Cellular Antioxidant Activity (CAA)
2.6.5. Evaluation of Antiadipogenic Potential
2.6.6. Triglyceride Quantification
2.6.7. Free Glycerol Measurement
2.6.8. qPCR
2.7. Statistical Analysis
3. Results and Discussion
3.1. UVB Exposure Optimization
3.2. Phytochemical Evaluation of UVB-Treated Vegetables
3.3. Impact of UVB Exposure on the Bioactivity of Vegetables
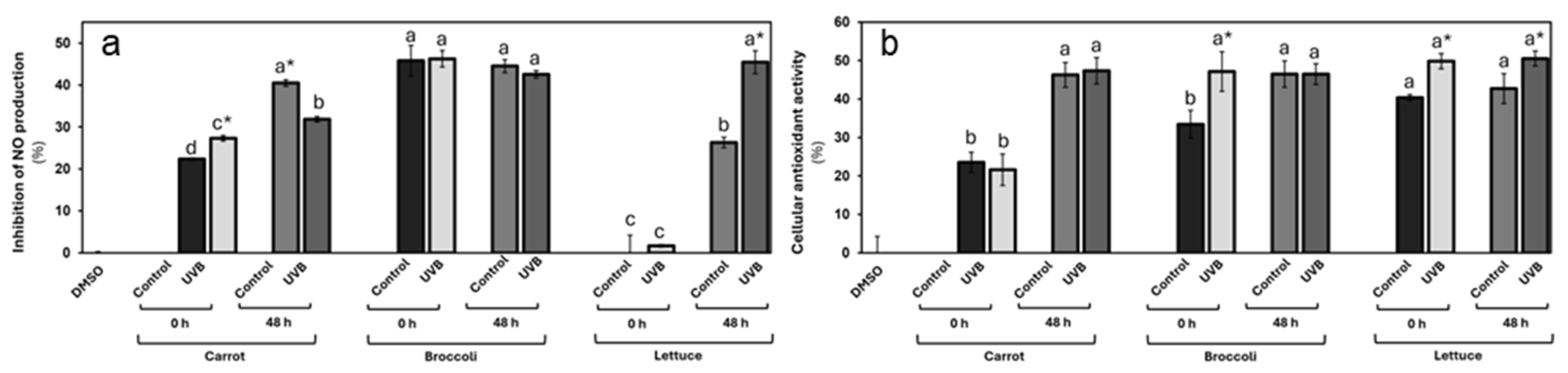
3.3.1. Anti-Inflammatory and Antioxidant Properties of UVB-Treated Vegetables
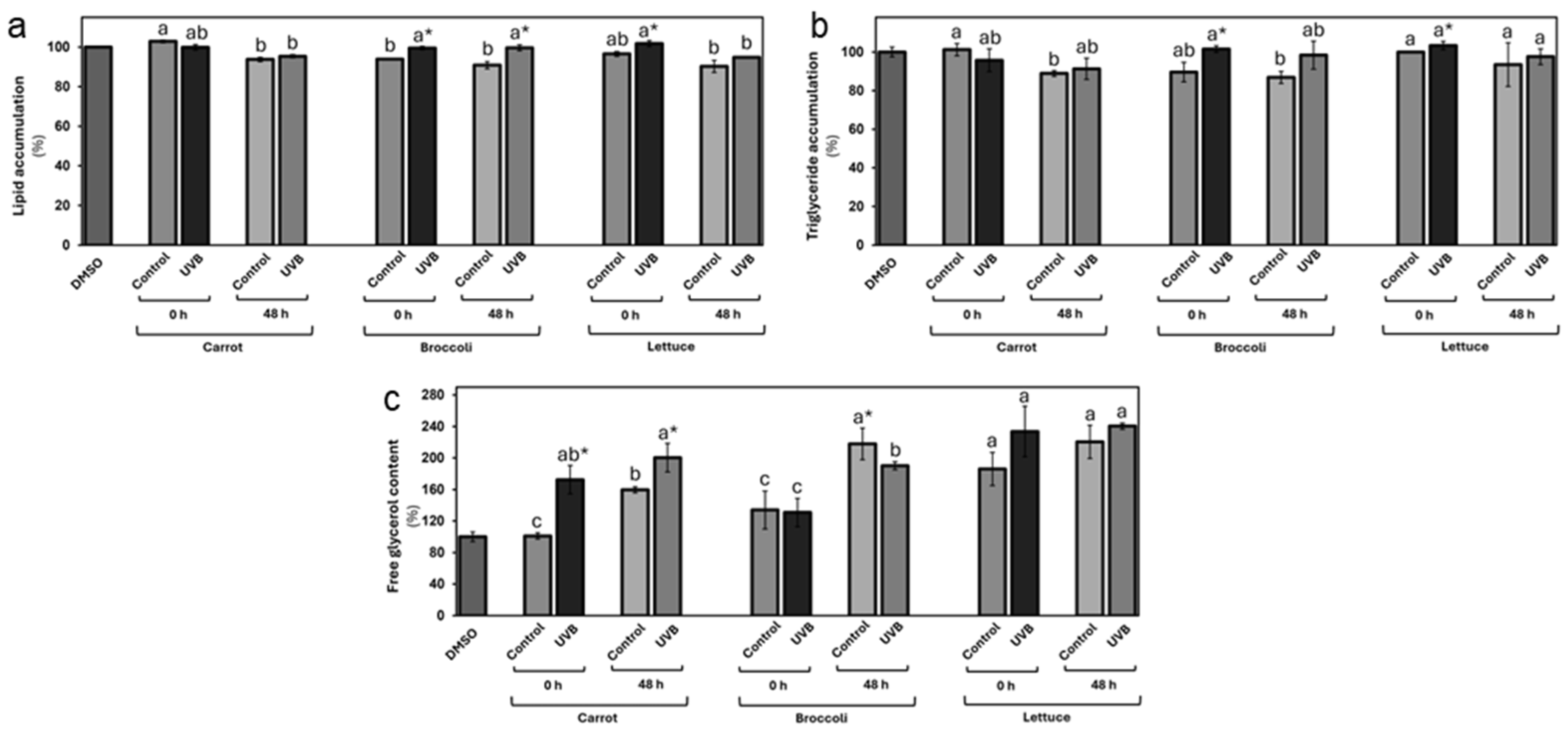
3.3.2. Effects of UVB-Treated Vegetables on Lipid Accumulation and Metabolism
3.3.3. Effect of Vegetable UVB Exposure on the Expression of Genes Related to Lipid Metabolism
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yip, C.S.C.; Chan, W.; Fielding, R. The Associations of Fruit and Vegetable Intakes with Burden of Diseases: A Systematic Review of Meta-Analyses. J. Acad. Nutr. Diet. 2019, 119, 464–481. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Luong, H.Q.; Li, H.-P.; Chiu, C.-H.; Hsieh, P.-C. Broccoli (Brassica oleracea L. Var. italica) Sprouts as the Potential Food Source for Bioactive Properties: A Comprehensive Study on In Vitro Disease Models. Foods 2019, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive Compounds in Lettuce: Highlighting the Benefits to Human Health and Impacts of Preharvest and Postharvest Practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef]
- FAOSTAT Online Database. Available online: https://www.fao.org/faostat/en/#home (accessed on 20 September 2024).
- Jacobo-Velázquez, D.A. Transformation of Carrots into Novel Food Ingredients and Innovative Healthy Foods. Appl. Food Res. 2023, 3, 100303. [Google Scholar] [CrossRef]
- Mamatha, B.S.; Sangeetha, R.K.; Baskaran, V. Provitamin-A and Xanthophyll Carotenoids in Vegetables and Food Grains of Nutritional and Medicinal Importance. Int. J. Food Sci. Technol. 2011, 46, 315–323. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.-H.; Hsieh, P.-C. Bioactive Compounds and Bioactivities of Brassica oleracea L. Var. italica Sprouts and Microgreens: An Updated Overview from a Nutraceutical Perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef]
- Kalmpourtzidou, A.; Eilander, A.; Talsma, E.F. Global Vegetable Intake and Supply Compared to Recommendations: A Systematic Review. Nutrients 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Controlled Abiotic Stresses Revisited: From Homeostasis through Hormesis to Extreme Stresses and the Impact on Nutraceuticals and Quality during Pre- and Postharvest Applications in Horticultural Crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar] [CrossRef]
- Han, C.; Li, J.; Jin, P.; Li, X.; Wang, L.; Zheng, Y. The Effect of Temperature on Phenolic Content in Wounded Carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Martínez-Hernández, G.B.; Del, C. Rodríguez, S.; Cao, C.-M.; Cisneros-Zevallos, L. Plants as Biofactories: Physiological Role of Reactive Oxygen Species on the Accumulation of Phenolic Antioxidants in Carrot Tissue under Wounding and Hyperoxia Stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Santacruz, A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Postharvest Wounding Stress in Horticultural Crops as a Tool for Designing Novel Functional Foods and Beverages with Enhanced Nutraceutical Content: Carrot Juice as a Case Study. J. Food Sci. 2019, 84, 1151–1161. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of Phenolic Antioxidants in Carrot Tissue Increases with Wounding Intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Ramírez, M.; Santana-Gálvez, J.; Santacruz, A.; Carranza-Montealvo, L.D.; Ortega-Hernández, E.; Tirado-Escobosa, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Using a Functional Carrot Powder Ingredient to Produce Sausages with High Levels of Nutraceuticals. J. Food Sci. 2018, 83, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Pérez-Carrillo, E.; Velázquez-Reyes, H.H.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Application of Wounding Stress to Produce a Nutraceutical-Rich Carrot Powder Ingredient and Its Incorporation to Nixtamalized Corn Flour Tortillas. J. Funct. Foods 2016, 27, 655–666. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Moreira-Rodríguez, M.; Benavides, J. UVA and UVB Radiation as Innovative Tools to Biofortify Horticultural Crops with Nutraceuticals. Horticulturae 2022, 8, 387. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Munzoor Hasan, S.M.; Angers, P.; Arul, J. UV-B Radiation Hormesis in Broccoli Florets: Glucosinolates and Hydroxy-Cinnamates Are Enhanced by UV-B in Florets during Storage. Postharvest Biol. Technol. 2020, 168, 111278. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C Combination on Phenolic Compounds Biosynthesis in Fresh-Cut Carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Use of Postharvest UV-B and UV-C Radiation Treatments to Revalorize Broccoli Byproducts and Edible Florets. Innov. Food Sci. Emerg. Technol. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-Talk between Signaling Pathways: The Link between Plant Secondary Metabolite Production and Wounding Stress Response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, A.; Choudhary, K.K. Revisiting the Role of Phenylpropanoids in Plant Defense against UV-B Stress. Plant Stress 2023, 7, 100143. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Physiological Role of Reactive Oxygen Species, Ethylene, and Jasmonic Acid on UV Light Induced Phenolic Biosynthesis in Wounded Carrot Tissue. Postharvest Biol. Technol. 2021, 172, 111388. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. In Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S.I., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Swizterland, 2017; pp. 15–23. ISBN 978-3-319-56017-5. [Google Scholar]
- Tilbrook, K.; Arongaus, A.B.; Binkert, M.; Heijde, M.; Yin, R.; Ulm, R. The UVR8 UV-B Photoreceptor: Perception, Signaling and Response. Arab. Book Am. Soc. Plant Biol. 2013, 11, e0164. [Google Scholar] [CrossRef]
- Bartley, G.E.; Avena-Bustillos, R.J.; Du, W.-X.; Hidalgo, M.; Cain, B.; Breksa, A.P. Transcriptional Regulation of Chlorogenic Acid Biosynthesis in Carrot Root Slices Exposed to UV-B Light. Plant Gene 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Redondo-Gil, M.; Benavides, J.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Combined Effect of Water Loss and Wounding Stress on Gene Activation of Metabolic Pathways Associated with Phenolic Biosynthesis in Carrot. Front. Plant Sci. 2015, 6, 837. [Google Scholar] [CrossRef]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as Biofactories: Postharvest Stress-Induced Accumulation of Phenolic Compounds and Glucosinolates in Broccoli Subjected to Wounding Stress and Exogenous Phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef]
- Fernando Reyes, L.; Emilio Villarreal, J.; Cisneros-Zevallos, L. The Increase in Antioxidant Capacity after Wounding Depends on the Type of Fruit or Vegetable Tissue. Food Chem. 2007, 101, 1254–1262. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Does Consumption of Tortured Fruit and Vegetables Improve Health, and Do They Taste Good? ACS Food Sci. Technol. 2023, 3, 1311–1313. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.J.; Du, W.-X.; Woods, R.; Olson, D.; Breksa, A.P., III; McHugh, T.H. Ultraviolet-B Light Treatment Increases Antioxidant Capacity of Carrot Products. J. Sci. Food Agric. 2012, 92, 2341–2348. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC Light Enhances the Biosynthesis of Phenolic Antioxidants in Fresh-Cut Carrot through a Synergistic Effect with Wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef] [PubMed]
- Torres-Contreras, A.M.; Senés-Guerrero, C.; Pacheco, A.; González-Agüero, M.; Ramos-Parra, P.A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Genes Differentially Expressed in Broccoli as an Early and Late Response to Wounding Stress. Postharvest Biol. Technol. 2018, 145, 172–182. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef] [PubMed]
- dos Nascimento, L.B.S.; Gori, A.; Cavigli, L.; Marino, G.; Brunetti, C.; Haworth, M.; Micheletti, F.; Pöhnl, T.; Neugart, S.; Agati, G. UVB Treatments of Packaged Ready-to-Eat Salads: Induced Enhancement of Quercetin Derivatives in Baby-Leaf Lettuce (Lactuca sativa L.) and Wild Rocket (Diplotaxis tenuifolia L.). Postharvest Biol. Technol. 2024, 207, 112606. [Google Scholar] [CrossRef]
- Saltveit, M.E. Anaerobic Exposure before or after Wounding Reduces the Production of Wound-Induced Phenolic Compounds in Fresh-Cut Lettuce. Postharvest Biol. Technol. 2018, 135, 77–82. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of Bioactive Compounds in Broccoli as Affected by Cutting Styles and Storage Time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-X.; Avena-Bustillos, R.J.; Breksa, A.P.; McHugh, T.H. Effect of UV-B Light and Different Cutting Styles on Antioxidant Enhancement of Commercial Fresh-Cut Carrot Products. Food Chem. 2012, 134, 1862–1869. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef]
- Reza-Zaldívar, E.E.; Bojorquez-Rodríguez, E.M.; Jacobo-Velázquez, D.A. Wounding Stress Enhances the Anti-Obesogenic, Anti-Inflammatory, and Antioxidant Properties of Carrots (Daucus carota). J. Agric. Food Res. 2024, 16, 101155. [Google Scholar] [CrossRef]
- Ning, W.; Peng, X.; Ma, L.; Cui, L.; Lu, X.; Wang, J.; Tian, J.; Li, X.; Wang, W.; Zhang, L. Enhanced Secondary Metabolites Production and Antioxidant Activity in Postharvest Lonicera Japonica Thunb. in Response to UV Radiation. Innov. Food Sci. Emerg. Technol. 2012, 13, 231–243. [Google Scholar] [CrossRef]
- Takshak, S.; Agrawal, S.B. Defense Potential of Secondary Metabolites in Medicinal Plants under UV-B Stress. J. Photochem. Photobiol. B 2019, 193, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic Acid: A Review on Its Mechanisms of Anti-Inflammation, Disease Treatment, and Related Delivery Systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Reza-Zaldivar, E.E.; Jacobo-Velázquez, D.A. Evaluating Bioactivity Retention in Metabolism: A Comparative Study of Chlorogenic Acid and Its Metabolite Dihydrocaffeic Acid on Antioxidant, Anti-Inflammatory, and Anti-Obesogenic Activities in Vitro. CyTA J. Food 2024, 22, 2390997. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-Inflammatory Effects of Chlorogenic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and Glucose Metabolism in White Adipocytes: Pathways, Dysfunction and Therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic Acid Derivatives: A Potential Class of Natural Compounds for the Management of Lipid Metabolism and Obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; Del Mar Contreras, M. Phenolic Compounds as Natural and Multifunctional Anti-Obesity Agents: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Managing Obesity through Natural Polyphenols: A Review. Future Foods 2020, 1–2, 100002. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Iqbal, M.S.; Srivastava, J.K. Therapeutic Promises of Chlorogenic Acid with Special Emphasis on Its Anti-Obesity Property. Curr. Mol. Pharmacol. 2020, 13, 7–16. [Google Scholar] [CrossRef]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Ke, P.-Y.; Wu, C.-Y.; Peng, H.-H.; Young, J.D. Hormetic Effects of Phytochemicals on Health and Longevity. Trends Endocrinol. Metab. 2019, 30, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lin, Z.; Zhang, Y.; Zheng, B.; Tan, B.K.; Zhang, Y.; Hu, J. Analysis of Transcriptomic, Lipidomic and Phospho-Kinase Profiles Reveals the Effects of Chlorogenic Acid on 3T3-L1 Preadipocytes Differentiation. J. Funct. Foods 2023, 110, 105828. [Google Scholar] [CrossRef]
- Peng, S.; Pang, Y.; Zhu, Q.; Kang, J.; Liu, M.; Wang, Z. Chlorogenic Acid Functions as a Novel Agonist of PPARγ2 during the Differentiation of Mouse 3T3-L1 Preadipocytes. BioMed Res. Int. 2018, 2018, 8594767. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Sathyanarayan, A.; Mashek, M.T.; Ong, K.T.; Wollaston-Hayden, E.E.; Mashek, D.G. ATGL-Catalyzed Lipolysis Regulates SIRT1 to Control PGC-1α/PPAR-α Signaling. Diabetes 2015, 64, 418–426. [Google Scholar] [CrossRef]
- Chakrabarti, P.; English, T.; Karki, S.; Qiang, L.; Tao, R.; Kim, J.; Luo, Z.; Farmer, S.R.; Kandror, K.V. SIRT1 Controls Lipolysis in Adipocytes via FOXO1-Mediated Expression of ATGL. J. Lipid Res. 2011, 52, 1693–1701. [Google Scholar] [CrossRef]



| Carrot | Broccoli | Lettuce | ||||
|---|---|---|---|---|---|---|
| 0 h | 48 h | 0 h | 48 h | 0 h | 48 h | |
| β0 1 | 42.56 | 197.28 | 140.56 | 118.33 | 16.39 | 11.80 |
| β1 1 | 6.24 | −38.40 | −1.93 | 0.98 | −0.63 | 0.94 |
| β2 1 | 3.76 | −32.68 | −8.57 | 5.42 | 0.54 | 0.67 |
| β3 1 | −0.36 | 3.32 | 0.18 | −0.14 | 0.07 | −0.07 |
| β4 1 | −0.85 | 5.97 | 1.83 | −1.28 | −0.17 | −0.12 |
| β5 1 | 0.34 | 7.12 | 0.65 | −0.01 | 0.06 | −0.53 |
| β6 1 | 0.01 | −0.07 | −0.01 | 0.01 | −0.01 | 0.01 |
| β7 1 | 0.05 | −0.28 | −0.10 | 0.07 | 0.01 | 0.01 |
| β8 1 | −0.01 | −0.21 | −0.01 | 0.01 | −0.01 | 0.02 |
| β9 1 | −0.03 | −0.52 | −0.06 | 0.01 | −0.01 | 0.05 |
| β10 1 | 0.01 | 0.02 | 0.01 | −0.01 | 0.01 | −0.01 |
| R2 | 0.97 | 0.79 | 0.89 | 0.79 | 0.77 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastélum-Estrada, A.; Reza-Zaldivar, E.E.; Jacobo-Velázquez, D.A. Boosting Health Benefits in Vegetables: A Novel Ultraviolet B (UVB) Device for Rapid At-Home Enhancement of Phytochemicals and Bioactivity. Foods 2024, 13, 3311. https://doi.org/10.3390/foods13203311
Gastélum-Estrada A, Reza-Zaldivar EE, Jacobo-Velázquez DA. Boosting Health Benefits in Vegetables: A Novel Ultraviolet B (UVB) Device for Rapid At-Home Enhancement of Phytochemicals and Bioactivity. Foods. 2024; 13(20):3311. https://doi.org/10.3390/foods13203311
Chicago/Turabian StyleGastélum-Estrada, Alejandro, Edwin E. Reza-Zaldivar, and Daniel A. Jacobo-Velázquez. 2024. "Boosting Health Benefits in Vegetables: A Novel Ultraviolet B (UVB) Device for Rapid At-Home Enhancement of Phytochemicals and Bioactivity" Foods 13, no. 20: 3311. https://doi.org/10.3390/foods13203311
APA StyleGastélum-Estrada, A., Reza-Zaldivar, E. E., & Jacobo-Velázquez, D. A. (2024). Boosting Health Benefits in Vegetables: A Novel Ultraviolet B (UVB) Device for Rapid At-Home Enhancement of Phytochemicals and Bioactivity. Foods, 13(20), 3311. https://doi.org/10.3390/foods13203311







